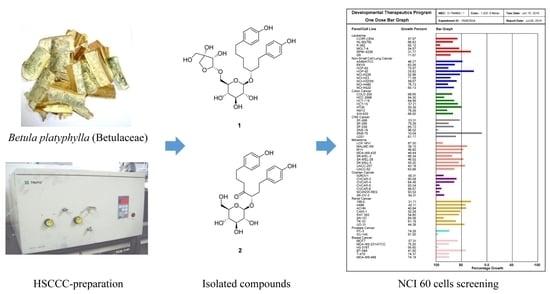
Preparative Purification of Anti-Proliferative Diarylheptanoids from Betula platyphylla by High-Speed Counter-Current Chromatography
Abstract
:1. Introduction
2. Results and Discussion
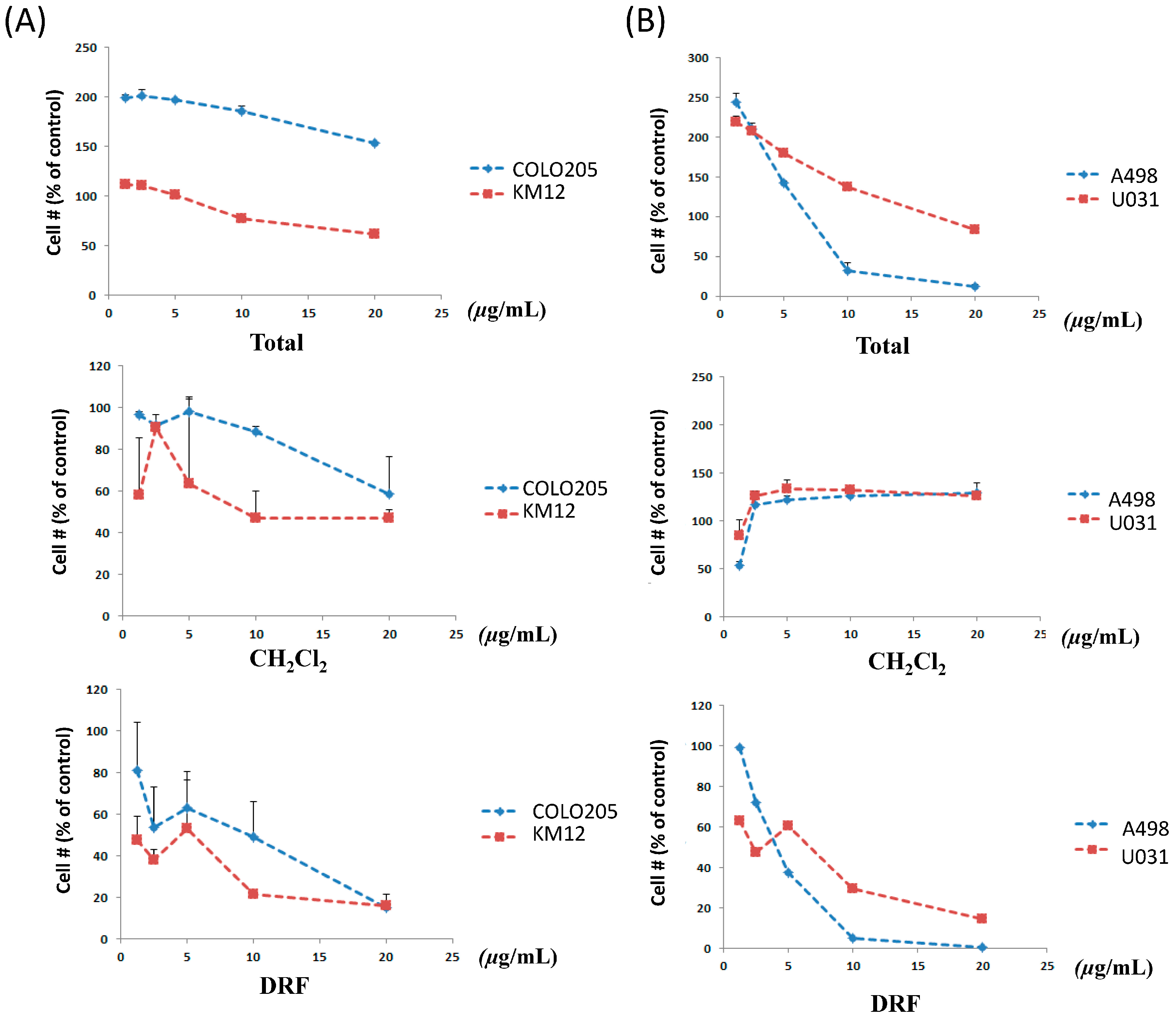
2.1. Effect of B. platyphylla Bark Extract and Fractions on Renal and Colon Cancer Cells
2.2. Solvent System Selection
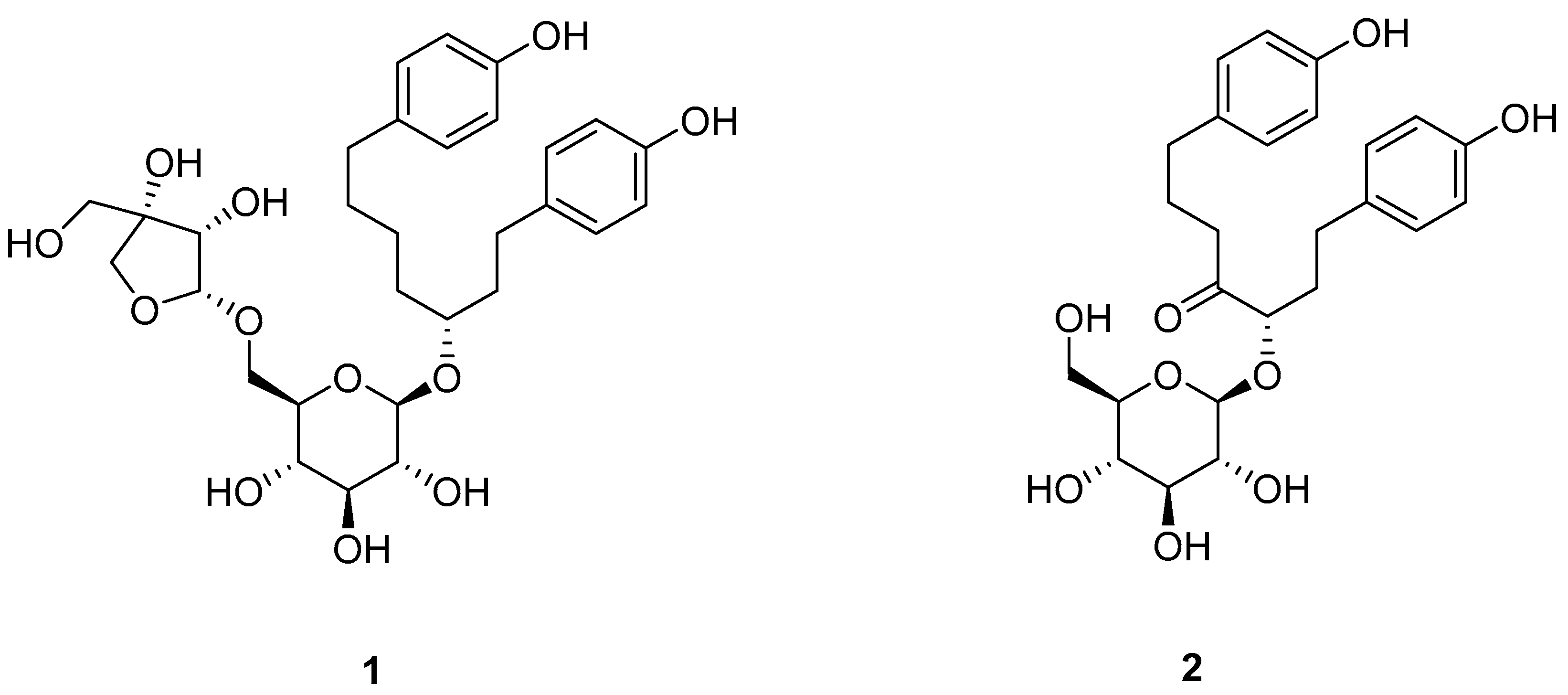
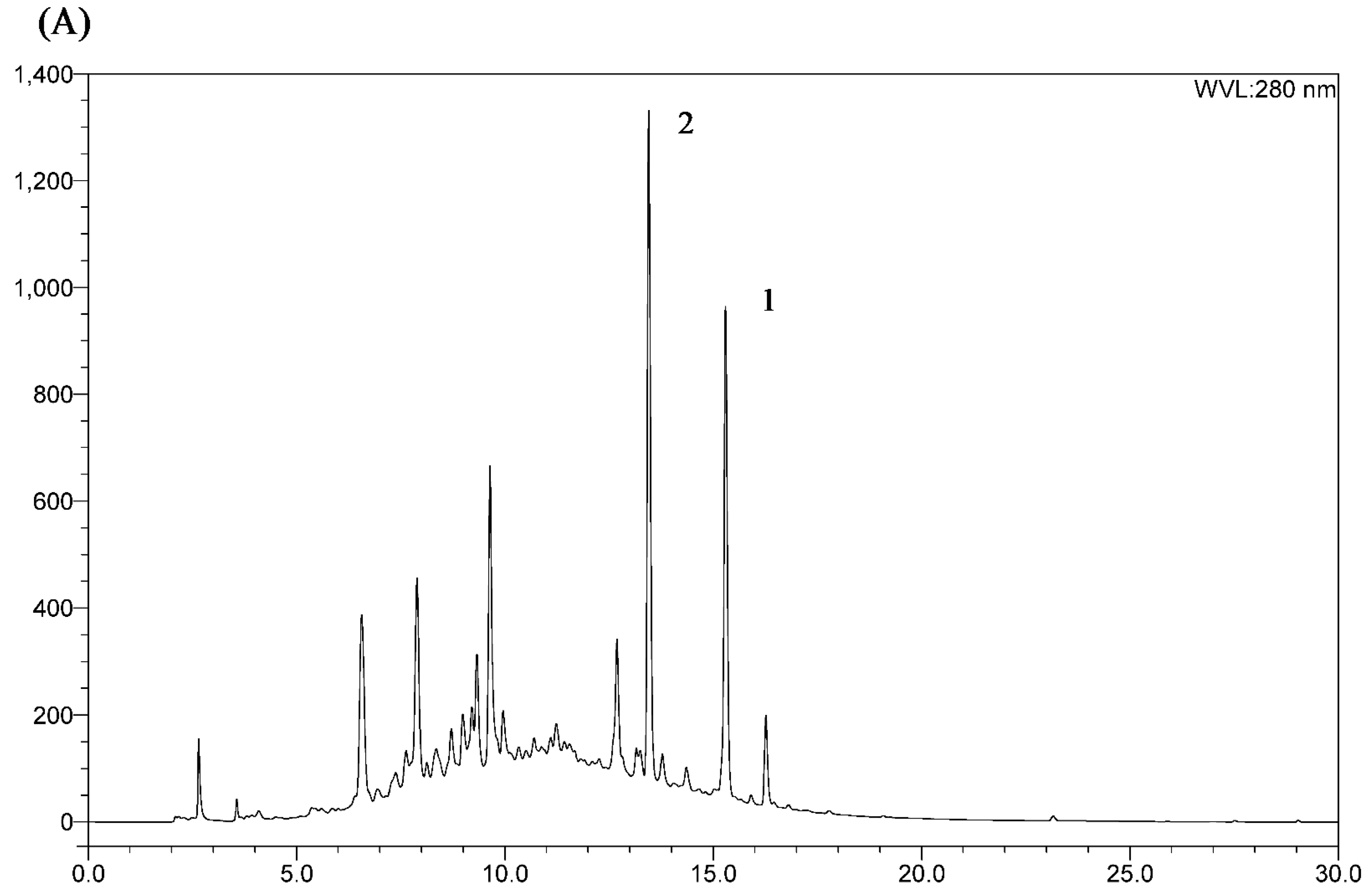
2.3. HSCCC Separation
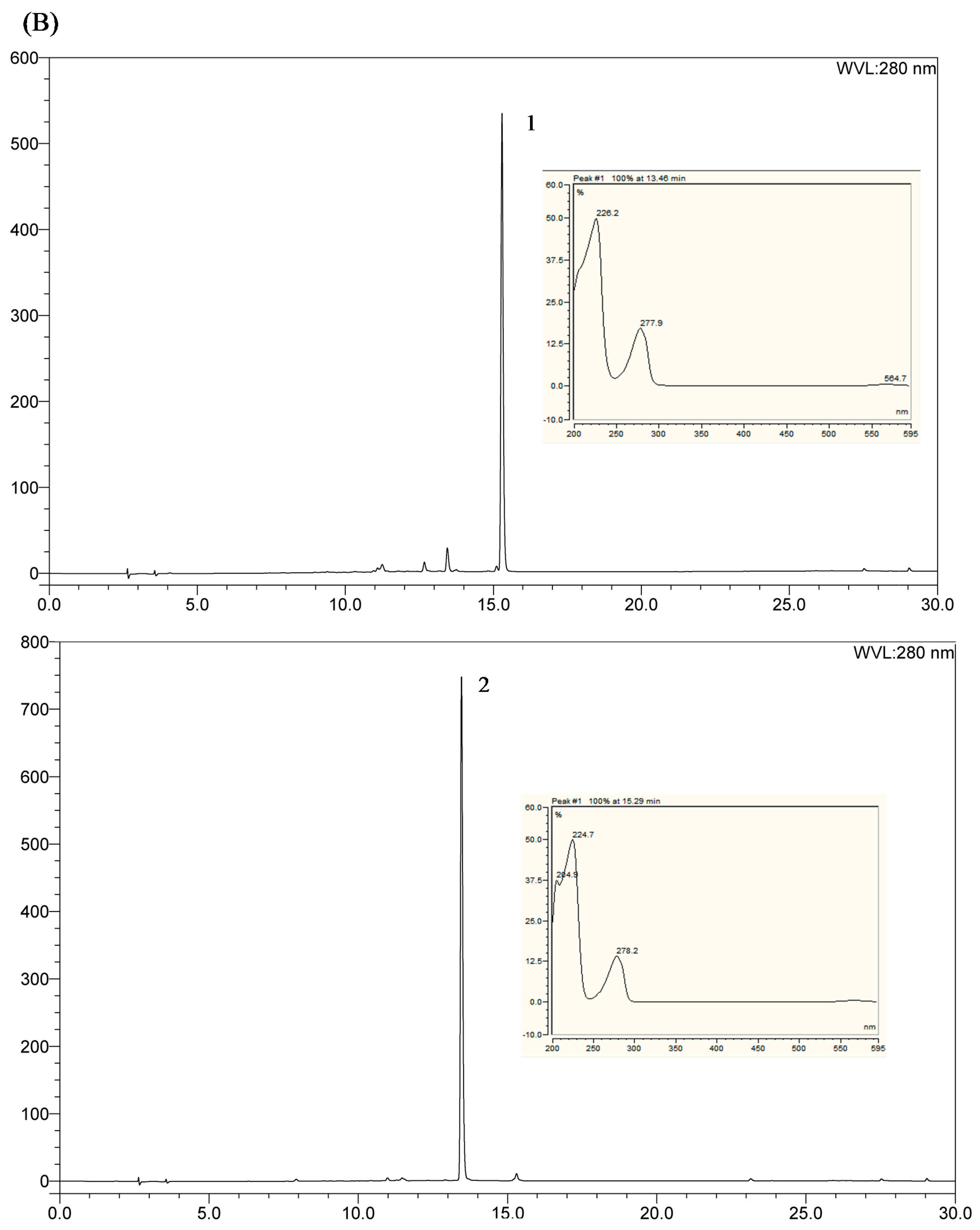
2.4. Comparison with HSCCC Separation and Traditional Isolation Techniques
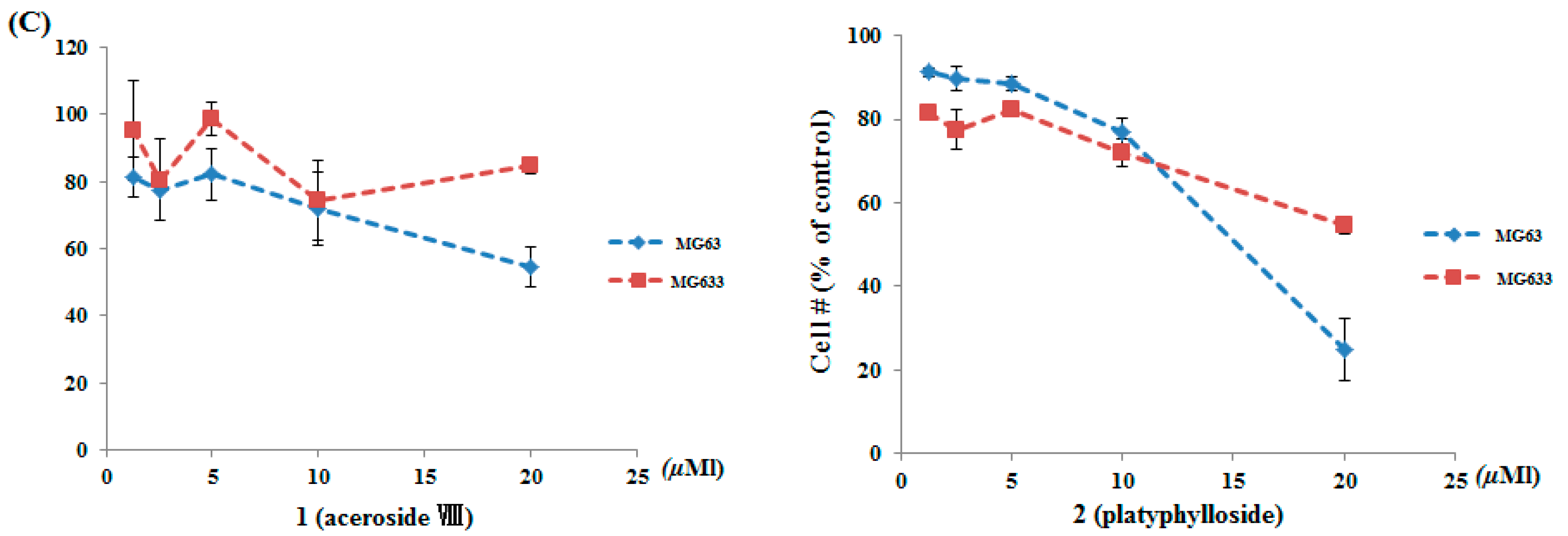
2.5. Anti-Proliferative Effects
3. Experimental Section
3.1. Materials and Reagents
3.2. Apparatus
3.3. HPLC Analysis
3.4. Determination of the Partition Coefficient Values (K-Value)
3.5. Preparation of the Two-Phase Solvent System and Sample Solution
3.6. HSCCC Separation Procedure
3.7. Identification of Isolated Peak Fraction
3.8. Cytotoxicity Assay for Colon, Renal, and Osteosarcoma Cancer Cells
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McKee, T.C.; Rabe, D.; Bokesch, H.R.; Grkovic, T.; Whitson, E.L.; Diyabalanage, T.; VanWyk, A.W.; Marcum, S.R.; Gardella, R.S.; Gustafson, K.R.; et al. Inhibition of hypoxia inducible factor-2 transcription: Isolation of active modulators from marine sponges. J. Nat. Prod. 2012, 75, 1632–1636. [Google Scholar] [CrossRef] [PubMed]
- Sourbier, C.; Scroggins, B.T.; Mannes, P.Z.; Liao, P.J.; Siems, K.; Wolf, D.; Beutler, J.A.; Linehan, W.M.; Neckers, L. Tonantzitlolone cytotoxicity toward renal cancer cells is PKC θ- and HSF1-dependent. Oncotarget 2015, 75, 1632–1636. [Google Scholar]
- Devkota, K.P.; Covell, D.; Ransom, T.; McMahon, J.B.; Beutler, J.A. Growth inhibition of human colon carcinoma cells by sesquiterpenoids and tetralones of Zygogynum calothyrsum. J. Nat. Prod. 2013, 76, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; San Martin, I.D.; Rose, R.; Beko, A.; Levea, C.; Sharratt, E.; Mazurchuk, R.; Hoffman, R.M.; Brattain, M.G.; Wang, J. Characterization of HCT116 human colon cancer cells in an orthotopic model. J. Surg. Res. 2008, 147, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Ishikado, A.; Nishida, N.; Ninomiya, K.; Fujiwara, H.; Kobayashi, Y.; Yoshikawa, M. Hepatoprotective, superoxide scavenging, and antioxidative activities of aromatic constituents from the bark of Betula platyphylla var. japonica. Bioorg. Med. Chem. Lett. 1998, 8, 2939–2944. [Google Scholar] [CrossRef]
- Huh, J.E.; Hong, J.M.; Baek, Y.H.; Lee, J.D.; Choi, D.Y.; Park, D.S. Anti-inflammatory and anti-nociceptive effect of Betula platyphylla var. japonica in human interleukin-1β-stimulated fibroblast-like synoviocytes and in experimental animal models. J. Ethnopharmacol. 2011, 135, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Jeong, E.J.; Huh, J.M.; Cho, N.K.; Kim, T.B.; Jeon, B.J.; Kim, S.H.; Kim, H.P.; Sung, S.H. Cognition-enhancing and neuroprotecive activities of the standardized extract of Betula platphylla bark and its major diarylheptanoids. Phytomedicine 2012, 19, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.; Lee, K.Y.; Huh, J.; Choi, J.H.; Yang, H.; Jeong, E.J.; Kim, H.P.; Sung, S.H. Cognitive-enhancing effects of Rhus verniciflua bark extract and its active flavonoids with neuroprotective and anti-inflammatory activities. Food Chem. Toxicol. 2013, 58, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Novaković, M.; Pešić, M.; Trifunović, S.; Vučković, I.; Todorović, N.; Podolski-Renić, A.; Dinić, J.; Stojković, S.; Tešević, V.; Vajs, V.; et al. Diarylheptanoids from the bark of black alder inhibit the growth of sensitive and multi-drug resistant non-small cell lung carcinoma cells. Phytochemistry 2014, 97, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Brand, S.; Hölscher, D.; Schierhorn, A.; Svatoš, A.; Schröder, J.; Scneider, B. A type III polyketide synthase from Wachendorfia thyrsiflora and its role in diarylheptanoid and phenylphenalenone biosynthesis. Planta 2006, 224, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Munde, T.; Brand, S.; Hidalgo, W.; Maddula, R.K.; Svatoš, A.; Scneider, B. Biosynthesis of tetraoxygenated phenylphenalenones in Wachendorfia thyrsiflora. Phytochemistry 2012, 91, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.E.; Kim, K.H.; Kwon, J.H.; Kim, S.B.; Kim, H.W.; Lee, M.W. Cytotoxic activities of diarylheptanoids from Alnus japonica. Arch. Pharm. Res. 2008, 31, 1287–1289. [Google Scholar] [CrossRef] [PubMed]
- Mshvildadze, V.; Legault, J.; Lavoie, S.; Gauthier, C.; Pichette, A. Anticancer diarylheptanoid glycosides from the inner bark of Betula papyrifera. Phytochemistry 2007, 68, 2531–2536. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.W.; Lee, D.H.; Shin, D.H.; Kim, S.H.; Kwon, S.H. Aceroside VIII is a new natural selective HDAC6 inhibitor that synergistically enhances the anticancer activity of HDAC inhibitor in HT29 cells. Planta Med. 2015, 81, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, Y.; Zhan, Y.; Liu, L.; Xu, Y.; Xu, T.; Liu, T. Preparative isolation and purification of five flavonoid glycosides and one benzophenone galloyl glycoside from Psidium guajava by high-speed counter-current chromatography (HSCCC). Molecules 2013, 18, 15648–15661. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Deng, L.; Cai, S.; Liu, J.; Li, W.; Du, L.; Cui, G.; Xu, X.; Lu, T.; et al. Systematic separation and purification of iridoid glycosides and crocetin derivatives from Gardenia jasminoides Ellis by high-speed counter-current chromatography. Phytochem. Anal. 2015, 26, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Wang, H.; He, Y.; Ding, C.; Wang, X.; Suo, Y. Separation and purification of four oligostilbenes from Iris lactea Pall. var. chinensis (Fisch.) Koidz by high-speed counter-currentchromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 988, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Lee, M.Y.; Jo, H.; Lim, S.S.; Jung, S.H. Preparative isolation and purification of neuroprotective compounds from Rhus verniciflua by high speed counter-current chromatography. Biol. Pharm. Bull. 2012, 35, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Luo, Q.; Ding, L.; Fang, F.; Yuan, Y.; Chen, J.; Zhang, J.; Jin, H.; He, S. Preparative isolation and purification of lignans from Justicia procumbens using high-speed counter-current chromatography in stepwise elution mode. Molecules 2015, 20, 7048–7058. [Google Scholar] [CrossRef] [PubMed]
- Henrich, C.J.; Beutler, J.A. Matching the power of high throughput screening to the chemical diversity of natural products. Nat. Prod. Rep. 2013, 30, 1284–1298. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Lee, M.Y.; Ahn, H.R.; Choi, S.J.; Lee, J.Y.; Jung, S.H. Preparative isolation and purification of antioxidative diarylheptanoid derivatives from Alnus japonica by high-speed counter-current chromatography. J. Sep. Sci. 2011, 34, 3344–3352. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatagr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, J.H.; Kim, T.B.; Lee, H.H.; Lee, K.Y.; Kim, Y.C.; Sung, S.H. Inhibition of antigen-induced degranulation by aryl compounds isolated from the bark of Betula platyphylla in RBL-2H3 cells. Bioorg. Med. Chem. 2010, 20, 2824–2827. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, M.G.; Rosalen, P.L.; Franchin, M.; de Alencar, S.M.; Ikegaki, M.; Ransom, T.; Beutler, J.A. Antiproliferative constituents of geopropolis from the bee Melipona scutellaris. Planta Med. 2016, 82, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Paull, K.D.; Shoemaker, R.H.; Hodes, L.; Monks, A.; Scudiero, D.A.; Rubinstein, L.; Plowman, J.; Boyd, M.R. Display and analysis of patterns of differential activity of drugs against human tumor cell lines: Development of mean graph and COMPARE algorithm. J. Natl. Cancer Inst. 1989, 81, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Ju, E.M.; Lee, S.E.; Hwang, H.J.; Kim, J.H. Antioxidant and anticancer activity of extract from Betula platyphylla var. japonica. Life Sci. 2004, 74, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer. 2006, 6, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Smite, E.; Lundgren, L.N.; Andersson, R. Arylbutanoid and diarylheptanoid glycosides from inner bark of Betula pendula. Phytochemistry 1993, 32, 365–369. [Google Scholar] [CrossRef]
- Sunnerheim-Sjoberg, K.; Knutsson, P.G. Platyphylloside: Metabolism and digestibility reduction in vitro. J. Chem. Ecol. 1995, 21, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.



| Solvent System | K-Value | Separation | |
|---|---|---|---|
| 1 | 2 | Factor | |
| Ethylacetate:Butanol:H2O (1:0.1:1, v/v/v) | 2.98 | 3.71 | 1.25 |
| Ethylacetate:Methanol:H2O (1:0.1:1, v/v/v) | 0.96 | 1.52 | 1.58 |
| Ethylacetate:Acetonitrile:H2O (1:0.1:1, v/v/v) | 1.08 | 1.67 | 1.54 |
| Compound | Mean Percent Inhibition at 10−5 M | Percent Range at 10−5 M | GI50 against COLO205 (μM) | GI50 against KM12 (μM) | GI50 against A498 (μM) | GI50 against U031 (μM) |
|---|---|---|---|---|---|---|
| Total extract | NT a | NT | >20 | >20 | 9 | >20 |
| MC | NT | NT | >20 | 9.1 | >20 | >20 |
| DRF | NT | NT | 9.6 | 5.4 | 3.8 | 6.7 |
| aceroside (1) | 3 | 41 | >20 | >20 | >20 | >20 |
| platyphylloside (2) | 72 | 166 | >20 | 11.8 | 17.5 | 16.6 |
| Adriamycin b | 40.0 ± 1.3 b (n = 16) | 105.5 ± 5.6 b | NT | NT | NT | NT |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, N.; Kim, H.W.; Kim, T.B.; Ransom, T.T.; Beutler, J.A.; Sung, S.H. Preparative Purification of Anti-Proliferative Diarylheptanoids from Betula platyphylla by High-Speed Counter-Current Chromatography. Molecules 2016, 21, 700. https://doi.org/10.3390/molecules21060700
Cho N, Kim HW, Kim TB, Ransom TT, Beutler JA, Sung SH. Preparative Purification of Anti-Proliferative Diarylheptanoids from Betula platyphylla by High-Speed Counter-Current Chromatography. Molecules. 2016; 21(6):700. https://doi.org/10.3390/molecules21060700
Chicago/Turabian StyleCho, Namki, Hyun Woo Kim, Tae Bum Kim, Tanya T. Ransom, John A. Beutler, and Sang Hyun Sung. 2016. "Preparative Purification of Anti-Proliferative Diarylheptanoids from Betula platyphylla by High-Speed Counter-Current Chromatography" Molecules 21, no. 6: 700. https://doi.org/10.3390/molecules21060700