The Mechanism by Which Amentoflavone Improves Insulin Resistance in HepG2 Cells
Abstract
:1. Introduction
2. Results
2.1. Subsection
2.1.1. Glucose Consumption in Each Group of Cells
2.1.2. The Impact of AME on PFK-1, GCK, and PK Activity
2.1.3. The Impact of AME on the Activity of GSK-3
2.1.4. The Impact of AME on the Activities of PEPCK and G-6-Pase
2.1.5. The Impact of AME on the Levels of IL-6, IL-8, TNF-α, and CRP
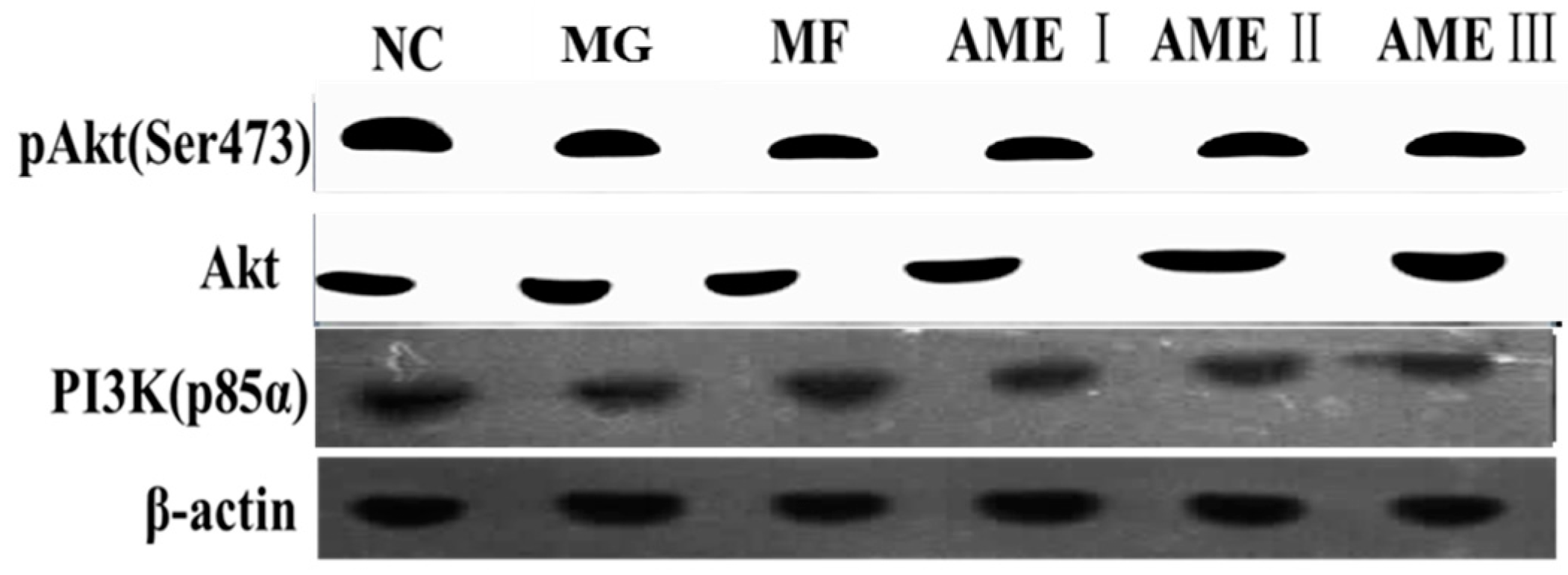
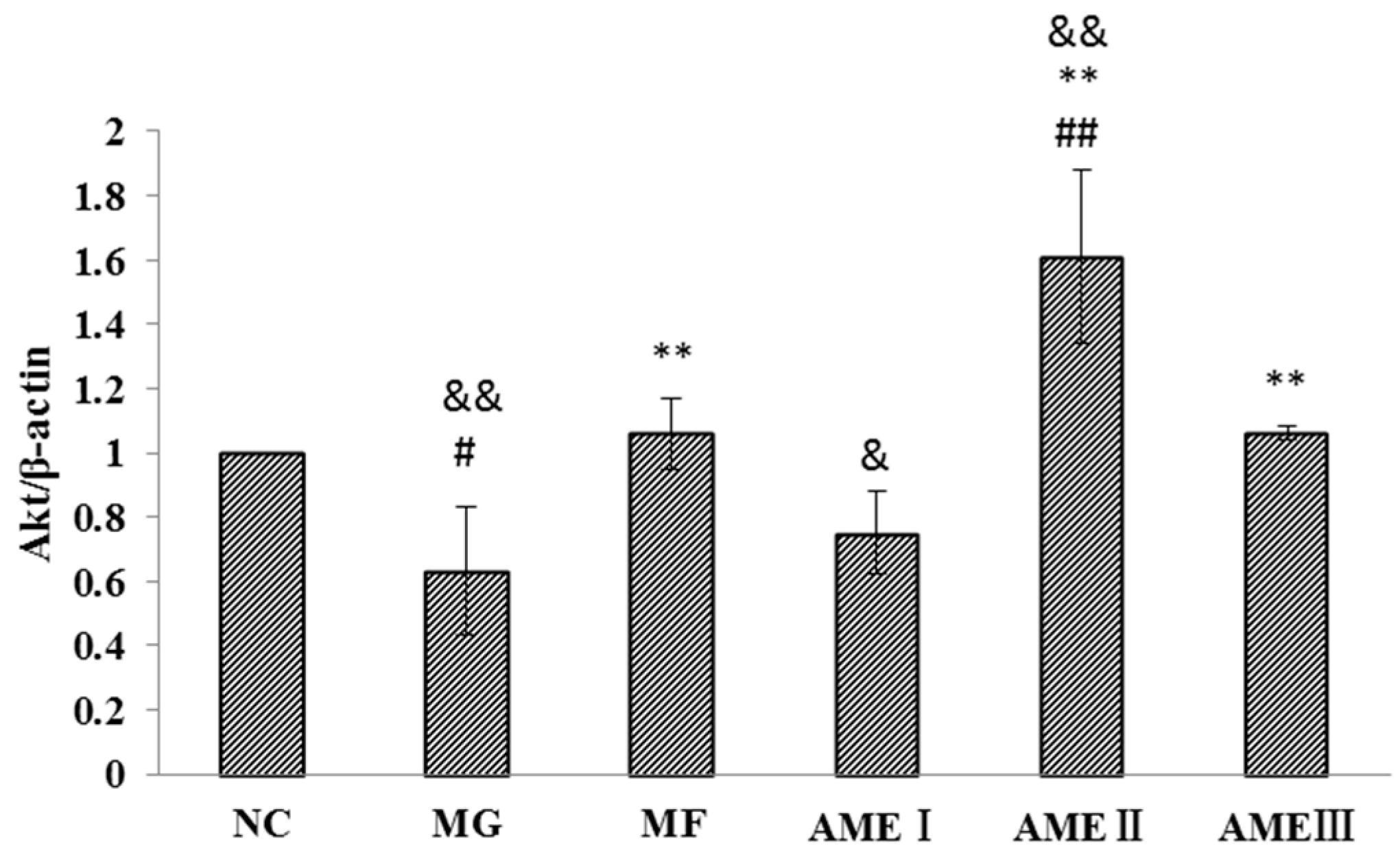
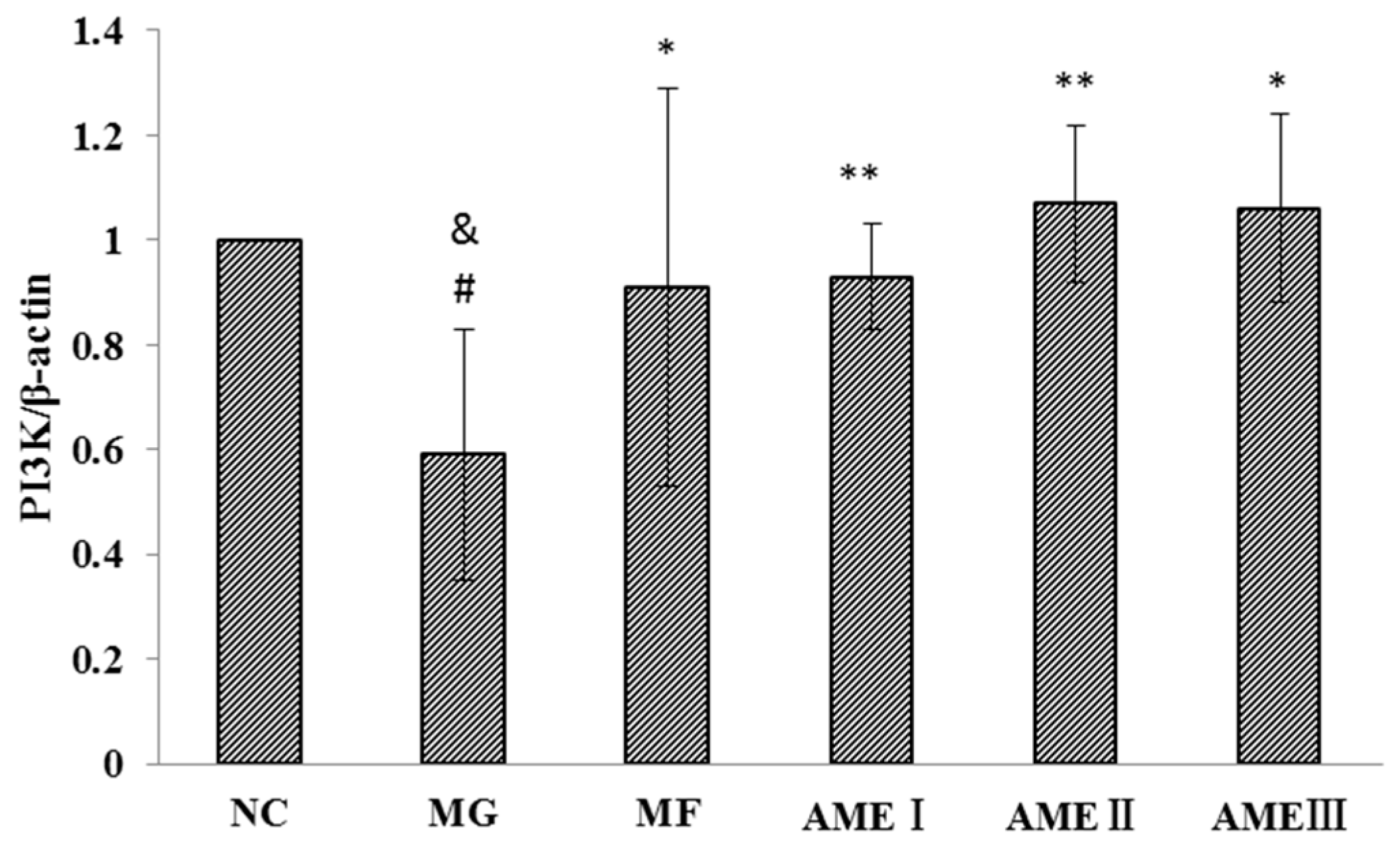
2.1.6. The Effect of AME on the PI3K, Akt, and pAkt Proteins
3. Discussion
4. Materials and Methods
4.1. Preparation of AME
4.2. Cell Culture
4.3. Reagents and Instruments
4.4. Methods
4.4.1. Generation of an Insulin Resistance Model
4.4.2. The Experimental Groups
4.4.3. Measurement of the Glucose Consumption Rate
4.4.4. ELISA
4.4.5. Western Blot Analysis
4.5. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AME | Amentoflavone |
| ELISA | Enzyme-linked immunosorbent assay |
| PI3K | Phosphatidyl inositol 3-kinase |
| PFK-1 | 6-Phosphofructokinase |
| GCK | Glucokinase |
| PK | Pyruvate kinase |
| GSK-3 | Glycogen synthase kinase-3 |
| PEPCK | Phosphoenolpyruvate carboxylase kinase |
| G-6-Pase | Glucose-6-phosphatase |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| TNF-α | Tumor necrosis factor-α |
| CRP | C reactive protein |
| NC group | Normal control group |
| MG | Model group |
| MF | Metformin group |
| GC | Glucose consumption |
| MTT | 3-(4, 5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| GLUT | Glucose transporter |
| DMSO | Dimethyl sulfoxide |
| BSA | Bovine serum albumin |
| PIP2 | Phosphatidylinositol-4,5-bisphosphate |
| PIP3 | Hydroxyl to form phosphatidylinositol-3,4,5-trisphosphate |
| PKB1 | Protein kinase B1 |
| PKB2 | Protein kinase B2 |
| TFST | Total Flavonoids of Selaginella tamariscina (Beauv.) Spring |
References
- Boden, G.; Merali, S. Measurement of the increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese insulin-resistant individuals. Methods Enzymol. 2011, 489, 67–82. [Google Scholar] [PubMed]
- Rungapamestry, V.; Mc Monagle, J. Inter-organ proteomic analysis reveals insights into the molecular mechanisms underlying the anti-diabetic effects of cis-9, trans-11-conjugated linoleic acid in ob/ob mice. Proteomics 2012, 12, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Milburn, M.V.; Lawton, K.A. Application of metabolomics to diagnosis of insulin resistance. Annu. Rev. Med. 2013, 64, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Cui, G.-Z.; Zhang, Y. The insulin signal transduction pathway in type 2 diabetes. J. Changchun Univ. Chin. Med. 2008, 24, 325–326. [Google Scholar]
- Alessi, D.R.; James, S.R.; Downes, C.P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 1997, 7, 261–269. [Google Scholar] [CrossRef]
- Choia, Y.J.; Sung, E. Involvement of visfatin in palmitate-induced upregulation of inflammatory cytokines in hepatocytes. Metab. Clin. Exp. 2011, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Leguisamo, N.M.; Alexandre M Lehnen, A.M. GLUT4 content decreases along with insulin resistance and high levels of inflammatory markers in rats with metabolic syndrome. Cardiovasc. Diabetol. 2012, 11, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Luo, T.; Zhang, Z.; Wang, T.; Kou, J.; Liu, B.; Huang, F. Modified Si-Miao-San extract inhibits inflammatory response and modulates insulin sensitivity in hepatocytes through an IKKβ/IRS-1/Akt-dependent pathway. J. Ethnopharmacol. 2011, 136, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-K.; Li, Y.-J. Antihyperglycemic activity of Selaginella tamariscina (Beauv.) Spring. J. Ethnopharmacol. 2011, 133, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.M. Studies on the Extracting and Purifying Technology and Chemical Constituents of the Flavone Fraction in Selaginellatamariscina. Master’s Thesis, Henan University of Traditional Chinese Medicine, Zhengzhou, China, 2009. [Google Scholar]
- Zheng, X.-K.; Li, Y.-J.; Zhang, L. Experimental study of Selaginella on the glucose metabolism in rat model of type 2 diabetes. J. Prev. Med. 2009, 36, 1918–1922. [Google Scholar]
- Zheng, X.-K.; Zhang, L.; Wang, W.-W. Anti-diabetic activity and potential mechanism of total flavonoids of Selaginella tamariscina (Beauv.) Spring in rats induced by high fat diet and low dose STZ. J. Ethnopharmacol. 2011, 137, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-K.; Wang, W.-W.; Zhang, L. Antihyperlipidaemic and antioxidant effect of the total flavonoids in Selaginella tamariscina (Beauv.) Spring in diabetic mice. J. Pharm. Pharmacol. 2013, 65, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-K.; Su, C.-F.; Zhang, L. The hypoglycemic effect of Amentotaxus in Selaginella. Chin. J. Exp. Tradit. Med. Formulae 2013, 19, 198–202. [Google Scholar]
- Maris, M.; Overbergh, L.; Mathieu, C. Type 2 diabetes: Gaining insight into the disease process using proteomics. Proteom. Clin. Appl. 2008, 2, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Sun, F. Apelin stimulates glucose uptake through the PI3K/Akt pathway and improves insulin resistance in 3T3-L1 adipocytes. Mol. Cell. Biochem. 2011, 353, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, X.-J. PI3K/AKT/mTOR signaling is involved in (−)-epigallocatechin-3-gallate-induced apoptosis of human pancreatic carcinoma cells. Am. J. Chin. Med. 2013, 41, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Antihyperglycemic Activity of Selaginella tamariscina. Master’s Thesis, Henan University of Traditional Chinese Medicine, Zhengzhou, China, 2009. [Google Scholar]
- Zheng, X.-K.; Liu, C.-X.; Zhai, Y.-Y.; Eli, L.L.; Wang, X.L.; Feng, W.S. Protection effect of amentoflavone in Selaginella tamariscina against TNF-α-induced vascular injure of endothelial cells. Acta Pharm. Sin. 2013, 48, 1503–1509. [Google Scholar]
- Chakraborty, C.; Roy, S.S.; Hsu, M.J. Landscape mapping of functional proteins in insulin signal transduction and insulin resistance: A network-based protein-protein interaction analysis. PLoS ONE 2011, 6, e16388. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-H.; Kim, K.-J. Effect of Pycnogenol® on glucose transport in mature 3T3-L1 adipocytes. Phytother. Res. 2010, 24, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mei, C. The PI3K-Akt pathway regulates calpain 6 expression, proliferation, and apoptosis. Cell. Signal. 2011, 23, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhang, H.; Yu, C. Effects of gold capsule on the blood glucose and expression of PI-3K, GLUT-4 of diabetic rats. J. Tradit. Chin. Med. 2016, 29, 50–54. [Google Scholar]
- Dai, B.; Wu, Q.; Xiao, Z.; Zeng, C.; Cao, L.; Ouyang, L.; Yang, M. Effects of Liuweidihuangtang and alcohol extraction on the PI3K/Akt signal pathway in the adipose tissue of diabetic rats. Chin. Tradit. Pat. Med. 2016, 38, 428–430. [Google Scholar]
- Liu, Y.; Zang, S.; Song, G.; Wang, C.; Ren, L.P.; Wang, Y. Effect of Jinlida on PI3K/AKT signal pathway in liver tissue of insulin resistant rats. Chin. J. Exp. Tradit. Med. Formulae 2015, 21, 72–76. [Google Scholar]
- Li, Y.-G.; Ji, D.-F.; Zhong, S. 1-Deoxynojirimycin inhibits glucose absorption and accelerates glucose metabolism in streptozotocin-induced diabetic mice. Sci. Rep. 2013, 28, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Tao, R.; DePinho, R.A. Deletion of hepatic FoxO1/3/4 genes in mice significantly impacts on glucose metabolism through downregulation of gluconeogenesis and upregulation of glycolysis. PLoS ONE 2013, 8, e74340. [Google Scholar] [CrossRef] [PubMed]
- Skepner, J.E.; Shelly, L.D.; Ji, C.; Reidich, B.; Luo, Y. Chronic treatment with epoxyeicosatrienoic acids modulates insulin signaling and prevent sinsulin resistance in hepatocytes. Prostaglandins Other Lipid Mediat. 2011, 94, 3–8. [Google Scholar] [CrossRef]
- Gong, L.; Jiang, X.; Huang, L.; Xing, D. Low-power laser irradiation (LPLI) can improve the insulin resistance of skeletal muscle and promote the glucose uptake and glycogen synthesis by activating the PI3-K/Akt2/GLUT4 signal pathway. In Proceedings of the 2013 Conference on Biophysical Society of Guangdong Province, Guangzhou, China, 10 December 2013; p. 98.
- Frayn, K.N.; Kingman, S.M. Dietary sugars and lipid metabolism in humans. Am. Soc. Clin. Nutr. 1995, 62, 250S–261S. [Google Scholar]
- Zhang, C.; Deng, K.; Yu, M.; Wei, X.; Qu, F.; Xu, G.; Sheng, J. Effects of Gegen Qinlian Tang-containing serum on glycometabolism in insulin-resistance HepG2 cell line. Chin. J. Exp. Tradit. Med. Formulae 2015, 21, 120–123. [Google Scholar]
- Esposito, D.; Kizelsztein, P.; Komarnytsky, S. Hypoglycemic effects of brassinosteroid in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2012, 303, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Wallerstedt, E.; Sandqvist, M. Anti-inflammatory effect of insulin in the human hepatoma cell line HepG2 involves decreased transcription of IL-6 target genes and nuclear exclusion of FOXO1. Mol. Cell. Biochem. 2011, 352, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Guzel1, S.; Seven, A. Osteoprotegerin, leptin and IL-6: Association with silent myocardial ischemia in type 2 diabetes mellitus. Diabetes Vasc. Dis. Res. 2014, 10, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Zhang, Y. FOXO1 involvement in insulin resistance-related pro-inflammatory cytokine production in hepatocytes. Inflamm. Res. 2012, 61, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wei, K.; Fu, L. Heat-clearing detoxifying method and inflammatory factors in type 2 diabetes interwention study. Shanxi J. Tradit. Chin. Med. 2016, 37, 89–91. [Google Scholar]
- Wei, Y. Experimental Study on Screening of Hypoglycemic Effect of Selaginella tamariscina and the Main Effective Component on Insulin Resistance. Master’s Thesis, Henan University of Traditional Chinese Medicine, Zhengzhou, China, 2008. [Google Scholar]
- Sample Availability: Sample of Amentoflavone is available from the authors.

| Group | Glucose Consumption (GC/MTT) |
|---|---|
| NC | 3.85 ± 0.75 |
| MG | 2.67 ± 0.94 #,&& |
| MF | 3.96 ± 0.53 ** |
| AMEI | 3.70 ± 0.34 * |
| AMEII | 3.81 ± 0.07 * |
| AMEIII | 3.66 ± 0.41 * |
| Group | PFK-1 (U·L−1) | GCK (U·L−1) | PK (mU·L−1) |
|---|---|---|---|
| NC | 1485.91 ± 97.55 | 230.9 ± 5.69 | 221.94 ± 8.36 |
| MG | 1111.60 ± 122.67 ##,&& | 154.48 ± 29.00 ##,&& | 189.10 ± 5.50 # |
| MF | 1464.34 ± 134.67 ** | 221.52 ± 16.74 ** | 216.53 ± 18.26 * |
| AMEI | 1458.57 ± 171.74 * | 259.05 ± 22.75 ** | 218.09 ± 19.26 * |
| AMEII | 1562.65 ± 171.74 ** | 267.10 ± 11.38 **,& | 218.05 ± 23.86 * |
| AMEIII | 1441.22 ± 196.27 * | 238.94 ± 5.69 ** | 217.58 ± 18.49 * |
| Group | GSK-3 (U·L−1) |
|---|---|
| NC | 131.69 ± 1.24 |
| MG | 142.43 ± 6.20 ## |
| MF | 134.55 ± 3.72 * |
| AMEI | 131.09 ± 3.14 ** |
| AMEII | 131.80 ± 6.11 ** |
| AMEIII | 134.66 ± 3.63 * |
| Group | PEPCK (IU·L−1) | G-6-Pase (mIU·mL−1) |
|---|---|---|
| NC | 38.50 ± 2.40 | 994.99 ± 62.15 |
| MG | 49.22 ± 3.34 ##,& | 1412.45 ± 93.22 # |
| MF | 41.27 ± 2.75 * | 1082.87 ± 62.15 |
| AMEI | 40.92 ± 6.25 * | 1024.28 ± 207.67 * |
| AMEII | 39.19 ± 4.52 * | 1060.90 ± 93.22 * |
| AMEIII | 40.57 ± 4.19 * | 1068.23 ± 166.37 * |
| Group | IL-6(ng·L−1) | IL-8(ng·L−1) | TNF-α (pg·mL−1) | CRP (μg·L−1) |
|---|---|---|---|---|
| NC | 6.24 ± 4.05 | 247.06 ± 53.08 | 331518.25 ± 24999.24 | 494.72 ± 91.22 |
| MG | 36.48 ± 8.01 ##,&& | 707.00 ± 45.07 ##,&& | 492280.22 ± 68203.98 ##,&& | 1461.54 ± 249.72 ##,&& |
| MF | 11.03 ± 2.54 ** | 247.06 ± 45.07 ** | 333865.14 ± 19046.96 ** | 540.45 ± 203.65 ** |
| AMEI | 34.83 ± 5.21 ##,&& | 694.31 ± 125.63 ##,&& | 433216.82 ± 28692.27 ##,*,&& | 1161.04 ± 133.35 ##,*,&& |
| AMEII | 12.91 ± 9.08 ** | 283.27 ± 99.23 ** | 371806.53 ± 26416.85 ** | 622.10 ± 129.63 ** |
| AMEIII | 28.66 ± 2.44 ##*&& | 524.61 ± 82.33 ##,**,&& | 436815.38 ± 37085.32 ##,*,&& | 1185.54 ± 161.40 ##,*,&& |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Ke, Y.; Feng, A.; Yuan, P.; Zhou, J.; Yu, Y.; Wang, X.; Feng, W. The Mechanism by Which Amentoflavone Improves Insulin Resistance in HepG2 Cells. Molecules 2016, 21, 624. https://doi.org/10.3390/molecules21050624
Zheng X, Ke Y, Feng A, Yuan P, Zhou J, Yu Y, Wang X, Feng W. The Mechanism by Which Amentoflavone Improves Insulin Resistance in HepG2 Cells. Molecules. 2016; 21(5):624. https://doi.org/10.3390/molecules21050624
Chicago/Turabian StyleZheng, Xiaoke, Yingying Ke, Aozi Feng, Peipei Yuan, Jing Zhou, Yang Yu, Xiaolan Wang, and Weisheng Feng. 2016. "The Mechanism by Which Amentoflavone Improves Insulin Resistance in HepG2 Cells" Molecules 21, no. 5: 624. https://doi.org/10.3390/molecules21050624





