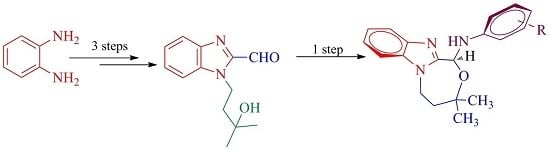
Synthesis, Spectroscopic, X-ray Diffraction and DFT Studies of Novel Benzimidazole Fused-1,4-Oxazepines
Abstract
:1. Introduction
2. Results and Discussion
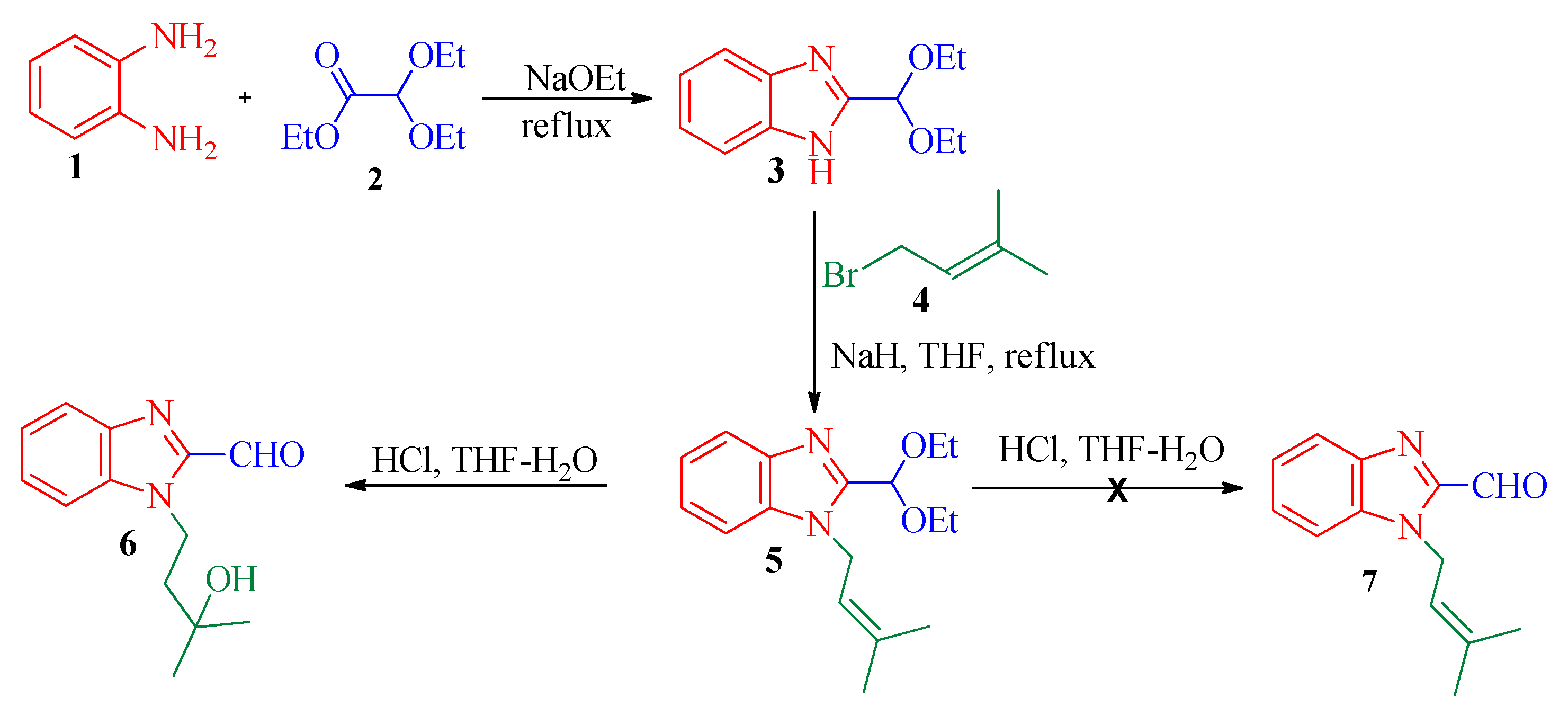
2.1. Synthesis
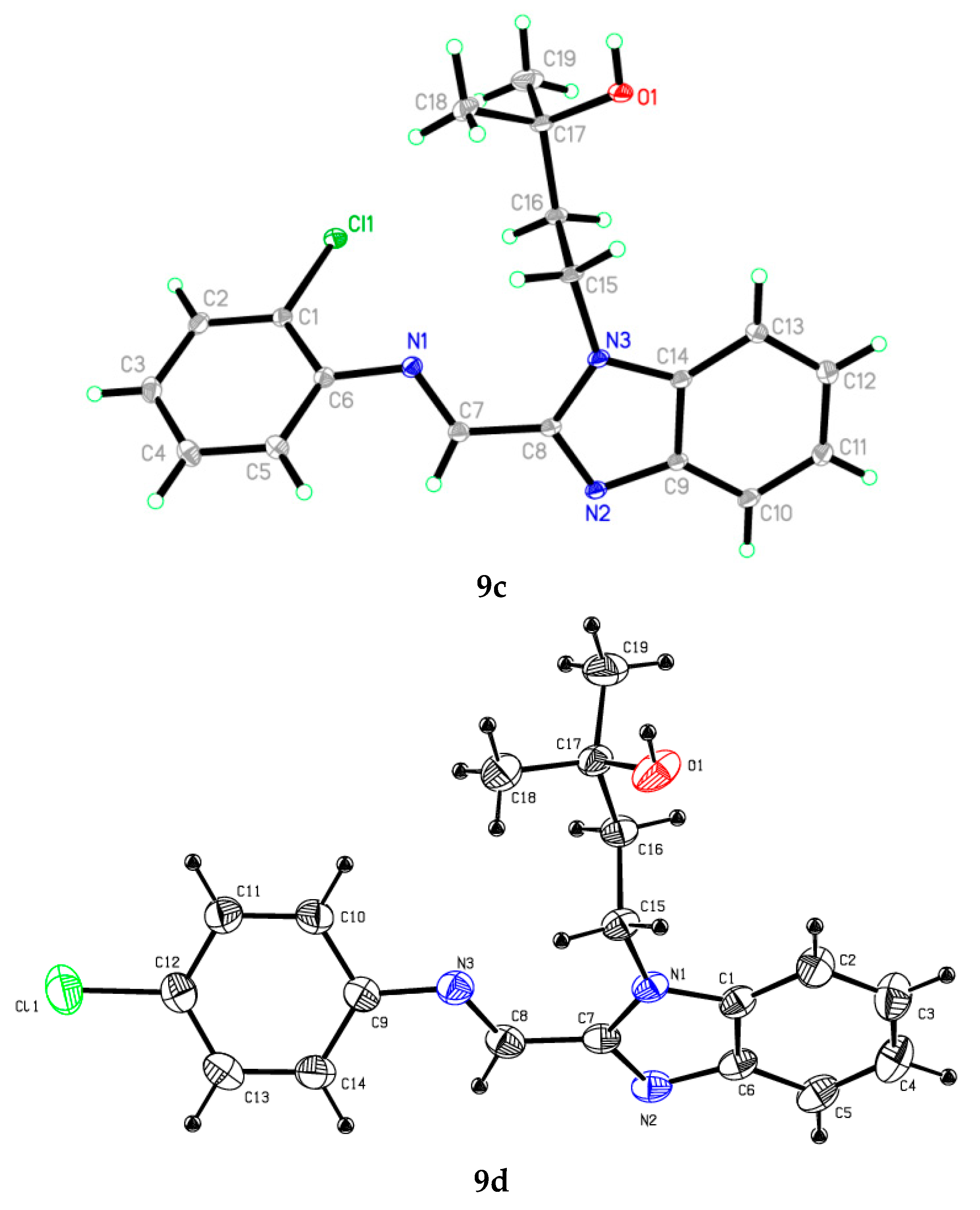
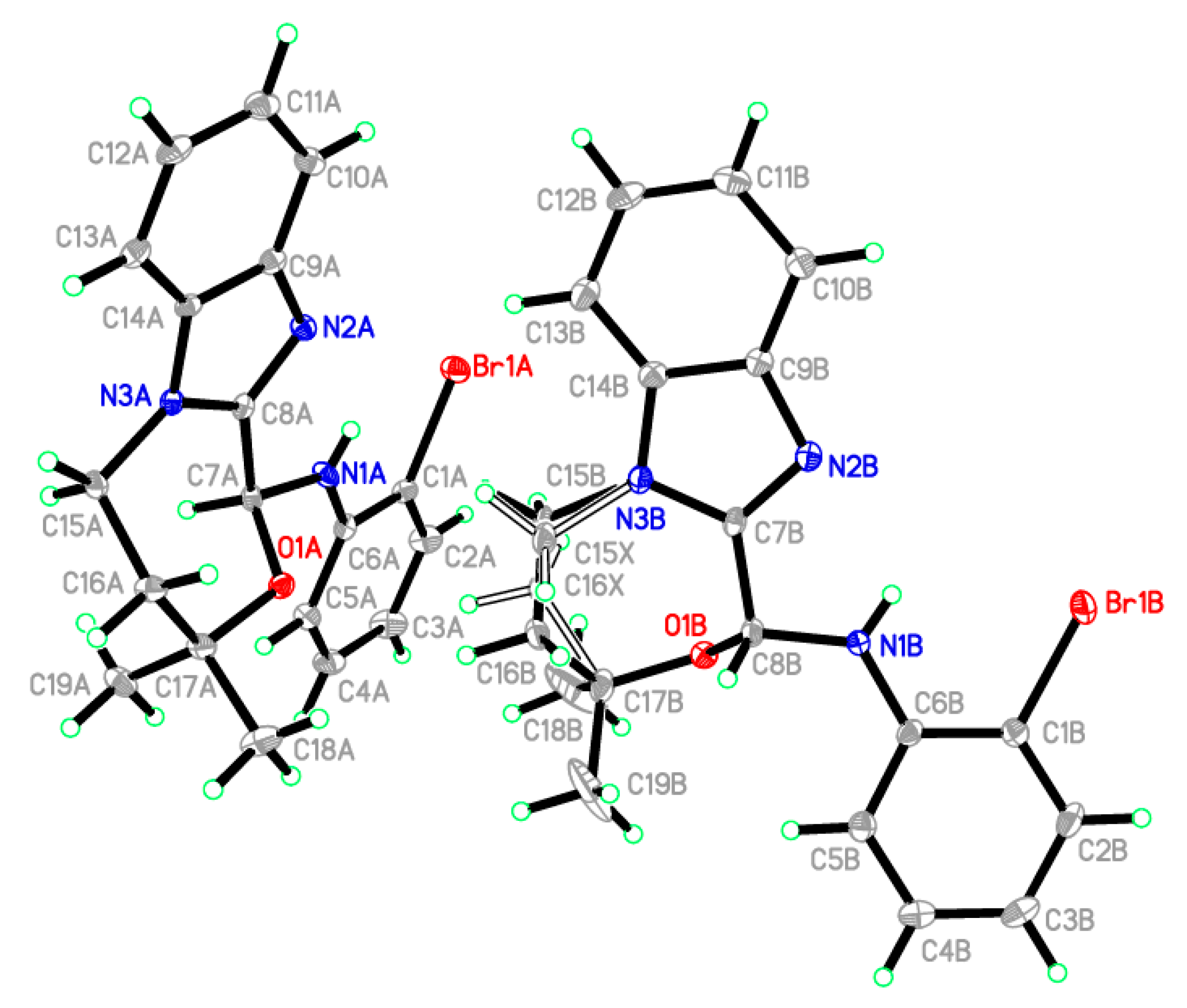
2.2. X-ray Crystallographic Studies
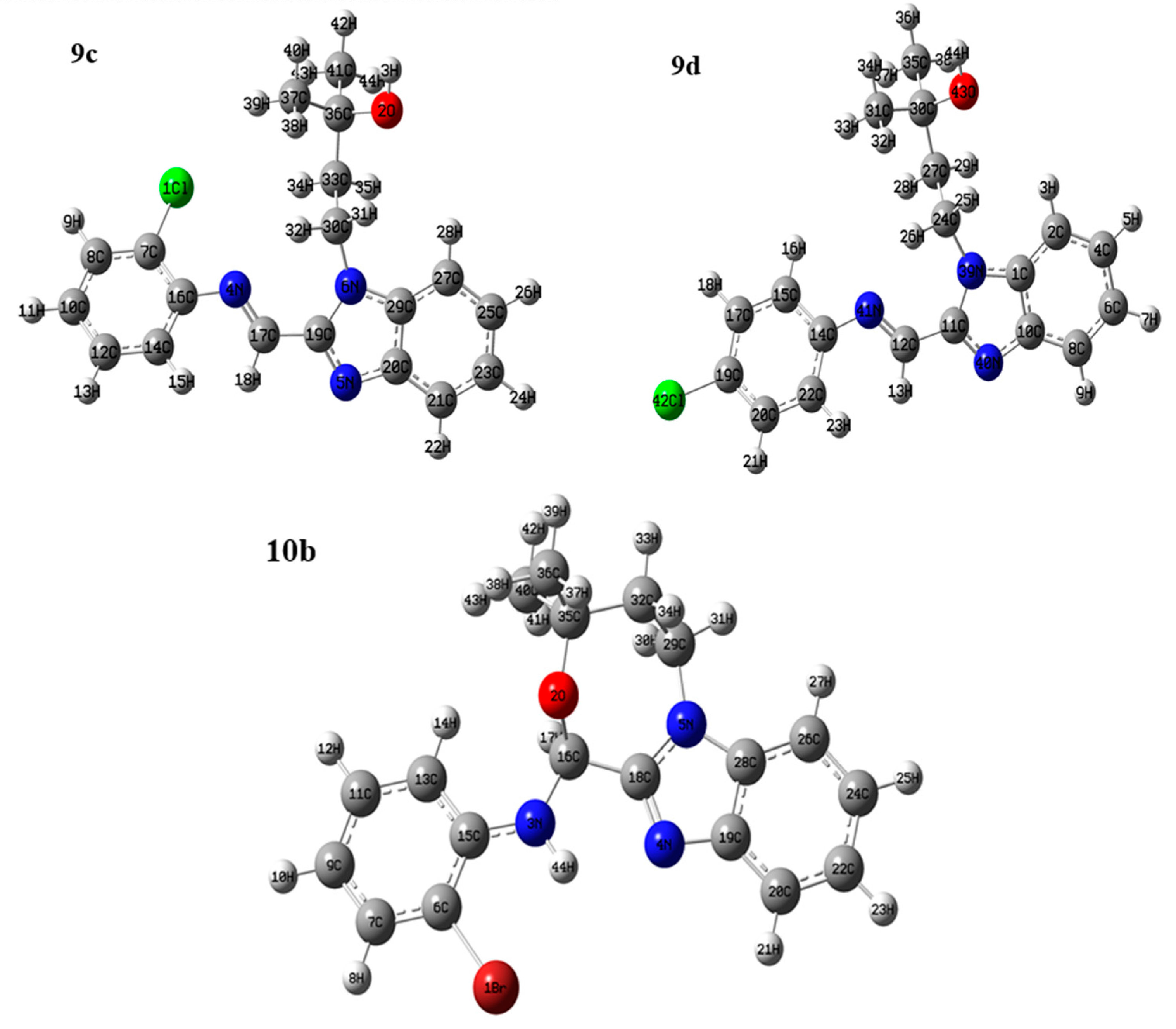
2.3. Optimized Molecular Geometry
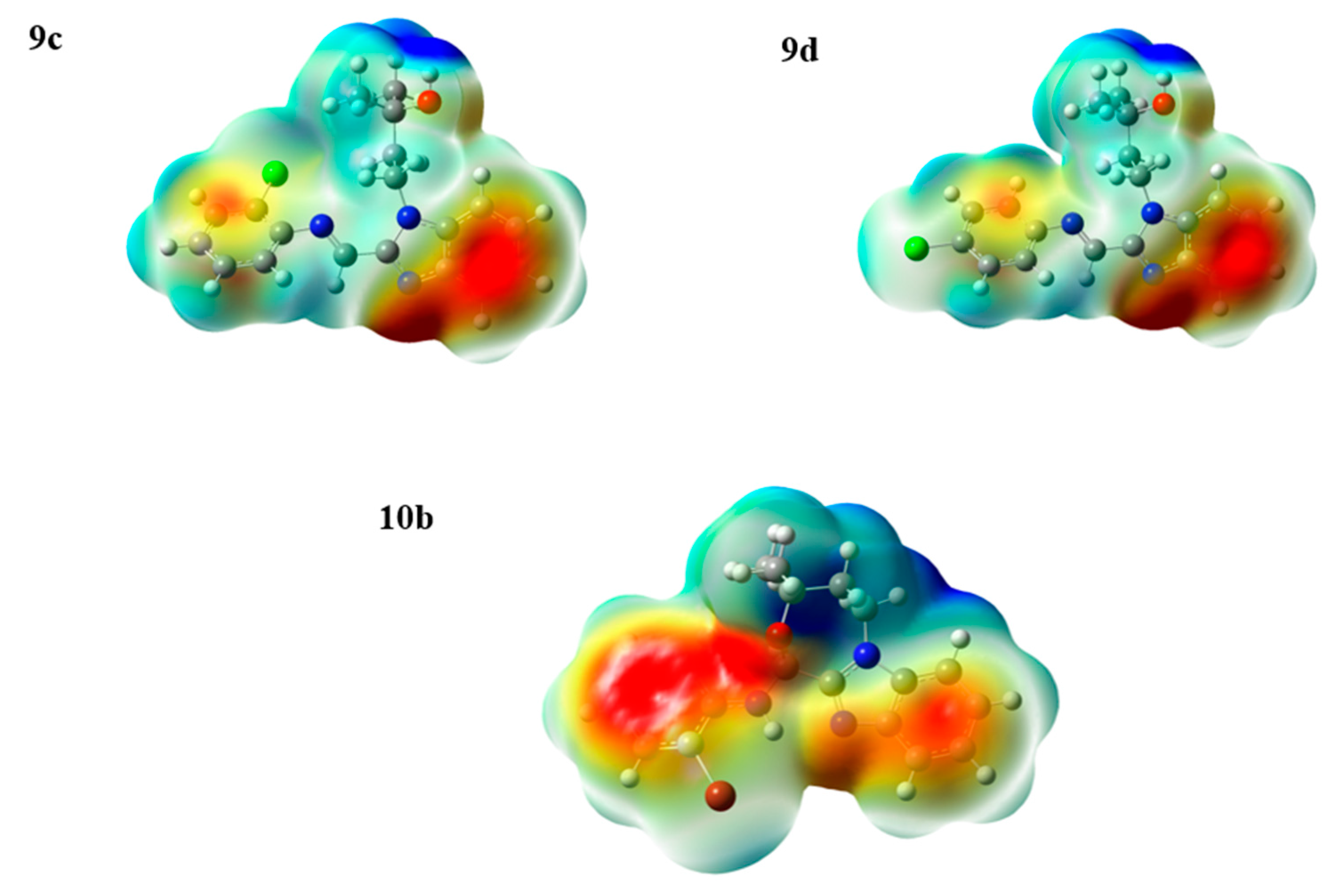
2.4. Natural Atomic Charges
2.5. Frontier Molecular Orbitals
2.6. Nonlinear Optical Properties
3. Materials and Methods
3.1. General Procedure
3.2. Synthesis of 2-(Diethoxymethyl)-1-(3-methylbut-2-en-1-yl)-1H-benzimidazole, 5
3.3. Synthesis of 1-Allyl-1H-benzimidazole-2-carboxaldehydes, 6
3.4. General Procedure for the Synthesis of (E)-2-Methyl-4-(2-((phenylimino)methyl)-1H-benzimidazol-1-yl)butan-2-ol, 9a–e
3.5. General Procedure for Synthesis of Benzimidazole Fused 1,4-Oxazepines, 10a–e
3.6. Computational Details
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Robl, J.A.; Simpkins, L.M.; Asaad, M.M. N-Formyl hydroxylamine containing dipeptides: Generation of a new class of vasopeptidase finhibitors. Bioorg. Med. Chem. Lett. 2000, 10, 257–260. [Google Scholar] [CrossRef]
- Murakami, Y.; Hara, H.; Okada, T.; Hashizume, H.; Kii, M.; Ishihara, Y.; Ishikawa, M.; Shimamura, M.; Mihara, S.; Kato, G.; et al. 1,3-Disubstituted benzazepines as novel, potent, selective neuropeptide Y Y1 receptor antagonists. J. Med. Chem. 1999, 42, 2621–2632. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H.; Ikegami, S.; Watanuki, M.; Maruyama, T.; Sumita, Y.; Inoguchi, K. Azepine Derivatives. Patent WO 0155121, 7 Augut 2001. [Google Scholar]
- Yukimasa, H.; Tozawa, R.; Kori, M.; Kitano, K. 4,1-benzoxazepin Derivatives and Their Use. U.S. Patent 5,726,306, 10 March 1998. [Google Scholar]
- Rodgers, J.D.; Cocuzza, A.J. 5,5-disubstituted-1,5-dihydro-4,1-benzoxazepin-2(3H)-ones Useful as HIV Reverse Transcriptase Inhibitors. U.S. Patent 6,140,320, 31 October 2000. [Google Scholar]
- Sica, D.A. Alpha1-adrenergic blockers: Current usage considerations. J. Clin. Hypertens. 2005, 7, 757–762. [Google Scholar] [CrossRef]
- Cale, A.D., Jr.; Gero, T.W.; Walker, K.R.; Lo, Y.S.; Welstead, W.J., Jr.; Jaques, L.W.; Johnson, A.F.; Leonard, C.A.; Nolan, J.C.; Johnson, D.N. Benzo- and pyrido-1,4-oxazepin-5-ones and -thiones: Synthesis and structure-activity relationships of a new series of H1-antihistamines. J. Med. Chem. 1989, 32, 2178–2199. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.-Q.; Wei, C.-X.; Li, F.-N.; Sun, Z.-G.; Quan, Z.-S. Design and synthesis of 10-alkoxy-5, 6-dihydro-triazolo[4,3-d]benzo[f][1,4]oxazepine derivatives with anticonvulsant activity. Eur. J. Med. Chem. 2010, 45, 3080–3086. [Google Scholar] [CrossRef] [PubMed]
- Kapples, K.J.; Effland, R.C. Pyrrolo[2,1-c][1,4]benzoxazepines. 2. Synthesis of 5-phenyl derivatives. J. Heterocycl. Chem. 1993, 30. [Google Scholar] [CrossRef]
- Serrano-Wu, M.H.; St Laurent, D.R.; Chen, Y.; Huang, S.; Lam, K.-R.; Matson, J.A.; Mazzucco, C.E.; Stickle, T.M.; Tully, T.P.; Wong, H.S.; et al. Sordarin Oxazepine Derivatives as Potent Antifungal Agents. Bioorg. Med. Chem. Lett. 2002, 12, 2757–2760. [Google Scholar] [CrossRef]
- Klunder, J.M.; Hargrave, K.D.; West, M.; Cullen, E.; Pal, K.; Behnke, M.L.; Kapadia, S.R.; McNeil, D.W.; Wu, J.C.; Chow, G.C. Novel non-nucleoside inhibitors of HIV-1 reverse transcriptase. 2. Tricyclic pyridobenzoxazepinones and dibenzoxazepinones. J. Med. Chem. 1992, 35, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.M.A.D.; Brigitte, J.B.F.; Hans, H.; Cor, W.K.; Hans, L.; Lourdes, O.; Jos, B.M.R.; Pedro, H.H.H. SAR study of 2,3,4,14b-tetrahydro-1H-dibenzo[b,f]pyrido[1,2-d][1,4]oxazepines as progesterone receptor agonists. Bioorg. Med. Chem. Lett. 2008, 18, 1461–1467. [Google Scholar]
- Xin-Hua, L.; Ying-Ming, J.; Bao-An, S.; Zhi-xiang, P.; Song, Y. Design and synthesis of novel 2-methyl-4,5-substitutedbenzo[f]-3,3a,4,5-tetrahydro-pyrazolo[1,5-d][1,4]oxazepin-8(7H)-one derivatives as telomerase inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 720–723. [Google Scholar]
- Yong, Y.; Yan-Qing, Z.; Biao, J.; Shao, S.; Xun, W.; Chetan, B.S.; She-Feng, W.; Fang, Q.; Ai-Min, Lu.; Peng-Cheng, L.; et al. 6,7-Dihydrobenzo[f]benzo[4,5]imidazo[1,2-d][1,4]oxazepine derivatives as selective inhibitors of PI3Kα. Bioorg. Med. Chem. 2015, 23, 1231–1240. [Google Scholar]
- Komazin, G.; Ptak, R.G.; Emmer, B.T.; Townsend, L.B.; Drach, J.C. Resistance of Human Cytomegalovirus to the Benzimidazole l-Ribonucleoside Maribavir Maps to UL27. Nucleosides Nucleotides. Nucl. 2003, 22, 1725–1727. [Google Scholar] [CrossRef] [PubMed]
- Mader, M.; De Dios, A.; Shih, C.; Bonjouklian, R.; Li, T.; White, W.; López de Uralde, B.; Sánchez-Martinez, C.; Prado, M.D.; Jaramillo, C.; et al. Imidazolyl benzimidazoles and imidazo[4,5-b]pyridines as potent p38α MAP kinase inhibitors with excellent in vivo antiinflammatory properties. Bioorg. Med. Chem. Lett. 2008, 18, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Chimirri, A.; Clercq, E.D.; Monforte, A.M.; Monforte, P.; Pannecouque, C.; Zappala, M. Synthesis and anti-HIV activity of 1-(2,6-difluorophenyl)-1H,3H-thiazolo[3,4-a]benzimidazole structurally-related 1,2-substituted benzimidazoles. II Farmaco 2002, 57, 819–823. [Google Scholar] [CrossRef]
- Goker, H.; Kus, C.; Boykin, D.W.; Yildiz, S.; Altanlar, N. Synthesis of some new 2-substituted-phenyl-1H-benzimidazole-5-carbonitriles and their potent activity against candida species. Bioorg. Med. Chem. 2002, 10, 2589–2596. [Google Scholar] [CrossRef]
- Penning, T.D.; Zhu, G.D.; Gandhi, V.B.; Gong, J.; Liu, X.; Shi, Y.; Klinghofer, V.; Johnson, E.F.; Donawho, C.K.; Frost, D.J.; et al. Discovery of the Poly(ADP-ribose) Polymerase (PARP) Inhibitor 2-[(R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-4-carboxamide (ABT-888) for the Treatment of Cancer. J. Med. Chem. 2009, 52, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Abonia, R.; Cortes, E.; Insuasty, B.; Quiroga, J.; Nogueras, M.; Cobo, J. Synthesis of novel 1,2,5-trisubstituted benzimidazoles as potential antitumor agents. Eur. J. Med. Chem. 2011, 46, 4062–4070. [Google Scholar] [CrossRef] [PubMed]
- Demirayak, S.; Usama, A.; Mohsen, A.C.; Agri, K. Synthesis and anticancer and anti-HIV testing of some pyrazino[1,2-a]benzimidazole derivatives. Eur. J. Med. Chem. 2002, 37, 255–260. [Google Scholar] [CrossRef]
- Kamal, A.; Praveen Kumar, P.; Sreekanth, K.; Seshadri, B.N.; Ramulu, P. Synthesis of new benzimidazole linked pyrrolo[2,1-c][1,4]benzodiazepine conjugates with efficient DNA- binding affinity and potent cytotoxicity. Bioorg. Med. Chem. Lett. 2008, 18, 2594–2598. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, N.; Almansour, A.I.; Suresh Kumar, R.; Perumal, S.; Ghabbour, H.G.; Fun, H.-K. A 1,3-dipolar cycloaddition-annulation protocol for the expedient regio-, stereo- and product-selective construction of novel hybrid heterocycles comprising seven rings and seven contiguous stereocentres. Tetrahedron Lett. 2013, 54, 2515–2519. [Google Scholar] [CrossRef]
- Almansour, A.I.; Arumugam, N.; Suresh Kumar, R.; Menéndez, J.C.; Ghabbour, H.A.; Fun, H.-K.; Ranjith Kumar, R. Straightforward synthesis of pyrrolo[3,4-b]quinolines through intramolecular Povarov reactions. Tetrahedron Lett. 2015, 56, 6900–6903. [Google Scholar] [CrossRef]
- Arumugam, N.; Raghunathan, R.; Almansour, A.I.; Karama, U. An efficient synthesis of highly functionalized novel chromeno[4,3-b]pyrroles and indolizino[6,7-b]indoles as potent antimicrobial and antioxidant agents. Bioorg. Med. Chem. Lett. 2012, 22, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, N.; Almansour, A.I.; Suresh Kumar, R.; Menéndez, J.C.; Sultan, M.A.; Karama, U.; Ghabbour, H.A.; Fun, H.-K. An Expedient Regio- and Diastereoselective Synthesis of Hybrid Frameworks with Embedded Spiro[9,10]dihydroanthracene [9,3′]-pyrrolidine and Spiro[oxindole-3,2′-pyrrolidine] Motifs via an Ionic Liquid-Mediated Multicomponent Reaction. Molecules 2015, 20, 16142–16153. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, N.; Abdul Rahim, A.S.; Hamid, S.A.; Osman, H. Straightforward Synthesis of Novel 1-(2′-α-O-D-Glucopyranosyl ethyl) 2-Arylbenzimidazoles. Molecules 2012, 17, 9887–9899. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rahim, A.S.; Salhimi, S.M.; Arumugam, N.; Pin, L.C.; Yee, N.S.; Muttiah, N.N.; Keat, W.B.; Hamid, S.A.; Osman, H.; Mat, I.B. Microwave-assisted synthesis of sec/tert-butyl 2-arylbenzimidazoles and their unexpected antiproliferative activity towards ER negative breast cancer cells. J. Enzym. Inhib. Med. Chem. 2013, 28, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Fettinger, J.C.; Kurth, M.J. Intramolecular Cycloaddition of Azomethine Ylides in the Preparation of Pyrrolidino[2‘,3‘:3,4]pyrrolidino[1,2-a]benzimidazoles. Org. Lett. 2007, 9, 5055–5058. [Google Scholar]
- Butler, P.; Gallagher, J.F.; Manning, A.R. Synthesis and characterisation of alkynylacetal and alkynylaldehyde derivatives of Ni(II) and Fe(II); the X-ray crystal structure of Ni(h5-C5H5)(PPh3)C-CCH(OEt)2. Inorg. Chem. Commun. 1998, 1, 343–345. [Google Scholar] [CrossRef]
- CCDC 1062849 (9c) and 974580 (9d) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: [email protected]).
- Govindaraj, J.; Raja, R.; Suresh, M.; Raghunathan, R.; SubbiahPandi, A. Crystal structures of methyl 3-phenyl-4,5-dihydro-1H,3H-benzo[4,5]imidazo[2,1-c][1,4]oxazepine-4-carboxylate and methyl 1-methyl-3-phenyl-4,5-dihydro-1H,3H-benzo[4,5]imidazo[2,1-c][1,4]oxazepine-4-carboxylate. Acta Crystallogr. Sect. E Struct. Rep. Online 2014, 70, 316–318. [Google Scholar] [CrossRef] [PubMed]
- CCDC 1402796 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: [email protected]).
- Murray, J.S.; Sen, K. Molecular Electrostatic Potentials, Concepts and Applications; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Scrocco, E.; Tomasi, J. Electronic Molecular Structure, Reactivity and Intermolecular Forces: An Euristic Interpretation by Means of Electrostatic Molecular Potentials. Adv. Quantum Chem. 1978, 11, 115–193. [Google Scholar]
- Fukui, K.; Yonezawa, T.; Shingu, H.J. A molecular-orbital theory of reactivity in aromatic hydrocarbons. J. Chem. Phys. 1952, 20. [Google Scholar] [CrossRef]
- Padmaja, L.; Ravikumar, C.; Sajan, D.; Joe, I.H.; Jayakumar, V.S.; Pettit, G.R.; Neilsen, F.O. Density functional study on the structural conformations and intramolecular charge transfer from the vibrational spectra of the anticancer drug combretastatin-A2. J. Raman Spectrosc. 2009, 40, 419–428. [Google Scholar] [CrossRef]
- Ravikumar, C.; Joe, I.H.; Jayakumar, V.S. Charge transfer interactions and nonlinear optical properties of push-pull chromophore benzaldehyde phenylhydrazone: A vibrational approach. Chem. Phys. Lett. 2008, 460, 552–558. [Google Scholar] [CrossRef]
- Geskin, V.M.; Lambert, C.; Bredas, J.-L. Origin of High Second- and Third-Order Nonlinear Optical Response in Ammonio/Borato Diphenylpolyene Zwitterions: The Remarkable Role of Polarized Aromatic Groups. J. Am. Chem. Soc. 2003, 125, 15651–15658. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.S. Observing High Second Harmonic Generation and Control of Molecular Alignment in One Dimension Cyclobutenediones as a Promising New Acceptor for Nonlinear Optical Materials. In Materials for Nonlinear Optics; ACS Symposium Series: Washington, DC, USA, 1991; Volume 455, p. 331. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian—03, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Dennington, R.; Keith, T., II; Millam, J. (Eds.) GaussView, Version 4.1; Semichem Inc.: Shawnee Mission, KS, USA, 2007.
- Sample Availability: Samples of the compounds 9 and 10 are available from the authors.



| Entry | Imine | Mp °C | Yield a % | Product | Mp °C | Yield b % |
|---|---|---|---|---|---|---|
| 1 |  9a 9a | 155–157 | 91 |  10a 10a | 197–199 | 60 |
| 2 |  9b 9b | 234–236 | 93 |  10b 10b | 220–222 | 63 |
| 3 |  9c 9c | 241–243 | 92 |  10c 10c | 225–227 | 62 |
| 4 |  9d 9d | 180–182 | 96 |  10d 10d | 252–254 | 64 |
| 5 |  9e 9e | 188–190 | 90 |  10e 10e | 222–224 | 67 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almansour, A.I.; Arumugam, N.; Suresh Kumar, R.; Soliman, S.M.; Altaf, M.; Ghabbour, H.A. Synthesis, Spectroscopic, X-ray Diffraction and DFT Studies of Novel Benzimidazole Fused-1,4-Oxazepines. Molecules 2016, 21, 724. https://doi.org/10.3390/molecules21060724
Almansour AI, Arumugam N, Suresh Kumar R, Soliman SM, Altaf M, Ghabbour HA. Synthesis, Spectroscopic, X-ray Diffraction and DFT Studies of Novel Benzimidazole Fused-1,4-Oxazepines. Molecules. 2016; 21(6):724. https://doi.org/10.3390/molecules21060724
Chicago/Turabian StyleAlmansour, Abdulrahman I., Natarajan Arumugam, Raju Suresh Kumar, Saied M. Soliman, Mohammad Altaf, and Hazem A. Ghabbour. 2016. "Synthesis, Spectroscopic, X-ray Diffraction and DFT Studies of Novel Benzimidazole Fused-1,4-Oxazepines" Molecules 21, no. 6: 724. https://doi.org/10.3390/molecules21060724