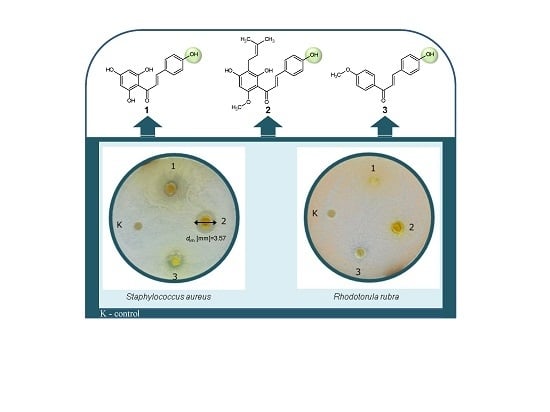
Antimicrobial Activity of Xanthohumol and Its Selected Structural Analogues
Abstract
:1. Introduction
2. Results
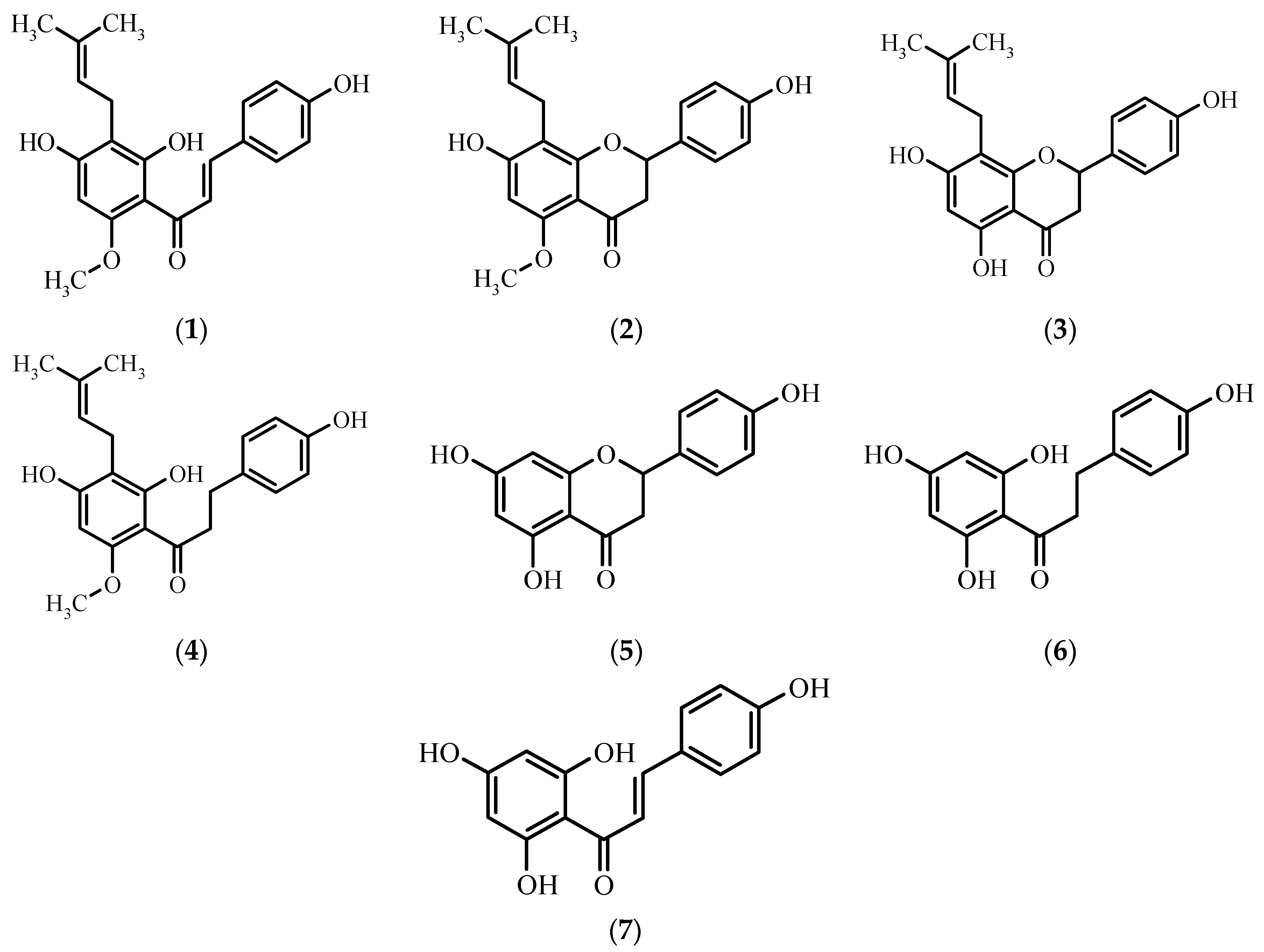
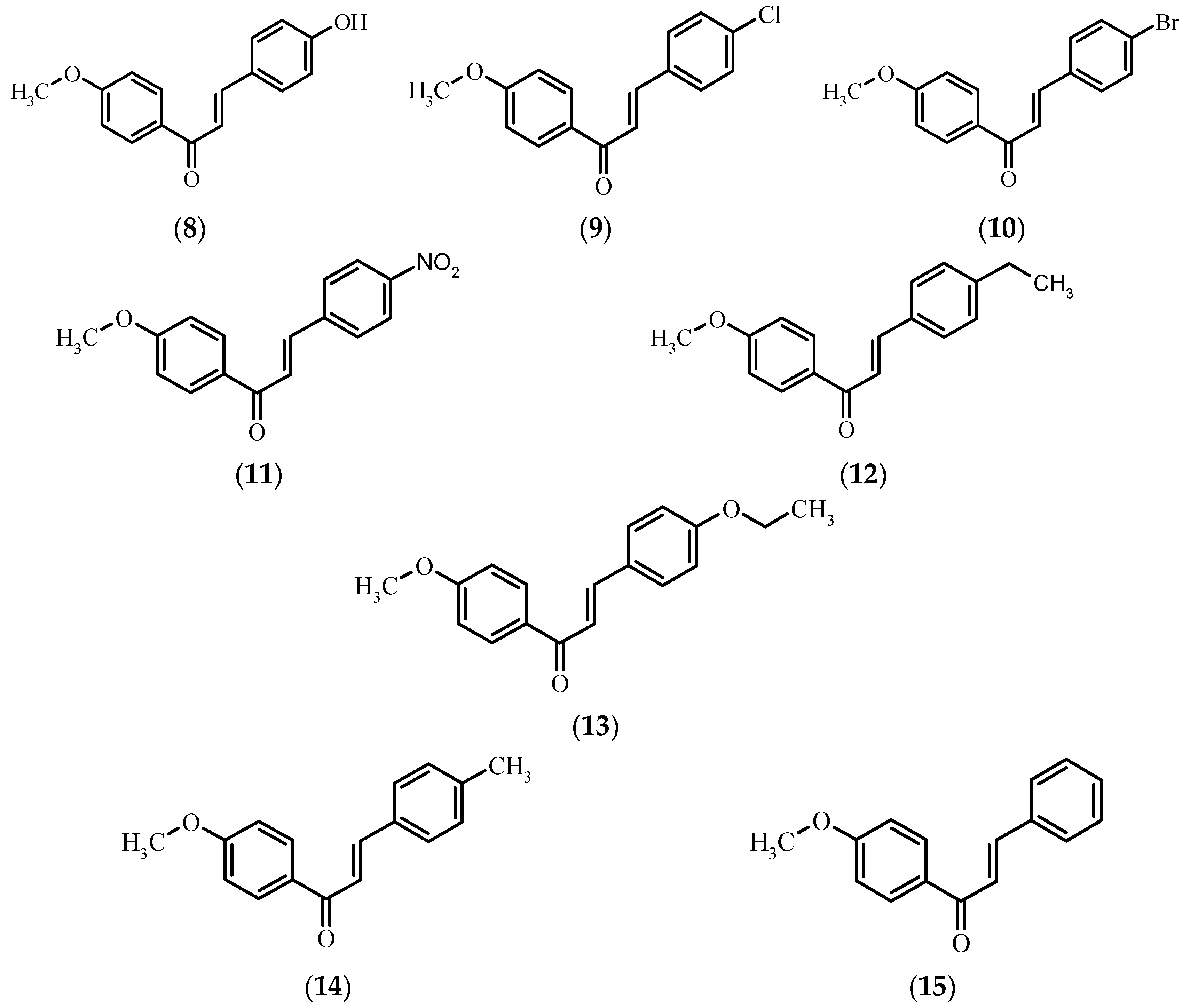
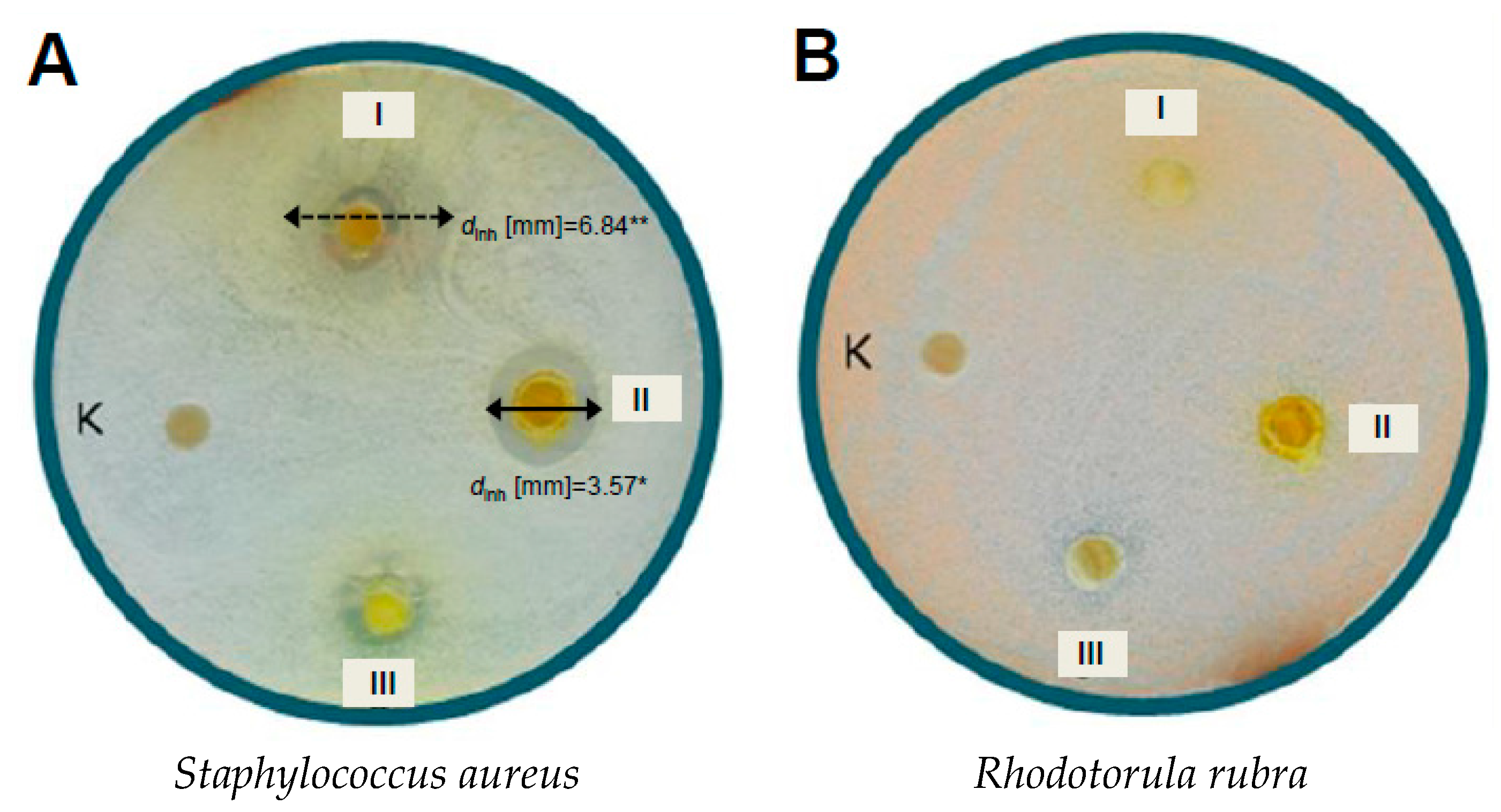
Antimicrobial Activity
3. Discussion
4. Materials and Methods
4.1. Antimicrobial Agents
4.1.1. Xanthohumol and Chalconaringenin
4.1.2. (E)-4′-Methoxy-4-Substituted Chalcones
4.2. Evaluation of Antimicrobial Activity
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Batovska, D.; Parushev, S.; Stamboliyska, B.; Tsvetkova, I.; Ninova, M.; Najdenski, H. Examination of growth inhibitory properties of synthetic chalcones for which antibacterial activity was predicted. Eur. J. Med. Chem. 2009, 4, 2211–2218. [Google Scholar] [CrossRef] [PubMed]
- Montes-Avila, J.; Díaz-Camacho, S.P.; Sicairos-Félix, J.; Delgado-Vargas, F.; Rivero, I.A. Solution-phase parallel synthesis of substituted chalcones and their antiparasitary activity against Giardia lamblia. Bioorg. Med. Chem. 2009, 17, 6780–6785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jin, H.; Ji, L.Z.; Tao, K.; Liu, W.; Zhao, H.Y.; Hou, T.P. Design, synthesis, and bioactivities screening of a diaryl ketone-inspired pesticide molecular library ad derived from natural products. Chem. Biol. Drug Des. 2011, 78, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Syam, S.; Abdelwahab, S.I.; Al-Mamary, M.A.; Mohan, S. Synthesis of chalcone with anticancer activities. Molecules 2012, 17, 6179–6195. [Google Scholar] [CrossRef] [PubMed]
- Mitić, S.S.; Paunović, D.D.; Pavlović, A.N.; Tošić, S.B.; Stojković, M.B.; Mitić, M.N. Phenolic profiles and total antioxidant capacity of marketed beers in Serbia. Int. J. Food Prop. 2014, 17, 908–922. [Google Scholar] [CrossRef]
- Lapcík, O.; Hill, M.; Hampl, R.; Wahala, K.; Adlercreutz, H. Identification of isoflavonoids in beer. Steroids 1998, 63, 14–20. [Google Scholar] [CrossRef]
- Mudura, E.; Paucean, A.; Tofana, M.; Socaci, S. The evaluation of prenylflavonoids compounds in Romanian beer. Bull. UASVM 2011, 68, 333–338. [Google Scholar]
- Etteldorf, N.; Etteldorf, N.; Becker, H. New chalcones from Humulus lupulus L. Z. Naturforsch. 1999, 54c, 610–612. [Google Scholar] [CrossRef]
- Toboła, D.; Stompor, M.; Błażewicz, J.; Anioł, M. Xanthohumol content in Polish beers. Przem. Chem. 2014, 93, 1447–1450. [Google Scholar]
- Gołąbczak, J.; Gendaszewska-Darmach, E. Ksantohumol i inne prenyloflawonoidy szyszek chmielu—Aspekty biologiczne i technologiczne. Biotechnologia 2010, 1, 75–89. [Google Scholar]
- Dhooghe, L.; Naessens, T.; Heyerick, A.; de Keukeleire, D.; Vlietinck, A.J.; Pieters, L.; Apers, S. Quantification of xanthohumol, isoxanthohumol, 8-prenylnaringenin, and 6-prenylnaringenin in hop extracts and derived capsules using secondary standards. Talanta 2010, 83, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, Q.; Jin, H.; Zhang, X.; Xu, Y.; Yu, A.; Zhang, H.; Ding, L. Determination of xanthohumol in beer based on cloud point extraction coupled with high performance liquid chromatography. Talanta 2010, 81, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Kramer, B.; Thielmann, J.; Hickisch, A.; Wunderlich, J.; Hauser, C. Antimicrobial activity of hop extracts against foodborne pathogens for meat applications. J. Appl. Microbiol. 2014, 118, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Stompor, M.; Gabryś, B.; Dancewicz, K.; Anioł, M. Insect antifeedant potential of xanthohumol and isoxanthohumol and their derivatives. J. Agric. Food Chem. 2015, 63, 6749–6756. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, L.; Balasooriya, B.A.I.S.; Padmini, W.C.; Hara, N.; Fujimoto, Y. Geranyl chalcone derivatives with antifungal and radical scavenging properties from the leaves of Artocarpus nobilis. Phytochemistry 2004, 65, 1287–1290. [Google Scholar] [CrossRef] [PubMed]
- Lόpez, S.N.; Castelli, M.V.; Zacchino, S.A.; Domínguez, J.N.; Lobo, G.; Charris-Charris, J.; Cortés, J.C.G.; Ribas, J.C.; Devia, C.; Rodríguez, A.M.; et al. In vitro antifungal evaluation and structure-activity relationship of a new series of chalcone derivatives and synthetic analogues, with inhibitory properties against polymers of the fungal cell wall. Bioorg. Med. Chem. 2001, 9, 1999–2013. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Ngameni, B.; Kuete, V.; Simo, I.K.; Ambassa, P.; Roy, R.; Bezabih, M.; Etoa, F.X.; Ngadjui, B.T.; Abegaz, B.M.; et al. Antimicrobial activity of crude extracts and five flavonoids from the twigs of Dorstenia barteri (Moracea). J. Ethnopharmacol. 2008, 116, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Kaveri, M.V.; Prabhakaran, R.; Karvembu, R.; Natarajan, K. Synthesis and spectral studies of [RuCl(CO)(L)(PPh3)(B)] (HL = 2′-hydroxychalcones and B = PPh3, pyridine or piperidine) and their catalytic and biological applications. Spectrochim. Acta Part A 2005, 61, 2915–2918. [Google Scholar] [CrossRef] [PubMed]
- Silva, W.A.; Andrade, C.K.Z.; Napolitano, H.B.; Vencato, I.; Lariucci, C.; Castro, M.R.C.; Camargo, A.J. Biological and structure-activity evaluation of chalcone derivatives against bacteria and fungi. J. Braz. Chem. Soc. 2013, 1, 133–144. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, G.T.; Baseer, M.; Shah, S.S. Study of partitioning and solubilization of some new chalcones between aqueous and micellar phases. J. Dispers. Sci. Technol. 2012, 33, 570–577. [Google Scholar] [CrossRef]
- Zhang, P.; Lin, R.; Yang, G.; Zhang, J.; Zhou, L.; Liu, T. Solubility of naringenin in ethanol and water mixtures. J. Chem. Eng. Data 2013, 58, 2402–2404. [Google Scholar] [CrossRef]
- Rozalski, M.; Micota, B.; Sadowska, B.; Stochmal, A.; Jedrejek, D.; Wieckowska-Szakiel, M.; Rozalska, B. Antiadherent and antibiofilm activity of Humulus lupulus L. derived products: New pharmacological properties. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Ávila, H.P.; Smânia, E.F.A.; Monache, F.D.; Júnior, A.S. Structure-activity relationship of antibacterial chalcones. Bioorg. Med. Chem. 2008, 16, 9790–9794. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, C.; Cai, Y.; Peng, J.; Liang, D.; Zhao, Y.; Yang, S.; Li, X.; Wu, X.; Liang, G. Synthesis and crystal structure of chalcones as well as on cytotoxicity and antibacterial properties. Med. Chem. Res. 2012, 21, 444–452. [Google Scholar] [CrossRef]
- Flythe, M.D.; Aiken, G.E. Effects of hop (Humulus lupulus L.) extract on volatile fatty acid production by rumen bacteria. J. Appl. Microbiol. 2010, 109, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Karaman, I.; Gezegen, H.; Gürdere, M.B.; Dingil, A.; Ceylan, M. Screening of biological activities of a series of chalcone derivatives against human pathogenic microorganisms. Chem. Biodivers. 2010, 7, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Kashiwada, Y.; Shibata, H.; Takaishi, Y. Prenylated flavonoids from Desmodium caudatum and evaluation of their anti-MRSA activity. Phytochemistry 2012, 82, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Ashida, H.; Danno, G.I. Rutin-enhanced antibacterial activities of flavonoids against Bacillus cereus and Salmonella enteritidis. Biosci. Biotechnol. Biochem. 2002, 66, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Chatzopoulou, A.; Katioti, A.; Gousiadou, C.; Vivancos, V.L.; Kyriazopoulos, P.; Golegou, S.; Skaltsa, H. Depsides and other polar constituents from Origanum dictamnus L. and their in vitro antimicrobial activity in clinical strains. J. Agric. Food Chem. 2010, 58, 6064–6068. [Google Scholar] [CrossRef] [PubMed]
- Shakil, N.A.; Singh, M.K.; Sathiyendiran, M.; Kumar, J. Microwave accelerated solvent-free synthesis and antifungal evaluations of flavanones. Arch. Phytopathol. Plant Prot. 2011, 44, 1958–1965. [Google Scholar] [CrossRef]
- Jeong, K.W.; Lee, J.Y.; Kang, D.I.; Lee, J.U.; Shin, S.Y.; Kim, Y. Screening of flavonoids as candidate antibiotics against Enterococcus faecalis. J. Nat. Prod. 2009, 72, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Herles, C.; Braune, A.; Blaut, M. First bacterial chalcone isomerise isolated from Eubacterium ramulus. Arch. Microbiol. 2004, 181, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Stompor, M.; Potaniec, B.; Szumny, A.; Zieliński, P.; Żołnierczyk, A.K.; Anioł, M. Microbial synthesis of dihydrochalcones using Rhodococcus and Gordonia species. J. Mol. Catal. B Enzym. 2013, 97, 283–288. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Laganà, G.; Ginestra, G.; Bisignano, C. Biochemical and antimicrobial activity of phloretin and its glicosilated derivatives present in apple and kumquat. Food Chem. 2014, 1, 160–292. [Google Scholar]
- Stompor, M.; Potaniec, B.; Szumny, A.; Zieliński, P.; Żołnierczyk, A.; Anioł, M. Microbiological reduction of xanthohumol and 4-methoxychalcone. Przem. Chem. 2013, 92, 574–578. [Google Scholar]
- Anioł, M.; Szymańska, K.; Żołnierczyk, A.K. An efficient synthesis of the phytoestrogen 8-prenylnaringenin from isoxanthohumol with magnesium iodide etherate. Tetrahedron 2008, 64, 9544–9547. [Google Scholar] [CrossRef]
- Le Bail, J.C.; Pouget, C.; Fagnere, C.; Basly, J.P.; Chulia, A.J.; Habrioux, G. Chalcones are potent inhibitors of aromatase and 17β-hydroxysteroid dehydrogenase activities. Life Sci. 2001, 68, 751–761. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1, 5, 7, 8–15 are available from the authors.
| Compound Number | Microorganism | ||||
|---|---|---|---|---|---|
| E. coli | S. aureus | C. albicans | R. rubra | Alternaria sp. | |
| dinh (mm) | |||||
| (1) | - | 3.57 * | - | - | - |
| (5) | - | 3.77 ** | - | - | - |
| (7) | - | 1.92 ** 6.84 ** | - | - | - |
| (8) | - | 2.86 * | - | 2.46 ** | - |
| (9) | - | - | - | - | - |
| (10) | - | - | - | - | - |
| (11) | - | - | - | - | - |
| (12) | - | - | - | - | - |
| (13) | - | - | - | - | - |
| (14) | - | - | - | - | - |
| (15) | - | - | - | - | - |
| oxytetracycline | 4.17 * | 9.18 * | |||
| cyklohexymide | 13.21 * | 11.32 * | 3.91 * | ||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stompor, M.; Żarowska, B. Antimicrobial Activity of Xanthohumol and Its Selected Structural Analogues. Molecules 2016, 21, 608. https://doi.org/10.3390/molecules21050608
Stompor M, Żarowska B. Antimicrobial Activity of Xanthohumol and Its Selected Structural Analogues. Molecules. 2016; 21(5):608. https://doi.org/10.3390/molecules21050608
Chicago/Turabian StyleStompor, Monika, and Barbara Żarowska. 2016. "Antimicrobial Activity of Xanthohumol and Its Selected Structural Analogues" Molecules 21, no. 5: 608. https://doi.org/10.3390/molecules21050608