Gold Incorporated Mesoporous Silica Thin Film Model Surface as a Robust SERS and Catalytically Active Substrate
Abstract
:1. Introduction
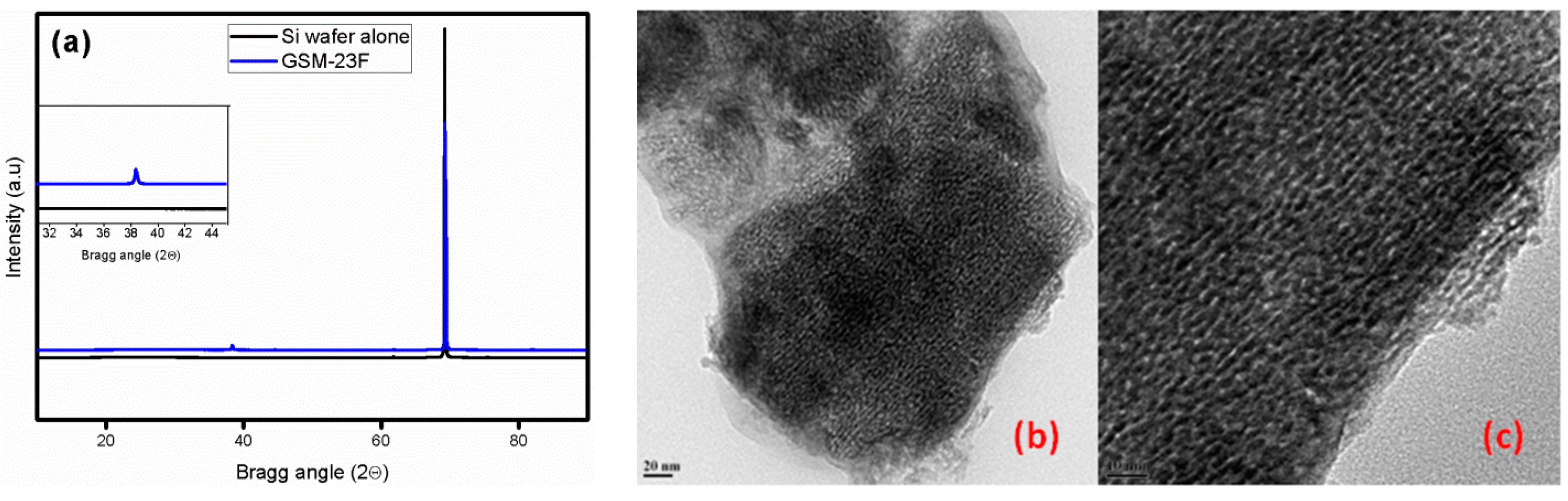
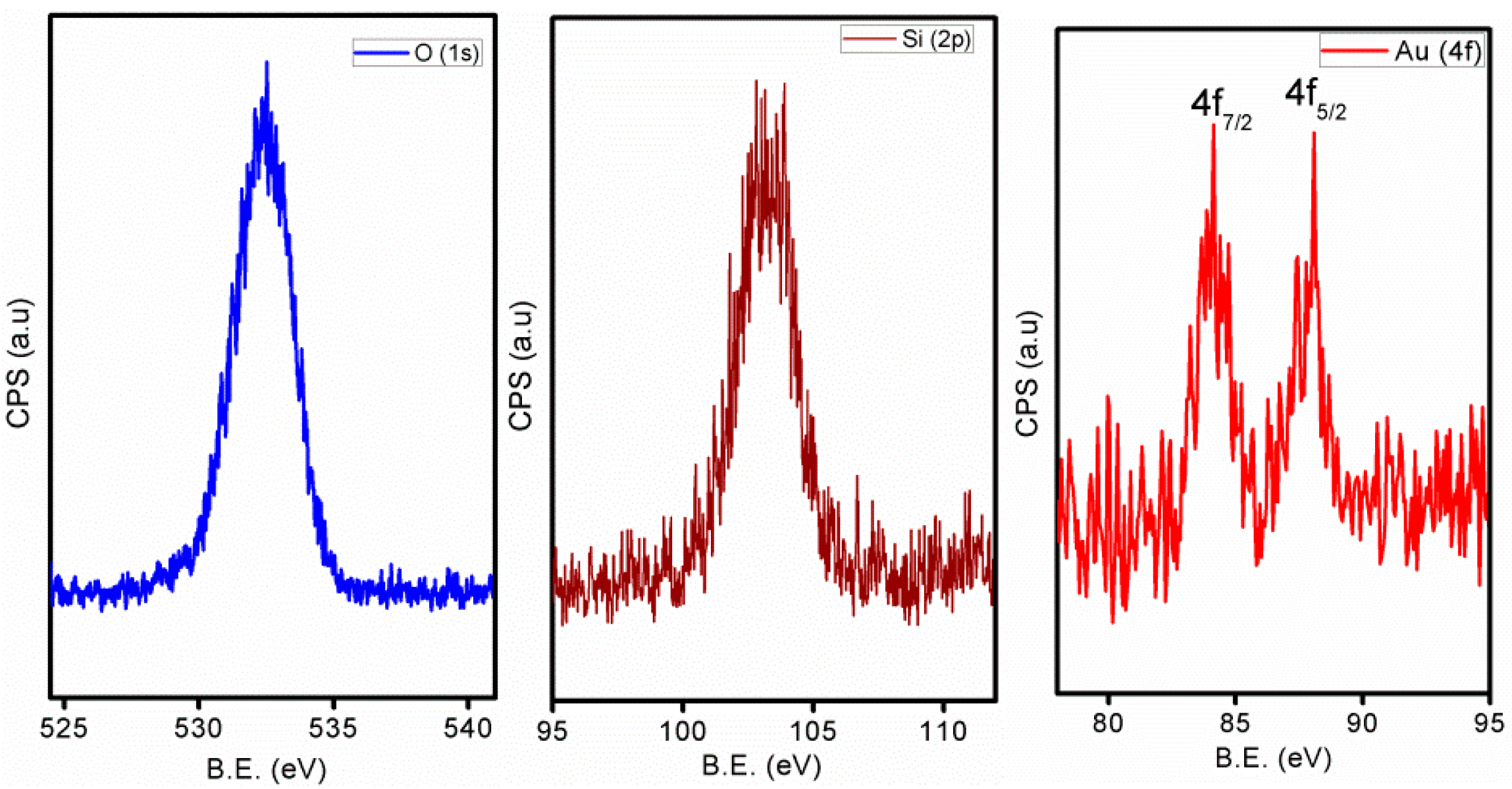
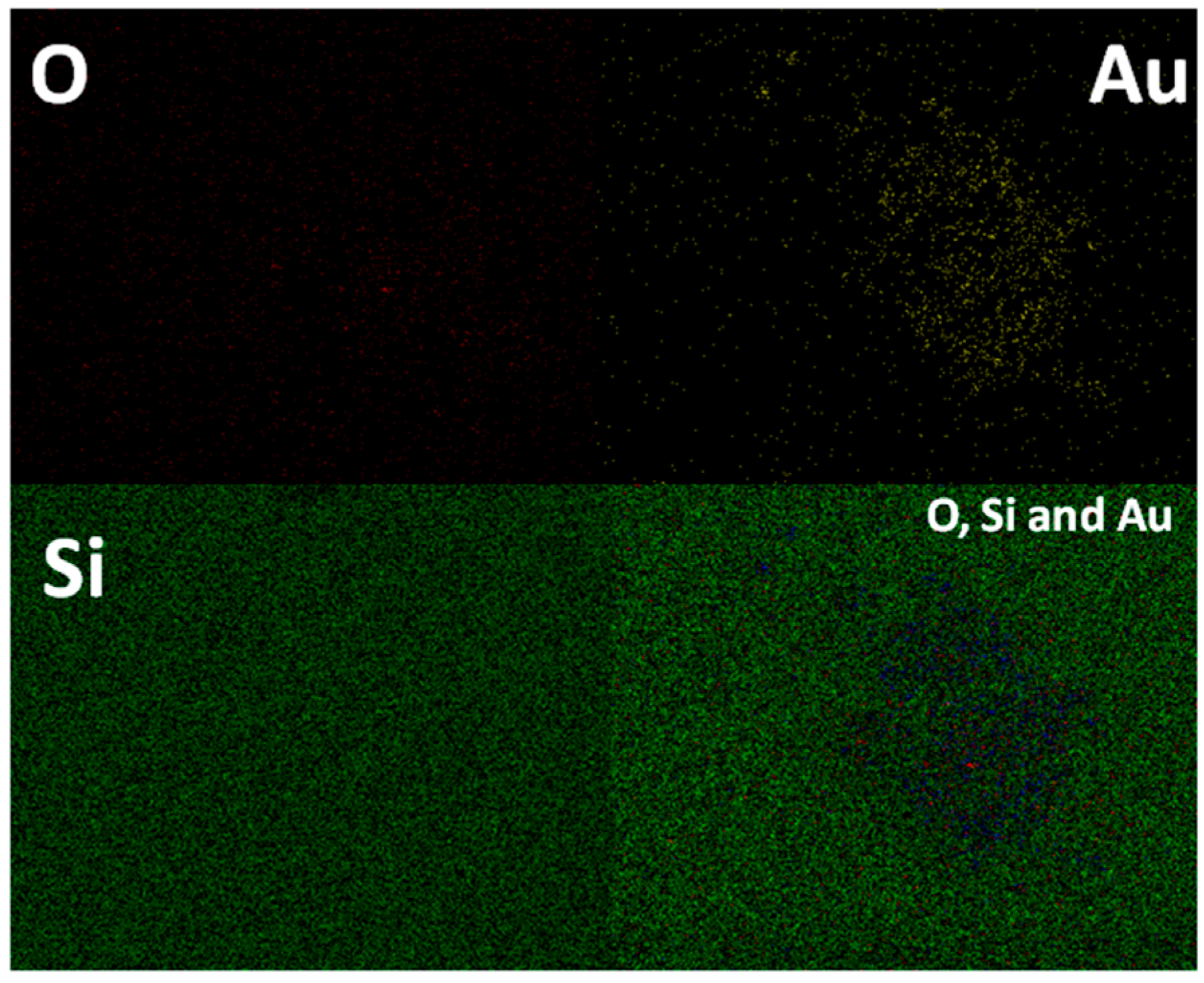
2. Results
Structural and Textural Characterisation
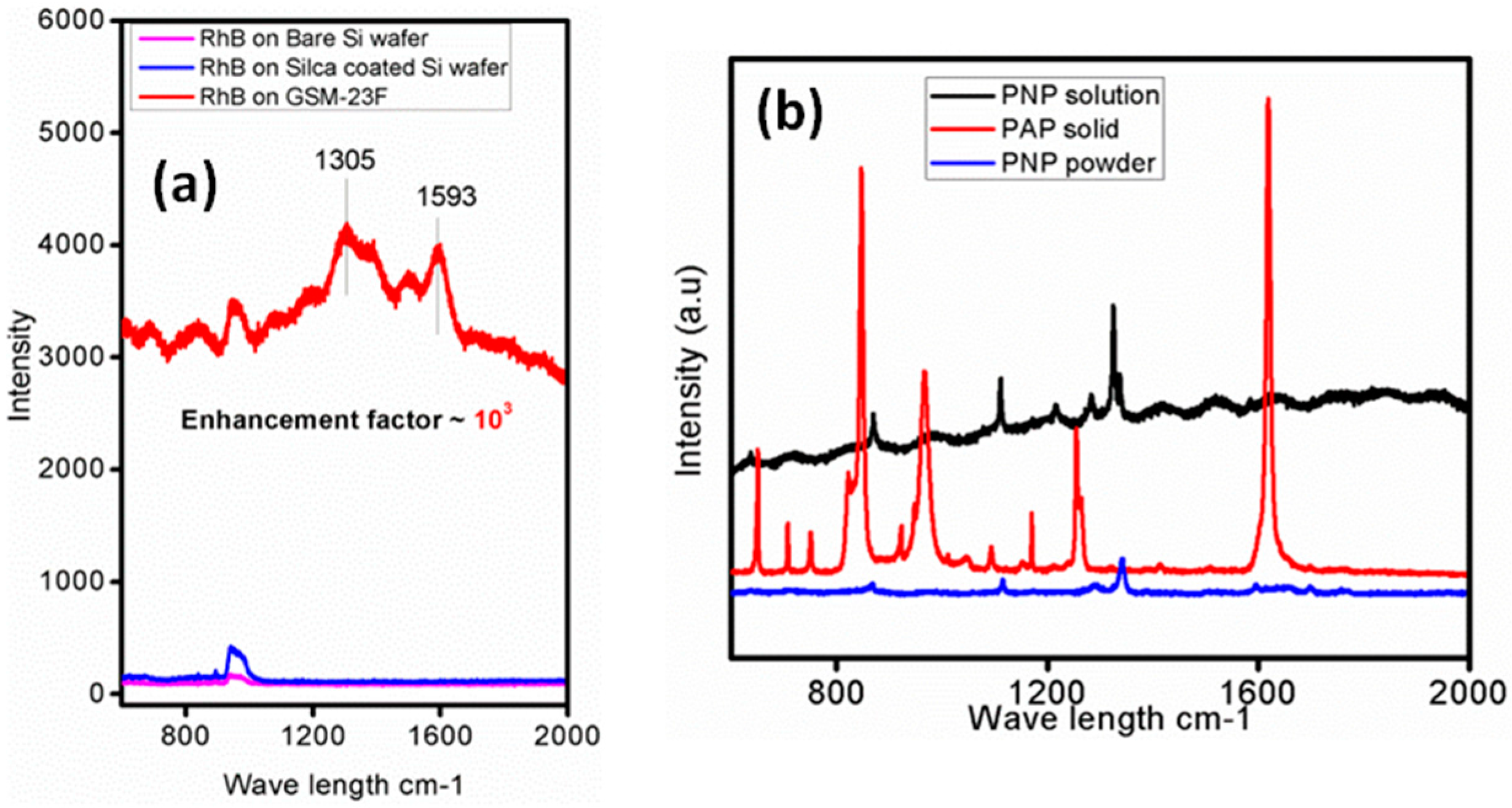
3. Discussion
3.1. GSM-23F as a Robust SERS Substrate
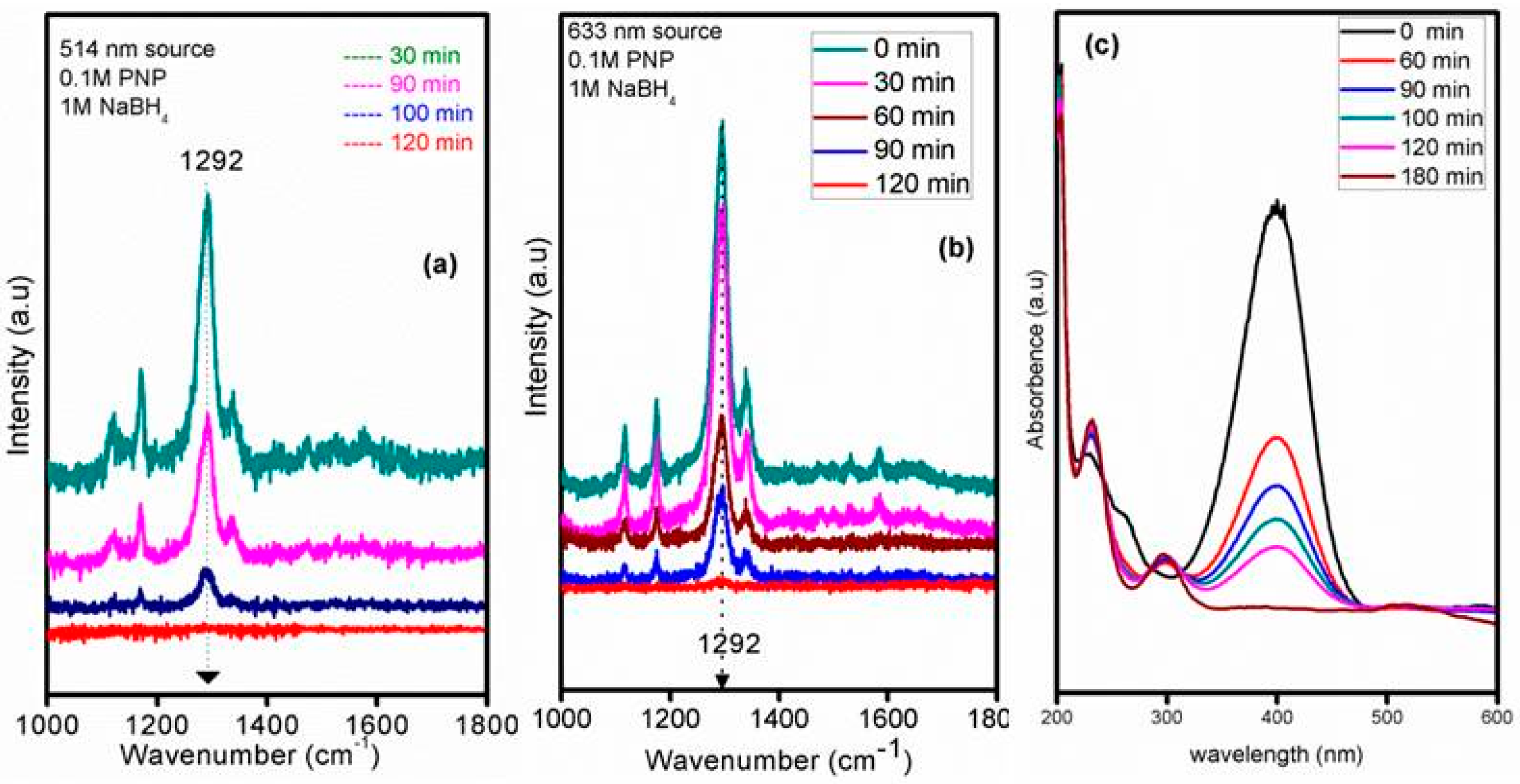
3.2. GSM-23F As a Catalytically Active Model Surface
4. Materials and Methods
4.1. Thin Film Synthesis
- (i)
- Pretreatment of the substrate
- (ii)
- Synthesis of the silica gold sol-gel and sol ageing
- (iii)
- Spin coating, ageing and high temperature calcinations
4.1.1. Pretreatment of the Substrate, Si (100)
4.1.2. Silica Gold Sol-Gel Synthesis
4.1.3. Film Casting on the Hydroxylated Si (100)
4.2. SERS Experimental
4.3. Characterizations
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single Molecule Detection Using Surface-Enhanced Raman Scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Lim, D.-K.; Jeon, K.-S.; Hwang, J.-H.; Kim, H.; Kwon, S.; Suh, Y.D.; Nam, J.-M. Highly uniform and reproducible surface-enhanced Raman scattering from DNA-tailorable nanoparticles with 1-nm interior gap. Nat. Nanotechnol. 2011, 6, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, A.I.; Rodriguez, J.A.; Chen, J.G. Synchrotron Techniques for In Situ Catalytic Studies: Capabilities, Challenges, and Opportunities. ACS Catal. 2012, 2, 2269–2280. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Hengst, T.; Lothschütz, C.; Rominger, F. New and Easily Accessible Nitrogen Acyclic Gold(I) Carbenes: Structure and Application in the Gold-Catalyzed Phenol Synthesis as well as the Hydration of Alkynes. Adv. Synth. Catal. 2010, 352, 1315–1337. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Lothschütz, C.; Ackermann, M.; Doepp, R.; Anantharaman, S.; Marchetti, B.; Bertagnolli, H.; Rominger, F. Gold Catalysis: In Situ EXAFS Study of Homogeneous Oxidative Esterification. Chem. A Eur. J. 2010, 16, 8012–8019. [Google Scholar] [CrossRef] [PubMed]
- Vinod, C.P. Surface science as a tool for probing nanocatalysis phenomena. Catal. Today 2010, 154, 113–117. [Google Scholar] [CrossRef]
- Freund, H.J.; Nilius, N.; Risse, T.; Schauermann, S. A fresh look at an old nano-technology: Catalysis. Phys. Chem. Chem. Phys. 2010, 16, 8148–8167. [Google Scholar] [CrossRef] [PubMed]
- Rainer, D.R.; Goodman, D.W. Metal clusters on ultrathin oxide films: Model catalysts for surface science studies. J. Mol. Catal. A 1998, 131, 259–283. [Google Scholar] [CrossRef]
- Freund, H.-J. Model Studies in Heterogeneous Catalysis. Chem. A Eur. J. 2010, 16, 9384–9397. [Google Scholar] [CrossRef] [PubMed]
- Orendorff, C.J.; Gole, A.; Sau, T.K.; Murphy, C.J. Surface-enhanced Raman spectroscopy of self-assembled monolayers: Sandwich architecture and nanoparticle shape dependence. Anal. Chem. 2005, 77, 3261–3266. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.A. Some Interesting Properties of Metals Confined in Time and Nanometer Space of Different Shapes. Acc. Chem. Res. 2001, 34, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Castillo, F.; De la Rosa, E.; Perez, E. Gold aggregates on silica templates and decorated silica arrays for SERS applications. Eur. Phys. J. D 2011, 63, 301–306. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Hutchings, G.J. Gold Catalysis. Angew. Chem. Int. Ed. 2006, 45, 7896–7936. [Google Scholar] [CrossRef] [PubMed]
- Pflasterer, D.; Hashmi, A.S.K. Gold catalysis in total synthesis—Recent achievements. Chem. Soc. Rev. 2016, 45, 1331–1367. [Google Scholar] [CrossRef] [PubMed]
- Roca, M.; Haes, A.J. Silica-Void-Gold Nanoparticles: Temporally Stable Surface-Enhanced Raman Scattering Substrates. J. Am. Chem. Soc. 2008, 130, 14273–14279. [Google Scholar] [CrossRef] [PubMed]
- Polavarapu, L.; Liz-Marzan, L.M. Towards low-cost flexible substrates for nanoplasmonic sensing. Phys Chem. Chem. Phys. 2013, 15, 5288–5300. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kuperman, A.; Coombs, N.; Mamiche-Afara, S.; Ozin, G.A. Synthesis of oriented films of mesoporous silica on mica. Nature 1996, 379, 703–705. [Google Scholar] [CrossRef]
- Teng, Z.G.; Zheng, G.F.; Dou, Y.Q.; Li, W.; Mou, C.Y.; Zhang, X.H.; Asiri, A.M.; Zhao, D.Y. Highly Ordered Mesoporous Silica Films with Perpendicular Mesochannels by a Simple Stober-Solution Growth Approach. Angew. Chem. Int. Ed. 2012, 51, 2173–2177. [Google Scholar] [CrossRef] [PubMed]
- Gawel, B.; Lambrechts, K.; Gawel, K.; Oye, G. One-pot synthesis of gold nanoparticle functionalised mesoporous silica—The double role of a tri-block copolymer and chitosan. Microporous Mesoporous Mater. 2012, 164, 32–37. [Google Scholar] [CrossRef]
- Pedrueza, E.; Valdes, J.L.; Chirvony, V.; Abargues, R.; Hernandez-Saz, J.; Herrera, M.; Molina, S.I.; Martinez-Pastor, J.P. Novel Method of Preparation of Gold-Nanoparticle-Doped TiO2 and SiO2 Plasmonic Thin Films: Optical Characterization and Comparison with Maxwell-Garnett Modeling. Adv. Funct. Mater. 2011, 21, 3502–3507. [Google Scholar] [CrossRef]
- Gutiérrez, L.-F.; Hamoudi, S.; Belkacemi, K. Synthesis of Gold Catalysts Supported on Mesoporous Silica Materials: Recent Developments. Catalysts 2011, 1, 97–154. [Google Scholar] [CrossRef]
- Akimov, Y.A.; Ostrikov, K.; Li, E.P. Surface Plasmon Enhancement of Optical Absorption in Thin-Film Silicon Solar Cells. Plasmonics 2009, 4, 107–113. [Google Scholar] [CrossRef]
- Akimov, Y.A.; Koh, W.S.; Ostrikov, K. Enhancement of optical absorption in thin-film solar cells through the excitation of higher-order nanoparticle plasmon modes. Opt. Express 2009, 17, 10195–10205. [Google Scholar] [CrossRef] [PubMed]
- Merlen, A.; Gadenne, V.; Romann, J.; Chevallier, V.; Patrone, L.; Valmalette, J.C. Surface enhanced Raman spectroscopy of organic molecules deposited on gold sputtered substrates. Nanotechnology 2009, 20, 215705. [Google Scholar] [CrossRef] [PubMed]
- Joseph, V.; Engelbrekt, C.; Zhang, J.; Gernert, U.; Ulstrup, J.; Kneipp, J. Characterizing the Kinetics of Nanoparticle-Catalyzed Reactions by Surface-Enhanced Raman Scattering. Angew. Chem. Int. Ed. 2012, 51, 7592–7596. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Kang, L.; Mack, N.H.; Schanze, K.S.; Han, X.; Wang, H.-L. Mechanistic understanding of surface plasmon assisted catalysis on a single particle: cyclic redox of 4-aminothiophenol. Sci. Rep. 2013, 3, 2997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Winget, S.A.; Wu, Y.; Su, D.; Sun, X.; Xie, Z.-X.; Qin, D. Ag@Au Concave Cuboctahedra: A Unique Probe for Monitoring Au-Catalyzed Reduction and Oxidation Reactions by Surface-Enhanced Raman Spectroscopy. ACS Nano 2016, 10, 2607–2616. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ma, B.; Yang, L.; Liu, J. Hybrid single nanoreactor for in situ SERS monitoring of plasmon-driven and small Au nanoparticles catalyzed reactions. Chem. Commun. 2015, 51, 11394–11397. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Polavarapu, L.; Liz-Marzan, L.M.; Pastoriza-Santos, I.; Perez-Juste, J. Gold nanoparticle-loaded filter paper: A recyclable dip-catalyst for real-time reaction monitoring by surface enhanced Raman scattering. Chem. Commun. 2015, 51, 4572–4575. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, V.; Acosta, B.; Miridonov, S.; Smolentseva, E.; Fuentes, S.; Simakov, A. Highly active Au-CeO2@ZrO2 yolk–shell nanoreactors for the reduction of 4-nitrophenol to 4-aminophenol. Appl. Catal. B 2015, 166–167, 518–528. [Google Scholar] [CrossRef]
- Ciganda, R.; Li, N.; Deraedt, C.; Gatard, S.; Zhao, P.; Salmon, L.; Hernandez, R.; Ruiz, J.; Astruc, D. Gold nanoparticles as electron reservoir redox catalysts for 4-nitrophenol reduction: A strong stereoelectronic ligand influence. Chem. Commun. 2014, 50, 10126–10129. [Google Scholar] [CrossRef] [PubMed]
- Van Schrojenstein Lantman, E.M.; Deckert-Gaudig, T.; Mank, A.J.G.; Deckert, V.; Weckhuysen, B.M. Catalytic processes monitored at the nanoscale with tip-enhanced Raman spectroscopy. Nat. Nanotechnol. 2012, 7, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Stavitski, E.; Weckhuysen, B.M. Infrared and Raman imaging of heterogeneous catalysts. Chem. Soc. Rev., 2010, 39, 4615–4625. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Alexandridis, P. Mechanism of Gold Metal Ion Reduction, Nanoparticle Growth and Size Control in Aqueous Amphiphilic Block Copolymer Solutions at Ambient Conditions. J. Phys. Chem. B 2005, 109, 7766–7777. [Google Scholar] [CrossRef] [PubMed]
- Alberius, P.C.A.; Frindell, K.L.; Hayward, R.C.; Kramer, E.J.; Stucky, G.D.; Chmelka, B.F. General predictive syntheses of cubic, hexagonal, and lamellar silica and titania mesostructured thin films. Chem. Mater. 2002, 14, 3284–3294. [Google Scholar] [CrossRef]
- Sunil Sekhar, A.C.; Sivaranjani, K.; Gopinath, C.S.; Vinod, C.P. A simple one pot synthesis of nano gold–mesoporous silica and its oxidation catalysis. Catal. Today 2012, 198, 92–97. [Google Scholar] [CrossRef]
- Cui, F.M.; Hua, Z.L.; Cui, X.Z.; Guo, L.M.; Wei, C.Y.; Bu, W.B.; Shi, J.L. Au nanoparticles incorporated mesoporous silica thin films with a high Au content: Preparation and third-order optical non-linearity. Dalton Trans. 2009, 15, 2679–2682. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.W.; Cai, M.; Pemberton, J.E. Insulating Ultrathin Silica Films Formed by a Room-Temperature Sol–Gel Process. Adv. Mater. 2001, 13, 662–667. [Google Scholar] [CrossRef]
- Shen, J.H.; Zhu, Y.H.; Yang, X.L.; Zong, J.; Li, C.Z. Multifunctional Fe3O4@Ag/SiO2/Au Core-Shell Microspheres as a Novel SERS-Activity Label via Long-Range Plasmon Coupling. Langmuir 2013, 29, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Hui, C.; Yang, T.; Xiao, C.; Tian, J.; Bao, L.; Chen, S.; Ding, H.; Gao, H. Monodisperse Noble-Metal Nanoparticles and Their Surface Enhanced Raman Scattering Properties. Chem. Mater. 2008, 20, 6939–6944. [Google Scholar] [CrossRef]
- Xie, J.P.; Zhang, Q.B.; Lee, J.Y.; Wang, D.I.C. The Synthesis of SERS-Active Gold Nanoflower Tags for in Vivo Applications. ACS Nano 2008, 2, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Budnyk, A.P.; Damin, A.; Agostini, G.; Zecchina, A. Gold Nanoparticle Aggregates Immobilized on High Surface Area Silica Substrate for Efficient and Clean SERS Applications. J. Phys. Chem. C 2010, 114, 3857–3862. [Google Scholar] [CrossRef]
- Xu, B.-B.; Zhang, R.; Liu, X.-Q.; Wang, H.; Zhang, Y.-L.; Jiang, H.-B.; Wang, L.; Ma, Z.-C.; Ku, J.-F.; Xiao, F.-S.; Sun, H.-B. On-chip fabrication of silver microflower arrays as a catalytic microreactor for allowing in situ SERS monitoring. Chem. Commun. 2012, 48, 1680–1682. [Google Scholar] [CrossRef] [PubMed]
- Khaing Oo, M.K.; Guo, Y.; Reddy, K.; Liu, J.; Fan, X. Ultrasensitive Vapor Detection with Surface-Enhanced Raman Scattering-Active Gold Nanoparticle Immobilized Flow-Through Multihole Capillaries. Anal. Chem. 2012, 84, 3376–3381. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-J. Physical Origins of Chemical Enhancement of Surface-Enhanced Raman Spectroscopy on a Gold Nanoparticle-Coated Polymer. J. Phys. Chem. C 2010, 114, 13979–13984. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, P.; Melosh, N.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Continuous Mesoporous Silica Films with Highly Ordered Large Pore Structures. Adv. Mater. 1998, 10, 1380–1385. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunil Sekhar, A.C.; Vinod, C.P. Gold Incorporated Mesoporous Silica Thin Film Model Surface as a Robust SERS and Catalytically Active Substrate. Molecules 2016, 21, 667. https://doi.org/10.3390/molecules21050667
Sunil Sekhar AC, Vinod CP. Gold Incorporated Mesoporous Silica Thin Film Model Surface as a Robust SERS and Catalytically Active Substrate. Molecules. 2016; 21(5):667. https://doi.org/10.3390/molecules21050667
Chicago/Turabian StyleSunil Sekhar, Anandakumari Chandrasekharan, and Chathakudath Prabhakaran Vinod. 2016. "Gold Incorporated Mesoporous Silica Thin Film Model Surface as a Robust SERS and Catalytically Active Substrate" Molecules 21, no. 5: 667. https://doi.org/10.3390/molecules21050667





