Fused 1,2,3-Dithiazoles: Convenient Synthesis, Structural Characterization, and Electrochemical Properties
Abstract
:1. Introduction
2. Results and Discussion
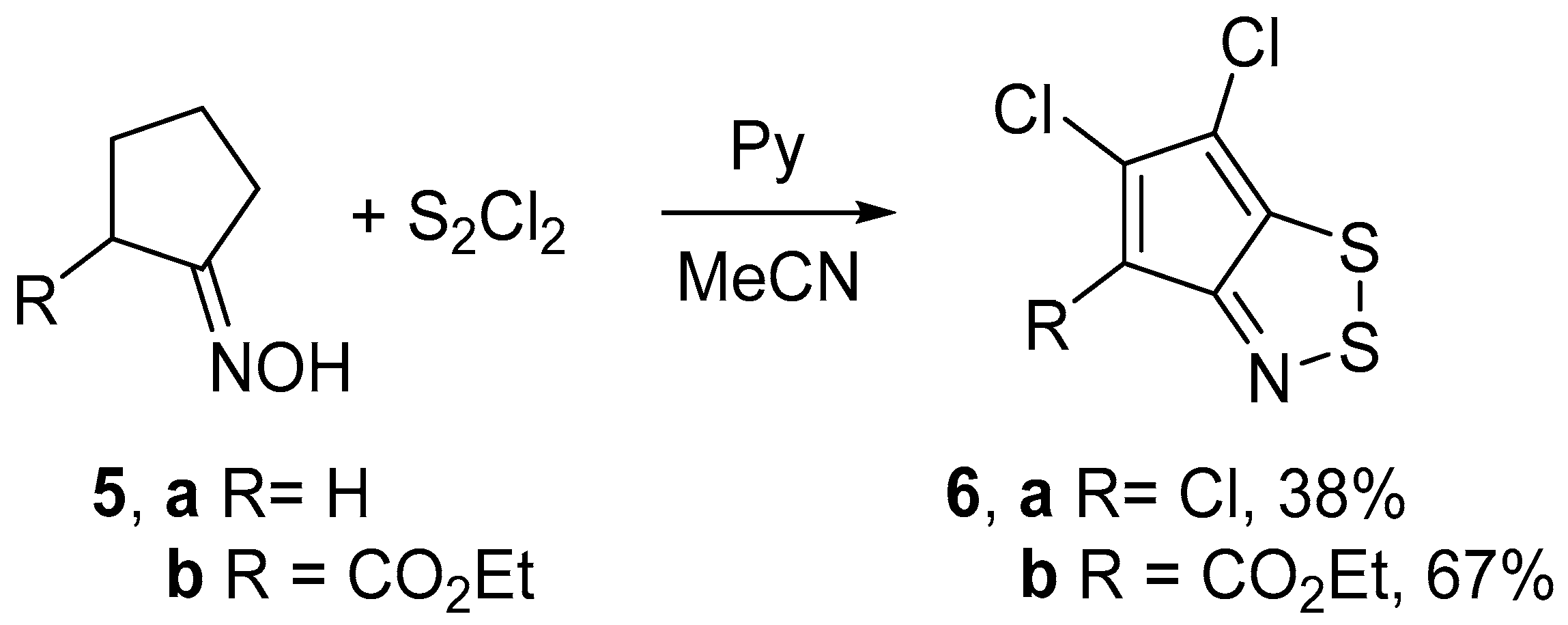
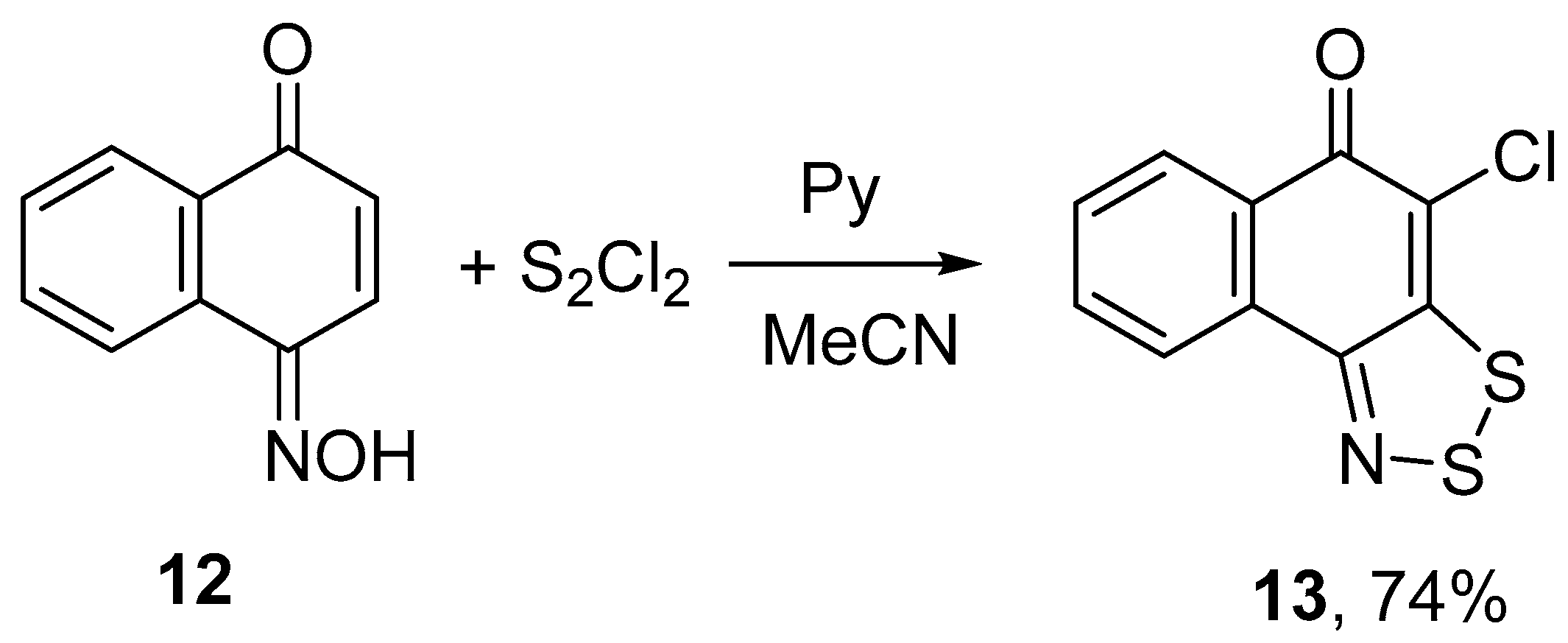
2.1. Syntheses
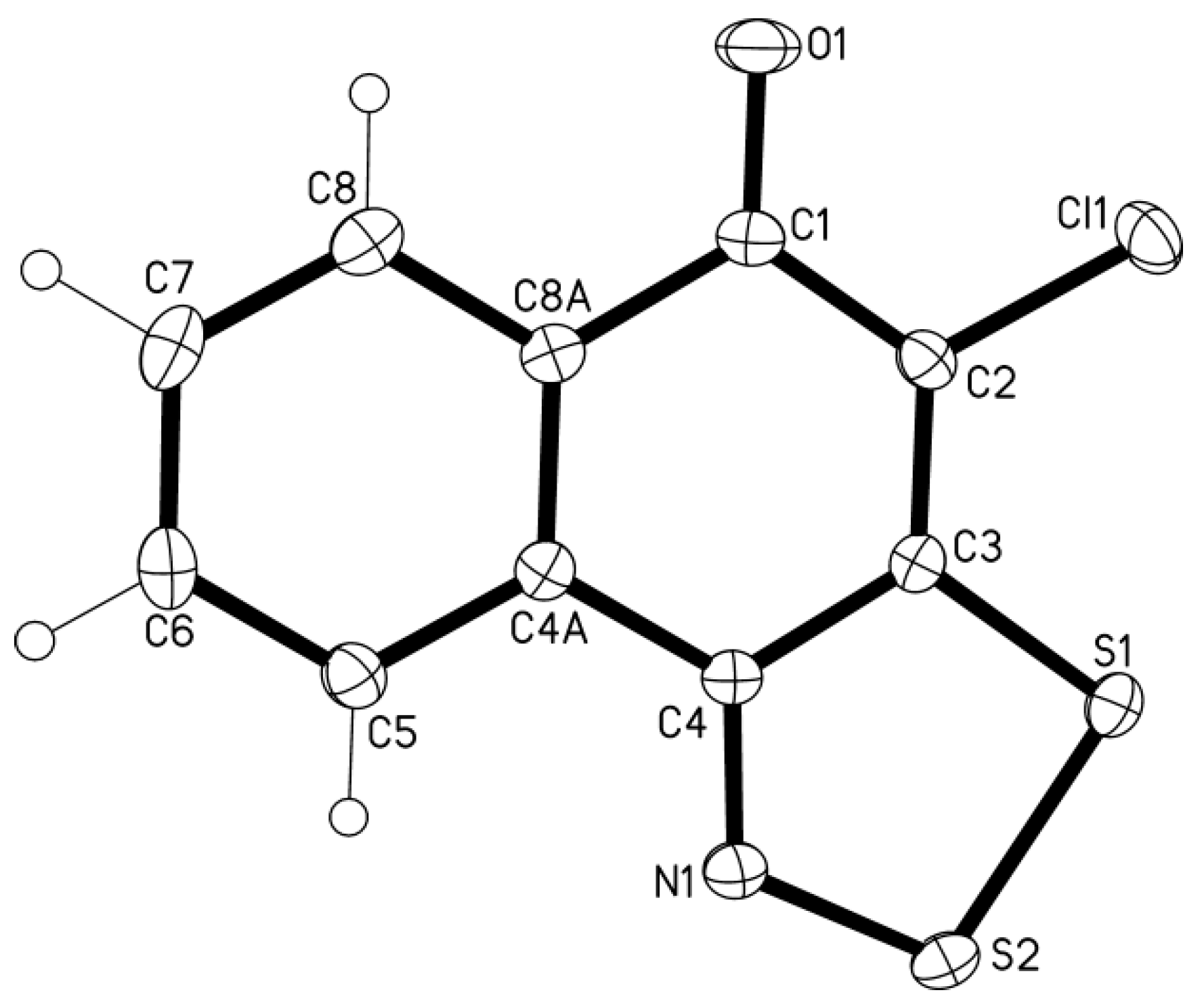
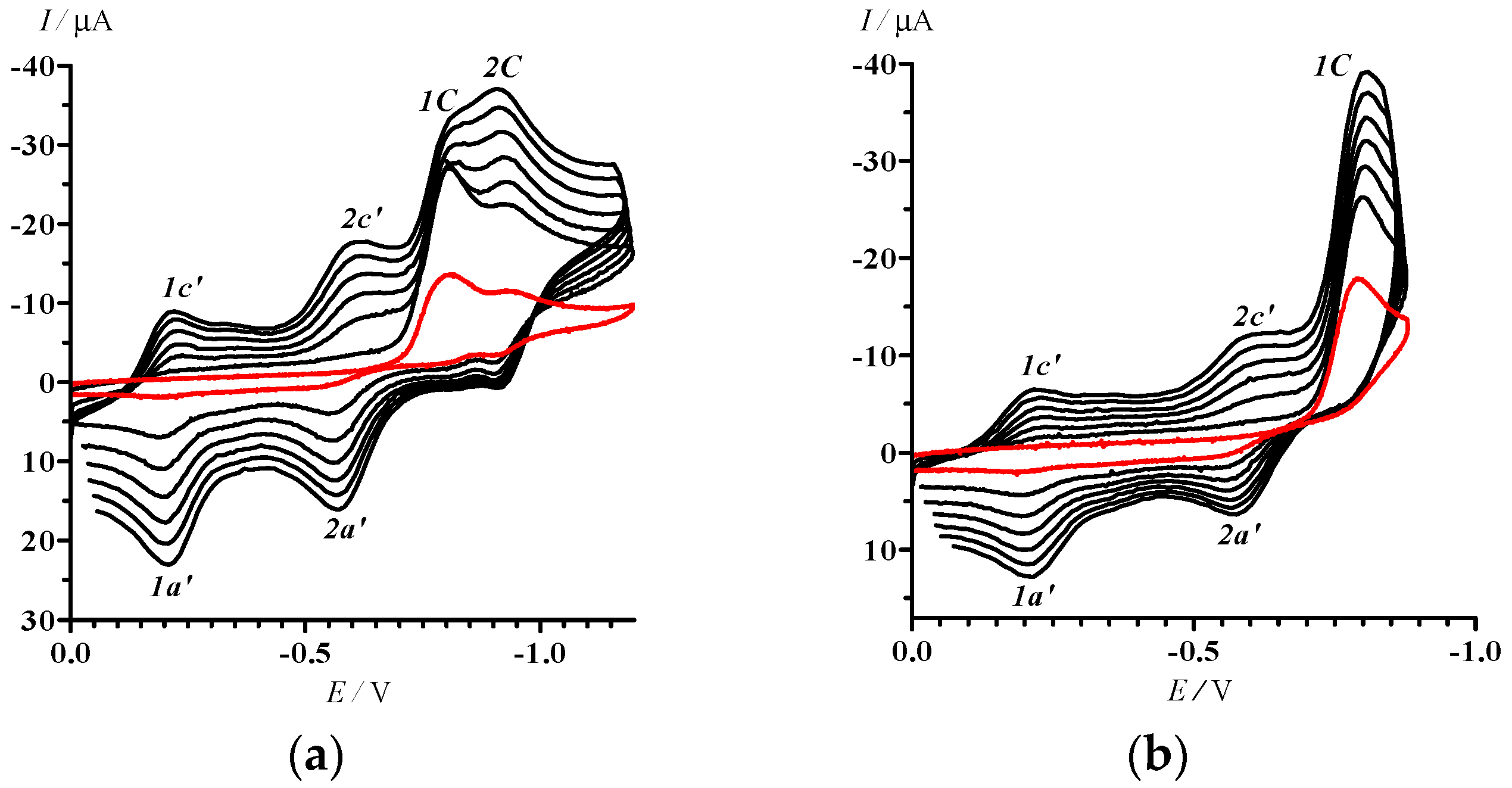
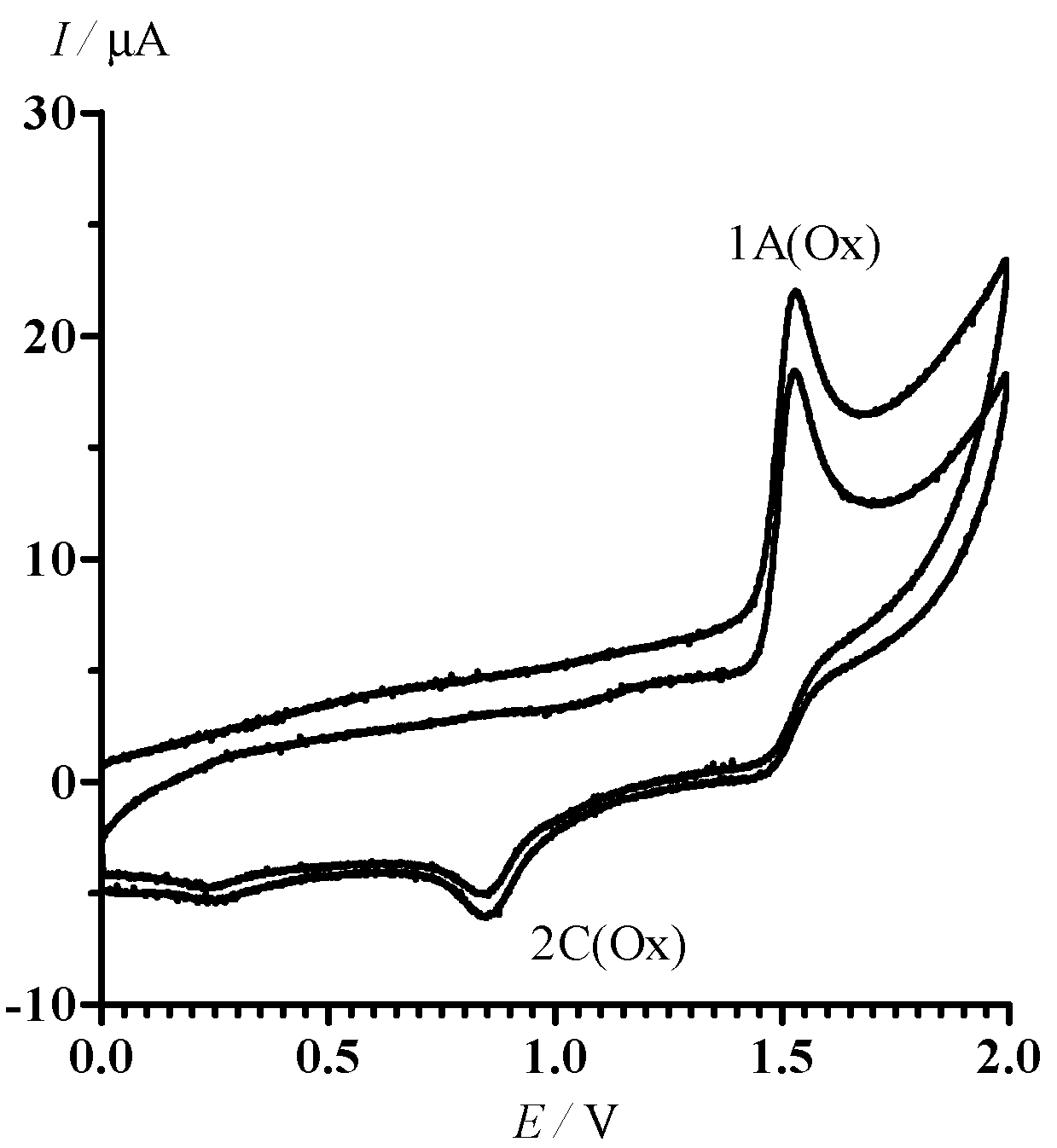
2.2. Electrochemical Reduction and Oxidation of Dithiazole 13
3. Experimental Section
3.1. General Information
3.2. General Procedure for the Reaction of Cyclic Oximes with S2Cl2 and Pyridine in Acetonitrile
3.3. Behavior of 1,2,3-Dithiazoles 13 and 15 in the S2Cl2/Pyridine System
3.4. X-ray Diffraction
3.5. Cyclic Voltammetry
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Khmelnitsky, L.I.; Rakitin, O.A. 1,2-Oxa-3-azoles and 1,2-thia-3-azoles. In Comprehensive Heterocyclic Chemistry II; Storr, R.C., Ed.; Pergamon: Oxford, UK, 1996; pp. 433–452. [Google Scholar]
- Rakitin, O.A. 1,2-Oxa/thia-3-azoles. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; pp. 1–36. [Google Scholar]
- Konstantinova, L.S.; Rakitin, O.A. Synthesis and properties of 1,2,3-dithiazoles. Russ. Chem. Rev. 2008, 77, 521–546. [Google Scholar] [CrossRef]
- Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds; Hicks, R.G. (Ed.) Wiley: Chichester, UK, 2010.
- Rakitin, O.A. Stable heterocyclic radicals. Russ. Chem. Rev. 2011, 80, 647–659. [Google Scholar] [CrossRef]
- Makarov, A.Y.; Chulanova, E.A.; Semenov, N.A.; Pushkarevsky, N.A.; Lonchakov, A.V.; Bogomyakov, A.S.; Irtegova, I.G.; Vasilieva, N.V.; Lork, E.; Gritsan, N.P.; et al. A novel sulfur-nitrogen π-heterocyclic radical anion, (6H-1,2,3-benzothiadiazole-6-ylidene)malononitrilidyl, and its homo- and heterospin salts. Polyhedron 2014, 72, 43–49. [Google Scholar] [CrossRef]
- Chulanova, E.A.; Irtegova, I.G.; Vasilieva, N.V.; Bagryanskaya, I.Y.; Gritsan, N.P.; Zibarev, A.V. Novel long-lived π-heterocyclic radical anion: A hybrid of 1,2,5-thiadiazo- and 1,2,3-dithiazolidyls. Mendeleev Commun. 2015, 25, 336–338. [Google Scholar] [CrossRef]
- Appel, R.; Janssen, H.; Siray, M.; Knoch, F. Synthese und reaktionen des 4,5-dichlor-1,2,3-dithiazolium chlorids. Chem. Ber. 1985, 118, 1632–1643. [Google Scholar] [CrossRef]
- Kim, K. Synthesis and reactions of 1,2,3-dithiazoles. Sulfur Rep. 1998, 21, 147–207. [Google Scholar] [CrossRef]
- Oakley, R.T.; Reed, R.W.; Robertson, C.M.; Richardson, J.F. Naphthalene-1,2,3-dithiazolyl and its selenium-containing variants. Inorg. Chem. 2005, 44, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Neo, A.G; Carrillo, R.M.; Marcos, C.F. A straightforward synthesis of 2-aminobenzothiazoles from Herz compounds. Org. Biomol. Chem. 2011, 9, 4850–4855. [Google Scholar] [CrossRef] [PubMed]
- Plater, M.J.; Rees, C.W.; Roe, D.G.; Torroba, T. Cyclopenta-1,2,3-dithiazoles and related compounds. J. Chem. Soc. Perkin Trans. 1 1993. [Google Scholar] [CrossRef]
- Polo, C.; Ramos, V.; Torroba, T.; Rakitin, O.A.; Rees, C. W. One-pot synthesis of 1,2,3-benzodithiazol-6-ones. Tetrahedron 1998, 54, 223–232. [Google Scholar] [CrossRef]
- Macho, S.; Rodriguez, T.; Torroba, T.; Rees, C.W. A novel oxime to pentathiepin cascade reaction. J. Chem. Soc. Chem. Commun. 2001. [Google Scholar] [CrossRef]
- Macho, S.; Miguel, D.; Gomez, T.; Rodriguez, T.; Torroba, T. From cyclopentanone oximes to bis[1,2,3]dithiazolo-s-indacenes, cyclopenta[c][1,2]thiazine, pentathiepino-, tetrathiino-, and thienocyclopenta[1,2,3]dithiazoles as a rich source of new materials. J. Org. Chem. 2005, 70, 9314–9325. [Google Scholar] [CrossRef] [PubMed]
- Rakitin, O.A.; Konstantinova, L.S. Sulfur monochloride in the synthesis of heterocyclic compounds. Adv. Heterocycl. Chem. 2008, 96, 175–229. [Google Scholar]
- Rakitin, O.A. One-pot synthesis of sulfur heterocycles from simple organic substrates. Arkivoc 2009, 1, 129–149. [Google Scholar]
- Rakitin, O.A.; Konstantinova, L.S. Design of sulfur heterocycles with sulfur monochloride: Retrosynthetic analysis and prospects. Mendeleev Commun. 2009, 19, 55–61. [Google Scholar]
- Rakitin, O.A.; Konstantinova, L.S. Sulfur monochloride in organic synthesis. Russ. Chem. Rev. 2014, 83, 225–250. [Google Scholar]
- Allen, F.H.; Kenard, O.; Watson, D.G.; Bramer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. II 1987. [Google Scholar] [CrossRef]
- In the crystal of 13, the intermolecular contacts S...O are shortened to 2.84–2.91 Å and form an infinite chains of molecules along the crystallographic axis c (normal contact S···O is 3.32 Å: Rowland, R.S.; Taylor, R. Intermolecular nonbonded contact distances in organic crystal structures: Comparison with distances expected from van der Waals radii. J. Phys. Chem. 1996, 100, 7384–7391. [Google Scholar]). These chains form infinite stacks along the axis b through the arene-arene π···π interactions (centroid-to-centroid distance is 3.64 Å, interplanar separation 3.51 Å). Additionally to the π…π interactions, the Cl···π interactions are observed, the atom-to-plane distance is 3.53 Å.
- Konstantinova, L.S.; Rakitin, O.A.; Rees, C.W. One-pot synthesis of fused pentathiepins. Chem. Commun. 2002, 2009, 1204–1205. [Google Scholar]
- Amelichev, S.A.; Konstantinova, L.S.; Lyssenko, K.A.; Rakitin, O.A.; Rees, C.W. Direct synthesis of fused 1,2,3,4,5-pentathiepins. Org. Biomol. Chem. 2005, 3, 3496–3501. [Google Scholar] [CrossRef] [PubMed]
- Konstantinova, L.S.; Amelichev, S.A.; Rakitin, O.A. Regioselective synthesis of pentathiepins fused with pyrroles, thiophene and indoles. Russ. Chem. Bull. 2006, 55, 2081–2084. [Google Scholar] [CrossRef]
- Lonchakov, A.V.; Rakitin, O.A.; Gritsan, N.P.; Zibarev, A.V. Breathing some new life into an old topic: Chalcogen-nitrogen π-heterocycles as electron acceptors. Molecules 2013, 18, 9850–9900. [Google Scholar] [CrossRef] [PubMed]
- Pushkarevsky, N.A.; Semenov, N.A.; Dmitriev, A.A.; Kuratieva, N.V.; Bogomyakov, A.S.; Irtegova, I.G.; Vasilieva, N.V.; Bode, B.E.; Gritsan, N.P.; Konstantinova, L.S.; et al. Synthesis and properties of the heterospin (S1 = S2 = 1/2) radical-ion salt bis(mesitylene)molibdenium(I) [1,2,5][thiadiazolo[3,4-c][1,2,5]thiadiazolidyl. Inorg. Chem. 2015, 54, 7007–7013. [Google Scholar] [CrossRef] [PubMed]
- Semenov, N.A.; Pushkarevsky, N.A.; Suturina, E.A.; Chulanova, E.A.; Kuratieva, N.V.; Bogomyakov, A.S.; Irtegova, I.G.; Vasilieva, N.V.; Konstantinova, L.S.; Gritsan, N.P.; et al. Bis(toluene)chromium(I) [1,2,5]thiadiazolo[3,4-c][1,2,5]thiadiazolidyl and [1,2,5]thiadiazolo[3,4-b]pyrazinidyl: New heterospin (S1 = S2 = 1/2) radical-ion salts. Inorg. Chem. 2013, 52, 6654–6663. [Google Scholar] [CrossRef] [PubMed]
- Semenov, N.A.; Pushkarevsky, N.A; Lonchakov, A.V.; Bogomyakov, A.S.; Pritchina, E.A.; Suturina, E.A.; Gritsan, N.P.; Konchenko, S.N.; Mews, R.; Ovcharenko, V.I.; et al. Heterospin π-heterocyclic radical-anion salt: Synthesis, structure and magnetic properties of decamethylchromocenium [1,2,5]thiadiazolo[3,4-c][1,2,5]thiadiazolidyl. Inorg. Chem. 2010, 49, 7558–7564. [Google Scholar] [CrossRef] [PubMed]
- Gritsan, N.P.; Zibarev, A.V. Chalcogen-nitrogen π-heterocyclic radical-anion salts: The synthesis and properties. Russ. Chem. Bull. 2011, 60, 2131–2140. [Google Scholar] [CrossRef]
- In the potential designation, i is a number of peak, j = A or C indicates the anode or cathode branch of the CV curve, respectively. An additional symbol Ox is related to CV in the area of oxidative potentials. The designation of corresponding currents is the same.
- Vasilieva, N.V.; Irtegova, I.V.; Gritsan, N.P.; Lonchakov, A.V.; Makarov, A.Y.; Shundrin, L.A.; Zibarev, A.V. Redox properties and radical anions of fluorinated 2,1,3-benzothia(selena)diazoles and related compounds. J. Phys. Org. Chem. 2010, 23, 536–543. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Knyazeva, E.A.; Obruchnikova, N.V.; Vasilieva, N.V.; Irtegova, I.G.; Nelyubina, Y.V.; Bagryanskaya, I.Y.; Shundrin, L.A.; Sosnovskaya, Z.Y.; Zibarev, A.V.; et al. 1,2,5-Thiadiazole 2-oxides, their benzo-fused derivatives and parent compounds: Selective synthesis, structural characterization and electrochemical properties. Tetrahedron 2014, 70, 5558–5568. [Google Scholar] [CrossRef]
- Banzi, V.; Gila, L.; Santi, R.; Biagini, P.; Borsotti, G. Process for Preparing Elastomeric ep(d)m Copolymers. EP 0806436, 12 November 1997. [Google Scholar]
- Kim, S.-H.; Kwon, J.-H.; Yoon, S.-H. An improved synthesis of 4′-hydroxydiclofenac. Bull. Korean Chem. Soc. 2010, 31, 3007–3009. [Google Scholar] [CrossRef]
- Meyer, K.H.; Lenhardt, S. Die Reaktionsweise der Enole und der Phenole; ein Beitrag zur Kenntnis der ungesättigten Verbindungen. Liebigs Ann. Chem. 1913, 398, 66–82. [Google Scholar] [CrossRef]
- Henriques, R.; Ilinski, M. Zur darstellung der nitrosonaphtole. Chem. Ber. 1885, 18, 704–706. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- SADABS. Version 2008-1; Bruker AXS: Madison, WI, USA, 2008.
- Spek, A.L. PLATON, A Multipurpose Crystallographic Tool (Version 10M); Utrecht University: Utrecht, The Netherlands, 2003. [Google Scholar]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Stree, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Sample Availability: Samples of all compounds are available from the authors.
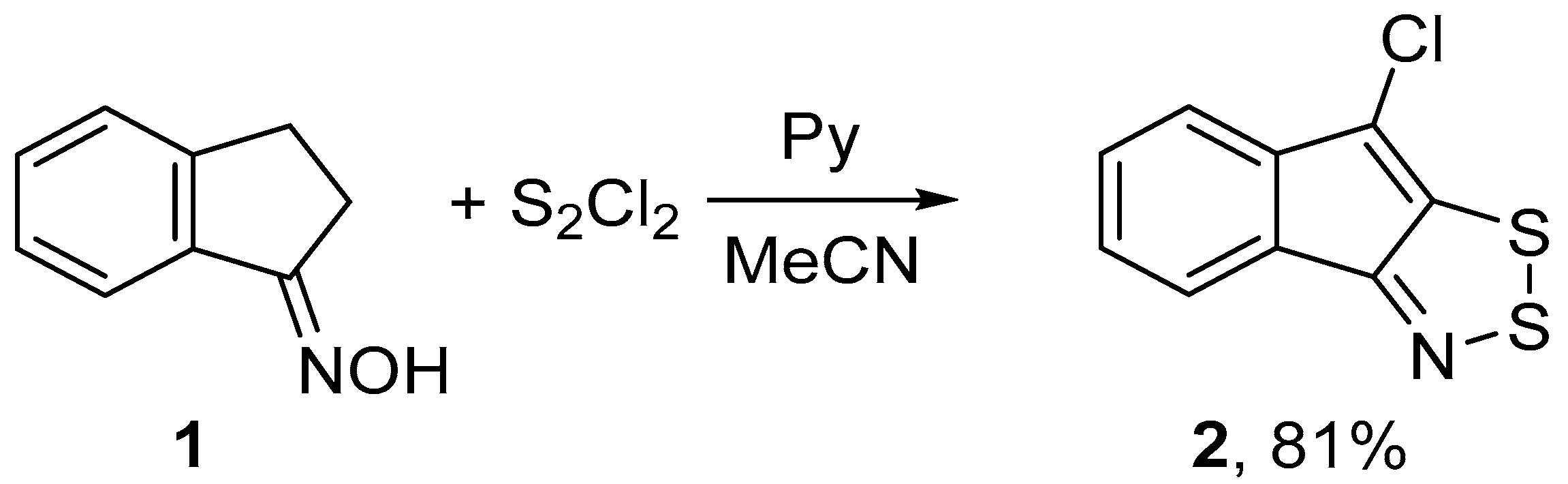


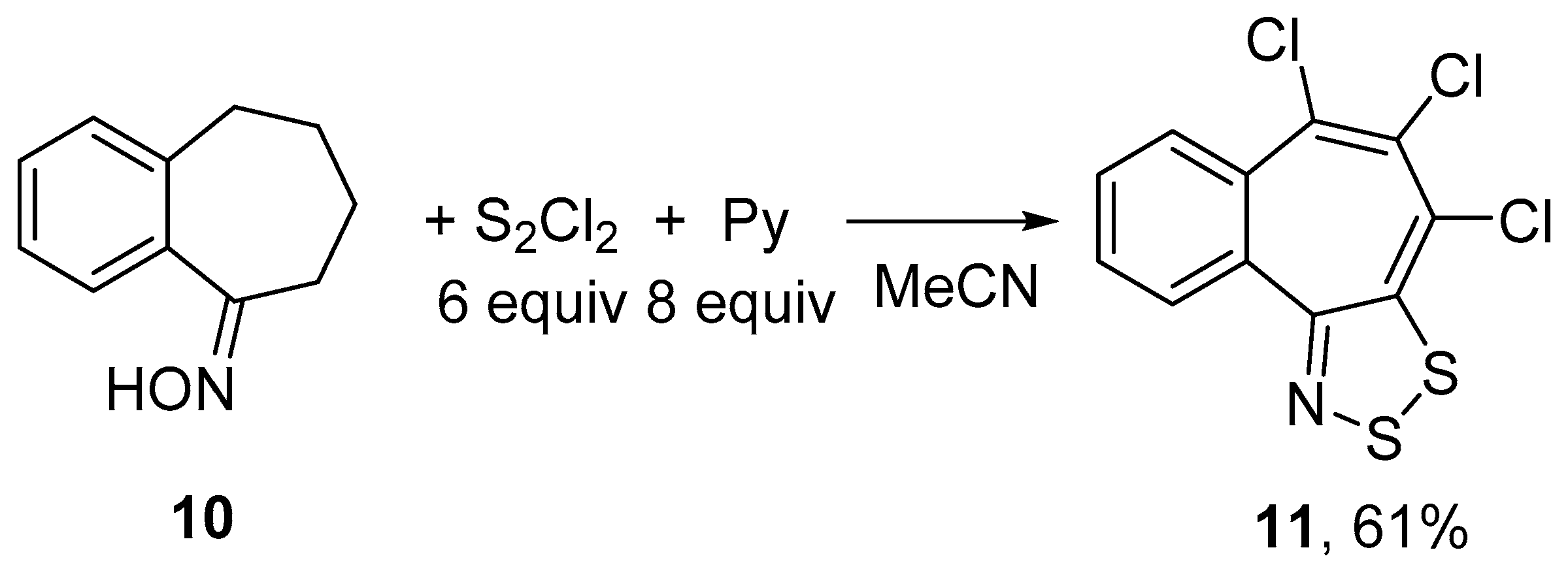


© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantinova, L.S.; Baranovsky, I.V.; Irtegova, I.G.; Bagryanskaya, I.Y.; Shundrin, L.A.; Zibarev, A.V.; Rakitin, O.A. Fused 1,2,3-Dithiazoles: Convenient Synthesis, Structural Characterization, and Electrochemical Properties. Molecules 2016, 21, 596. https://doi.org/10.3390/molecules21050596
Konstantinova LS, Baranovsky IV, Irtegova IG, Bagryanskaya IY, Shundrin LA, Zibarev AV, Rakitin OA. Fused 1,2,3-Dithiazoles: Convenient Synthesis, Structural Characterization, and Electrochemical Properties. Molecules. 2016; 21(5):596. https://doi.org/10.3390/molecules21050596
Chicago/Turabian StyleKonstantinova, Lidia S., Ilia V. Baranovsky, Irina G. Irtegova, Irina Y. Bagryanskaya, Leonid A. Shundrin, Andrey V. Zibarev, and Oleg A. Rakitin. 2016. "Fused 1,2,3-Dithiazoles: Convenient Synthesis, Structural Characterization, and Electrochemical Properties" Molecules 21, no. 5: 596. https://doi.org/10.3390/molecules21050596






