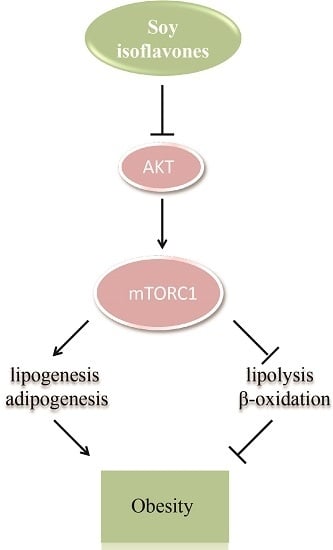
Soy Isoflavones Regulate Lipid Metabolism through an AKT/mTORC1 Pathway in Diet-Induced Obesity (DIO) Male Rats
Abstract
:1. Introduction
2. Results
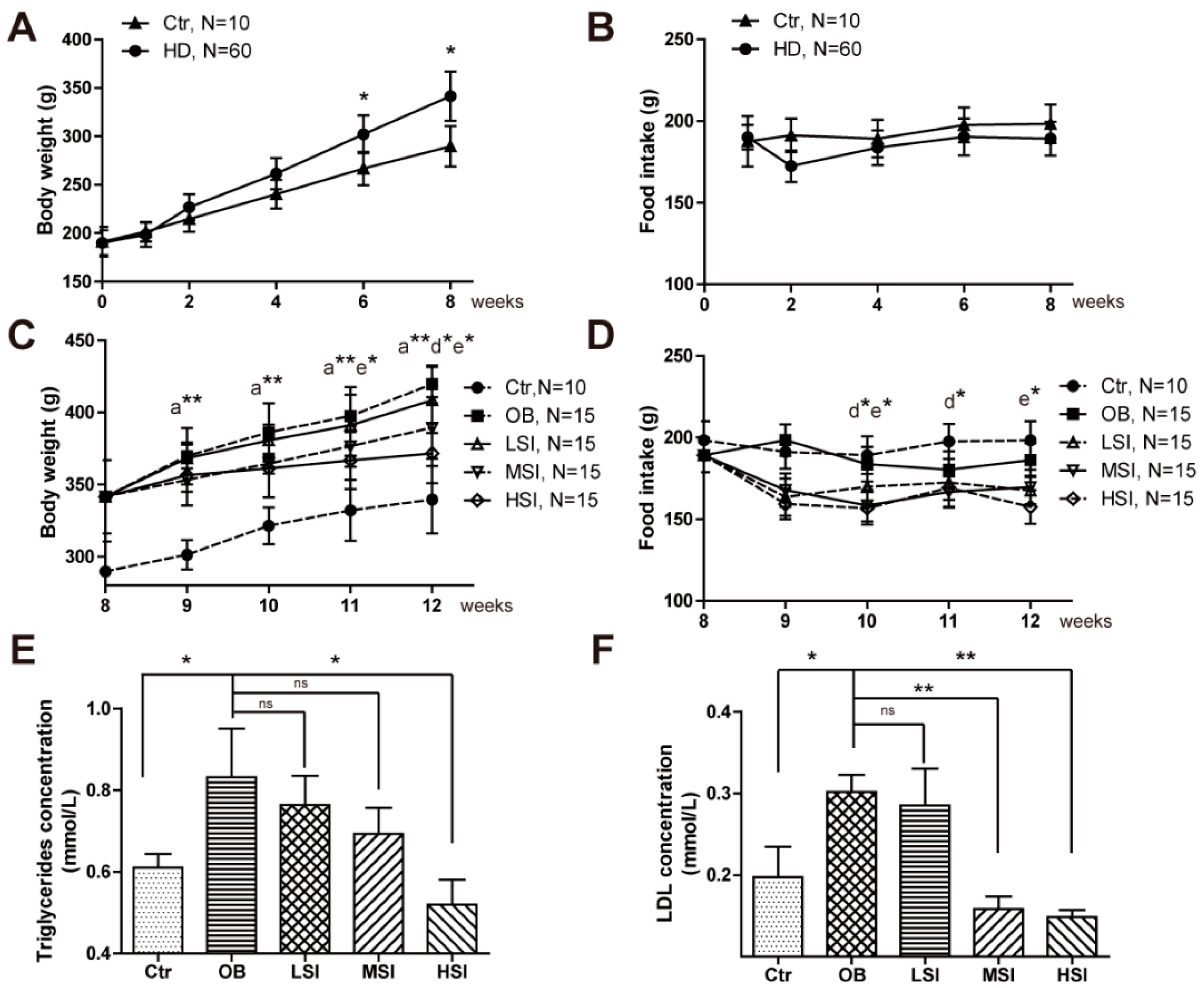
2.1. Soy Isoflavones Exhibited Remarkable Effects on Body Weight and Adiposity in DIO Male Rats
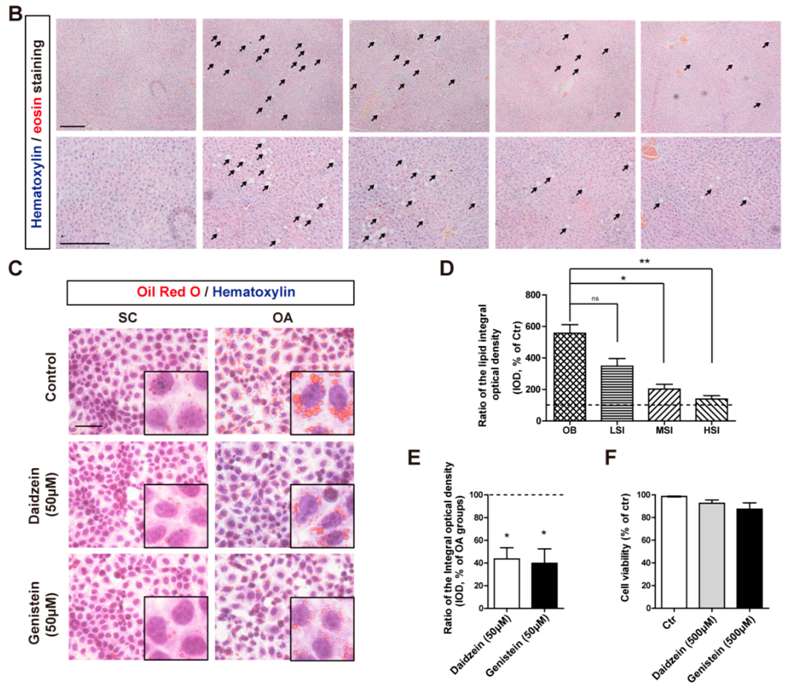
2.2. Soy Isoflavones Could Reduce Lipid Accumulation in Livers of DIO Rats
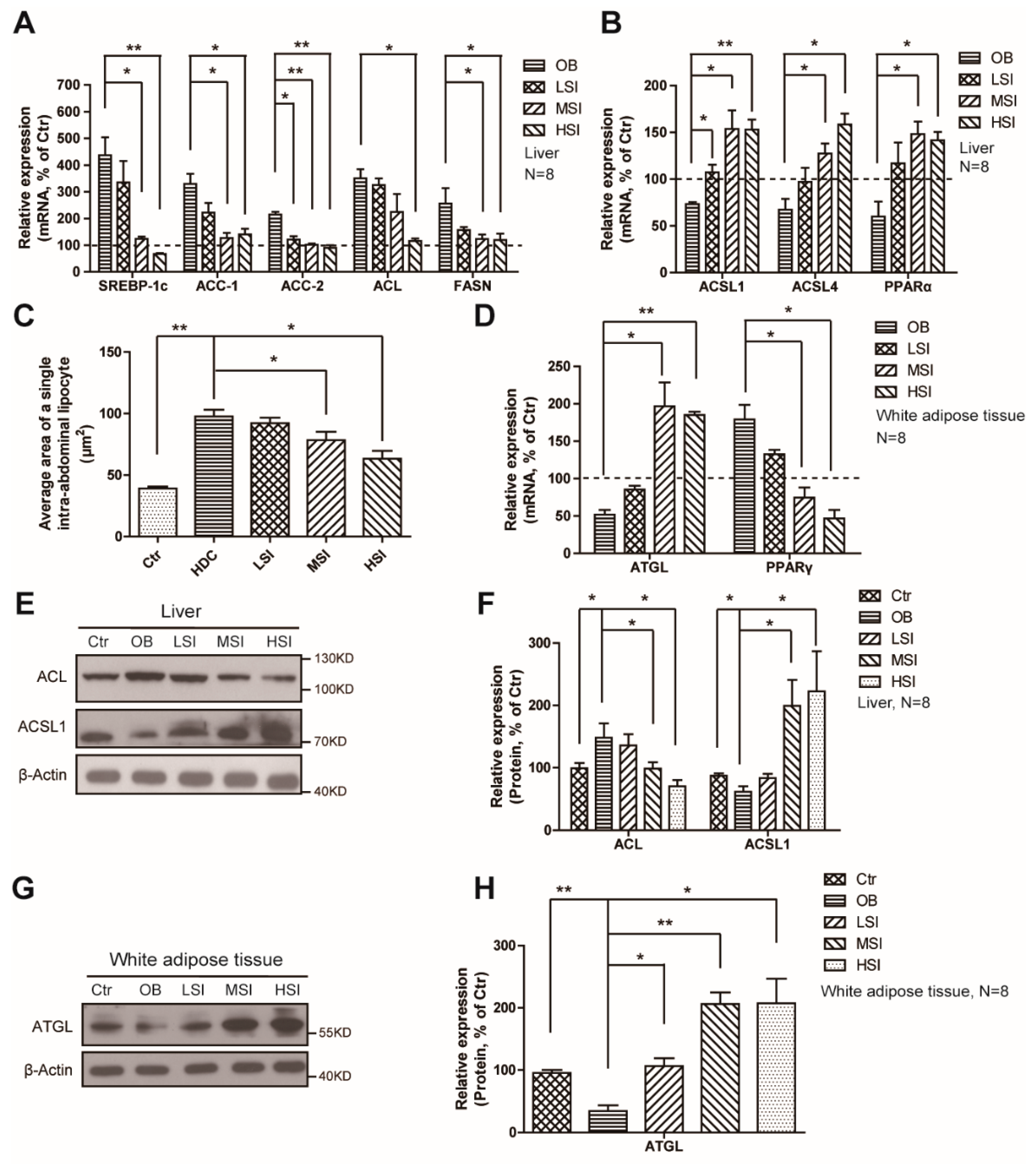
2.3. Effects of Soy Isoflavones on the Lipid Metabolism in the Liver and Adipose Tissue
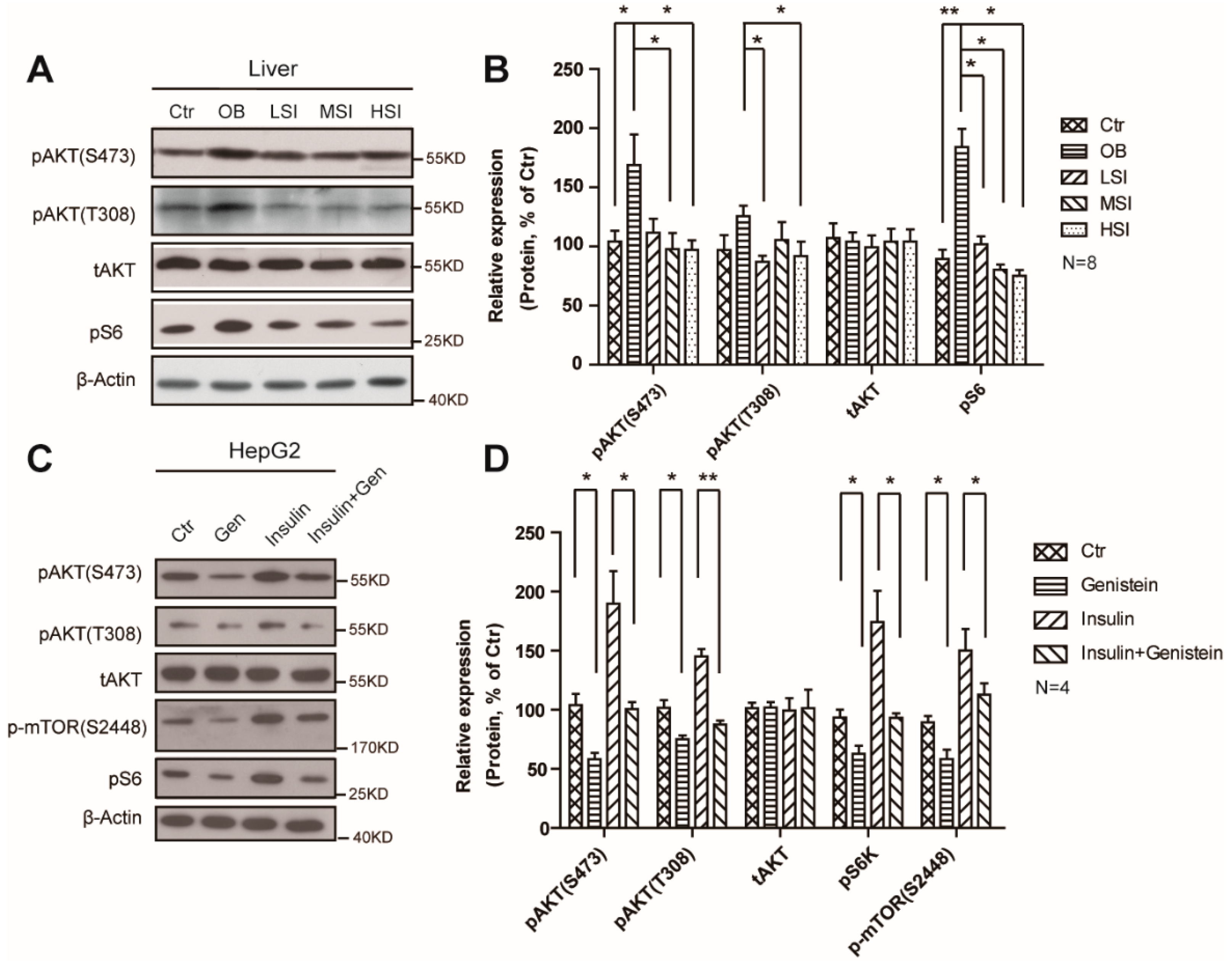
2.4. Soy Isoflavones Suppressed the Activity of mTORC1
3. Discussion
4. Materials and Methods
4.1. Animal Care and Maintenance
4.2. Body Weight, Food Intake, and Plasma Measurements
4.3. Histopathologic Evaluation
4.4. OA/BSA Complex Solution Preparation
4.5. Cell Culture
4.6. Quantitative Realtime PCR
4.7. Cell Viability Assay
4.8. Western Blotting
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DIO | diet induced obesity |
| mTORC1 | mechanistic target of rapamycin complexes 1 |
| Ctr | Control |
| H and E staining | hematoxylin and eosin staining |
| OA | oleic acid |
| OB | obesity control group |
| LSI | low dose of soy isoflavones |
| MSI | middle dose of soy isoflavones |
| HSI | high dose of soy isoflavones |
| TG | triglycerides |
| LDL | low-density lipoprotein |
| SREBP1 | Sterol regulatory element-binding transcription factor 1 |
| ACC | Acetyl-CoA carboxylase |
| ACL | ATP citrate lyase |
| FASN | Fatty acid synthase |
| ACSL | Long chain fatty acid-CoA ligase |
| ATGL | Adipose triglyceride lipase |
| HSL | Hormone sensitive lipase |
| PPARs | peroxisome proliferator-activated receptors |
References
- Haslam, D.W.; James, W.P. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef]
- Pi-Sunyer, F.X. Comorbidities of overweight and obesity: Current evidence and research issues. Med. Sci. Sports Exerc. 1999, 31 (Suppl. 11), S602–S608. [Google Scholar] [CrossRef] [PubMed]
- Rohde, U.; Hedback, N.; Gluud, L.L.; Vilsboll, T.; Knop, F.K. Effect of the EndoBarrier Gastrointestinal Liner on obesity and type 2 diabetes: Systematic review and meta-analysis. Diabetes Obes. Metab. 2016, 18, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A. Diet beverages and the risk of obesity, diabetes, and cardiovascular disease: A review of the evidence. Nutr. Rev. 2013, 71, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Sterry, W.; Strober, B.E.; Menter, A. Obesity in psoriasis: The metabolic, clinical and therapeutic implications. Report of an interdisciplinary conference and review. Br. J. Dermatol. 2007, 157, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Commodore-Mensah, Y.; Samuel, L.J.; Dennison-Himmelfarb, C.R.; Agyemang, C. Hypertension and overweight/obesity in Ghanaians and Nigerians living in West Africa and industrialized countries: A systematic review. J. Hypertens. 2014, 32, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Aballay, L.R.; Eynard, A.R.; Diaz Mdel, P.; Navarro, A.; Munoz, S.E. Overweight and obesity: A review of their relationship to metabolic syndrome, cardiovascular disease, and cancer in South America. Nutr. Rev. 2013, 71, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.S.; Palmieri, R.T.; Nyante, S.J.; Koralek, D.O.; Kim, S.; Bradshaw, P.; Olshan, A.F. Obesity and screening for breast, cervical, and colorectal cancer in women: A review. Cancer 2008, 112, 1892–1904. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [PubMed]
- Marchesini, G.; Moscatiello, S.; Di Domizio, S.; Forlani, G. Obesity-associated liver disease. J. Clin. Endocrinol. Metab. 2008, 93 (Suppl. 1), S74–S80. [Google Scholar]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar]
- Huang, J.; Manning, B.D. The TSC1-TSC2 complex: A molecular switchboard controlling cell growth. Biochem. J. 2008, 412, 179–190. [Google Scholar]
- Lamming, D.W.; Sabatini, D.M. A Central role for mTOR in lipid homeostasis. Cell Metab. 2013, 18, 465–469. [Google Scholar]
- Duvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S.; et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 2010, 39, 171–183. [Google Scholar]
- Zhang, H.H.; Huang, J.; Duvel, K.; Boback, B.; Wu, S.; Squillace, R.M.; Wu, C.L.; Manning, B.D. Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS ONE 2009, 4, e6189. [Google Scholar]
- Peng, T.; Golub, T.R.; Sabatini, D.M. The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol. Cell. Biol. 2002, 22, 5575–5584. [Google Scholar] [CrossRef] [PubMed]
- Um, S.H.; Frigerio, F.; Watanabe, M.; Picard, F.; Joaquin, M.; Sticker, M.; Fumagalli, S.; Allegrini, P.R.; Kozma, S.C.; Auwerx, J.; et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 2004, 431, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, P.; English, T.; Shi, J.; Smas, C.M.; Kandror, K.V. Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage. Diabetes 2010, 59, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Peterson, T.R.; Sengupta, S.S.; Harris, T.E.; Carmack, A.E.; Kang, S.A.; Balderas, E.; Guertin, D.A.; Madden, K.L.; Carpenter, A.E.; Finck, B.N.; et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 2011, 146, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Pyo, S.; Um, S.H. S6 kinase 2 deficiency enhances ketone body production and increases peroxisome proliferator-activated receptor alpha activity in the liver. Hepatology 2012, 55, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Nutritional benefits of soy protein and soy fiber. J. Am. Diet. Assoc. 1991, 91, 816–819. [Google Scholar] [PubMed]
- Munro, I.C.; Harwood, M.; Hlywka, J.J.; Stephen, A.M.; Doull, J.; Flamm, W.G.; Adlercreutz, H. Soy isoflavones: A safety review. Nutr. Rev. 2003, 61, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Higginbotham, A.; O’Connor, T.; Moustaid-Moussa, N.; Tebbe, A.; Kim, Y.C.; Cho, K.W.; Shay, N.; Adler, S.; Peterson, R.; et al. Soy protein and isoflavones influence adiposity and development of metabolic syndrome in the obese male ZDF rat. Ann. Nutr. Metab. 2007, 51, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Zhang, J.; Lazarenko, O.P.; Cao, J.J.; Blackburn, M.L.; Badger, T.M.; Ronis, M.J. Soy protein isolates prevent loss of bone quantity associated with obesity in rats through regulation of insulin signaling in osteoblasts. FASEB J. 2013, 27, 3514–3523. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D. Phytoestrogens: The biochemistry, physiology, and implications for human health of soy isoflavones. Am. J. Clin. Nutr. 1998, 68, 1333S–1346S. [Google Scholar] [PubMed]
- Taku, K.; Umegaki, K.; Sato, Y.; Taki, Y.; Endoh, K.; Watanabe, S. Soy isoflavones lower serum total and LDL cholesterol in humans: A meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2007, 85, 1148–1156. [Google Scholar] [PubMed]
- Brown, N.F.; Stefanovic-Racic, M.; Sipula, I.J.; Perdomo, G. The mammalian target of rapamycin regulates lipid metabolism in primary cultures of rat hepatocytes. Metab. Clin. Exp. 2007, 56, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Leavens, K.F.; Saleh, D.; Easton, R.M.; Guertin, D.A.; Peterson, T.R.; Kaestner, K.H.; Sabatini, D.M.; Birnbaum, M.J. Postprandial hepatic lipid metabolism requires signaling through Akt2 independent of the transcription factors FoxA2, FoxO1, and SREBP1c. Cell Metab. 2011, 14, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.W.; Wood, C.M.; Weber, D.; Aziz, S.A.; Mehta, R.; Griffin, P.; Cockell, K.A. Dietary supplementation with soy isoflavones or replacement with soy proteins prevents hepatic lipid droplet accumulation and alters expression of genes involved in lipid metabolism in rats. Genes Nutr. 2014, 9, 373. [Google Scholar] [CrossRef] [PubMed]
- Murosaki, S.; Lee, T.R.; Muroyama, K.; Shin, E.S.; Cho, S.Y.; Yamamoto, Y.; Lee, S.J. A combination of caffeine, arginine, soy isoflavones, and L-carnitine enhances both lipolysis and fatty acid oxidation in 3T3-L1 and HepG2 cells in vitro and in KK mice in vivo. J. Nutr. 2007, 137, 2252–2257. [Google Scholar] [PubMed]
- Ascencio, C.; Torres, N.; Isoard-Acosta, F.; Gomez-Perez, F.J.; Hernandez-Pando, R.; Tovar, A.R. Soy protein affects serum insulin and hepatic SREBP-1 mRNA and reduces fatty liver in rats. J. Nutr. 2004, 134, 522–529. [Google Scholar] [PubMed]
- Dang, Z.C.; Audinot, V.; Papapoulos, S.E.; Boutin, J.A.; Lowik, C.W. Peroxisome proliferator-activated receptor gamma (PPARgamma) as a molecular target for the soy phytoestrogen genistein. J. Biol. Chem. 2003, 278, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shin, H.J.; Kim, S.Y.; Kim, J.H.; Lee, Y.S.; Kim, D.H.; Lee, M.O. Genistein enhances expression of genes involved in fatty acid catabolism through activation of PPARalpha. Mol. Cell. Endocrinol. 2004, 220, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Sohn, I.; Lee, Y.S.; Lee, Y.S. Hepatic gene expression profiles are altered by genistein supplementation in mice with diet-induced obesity. J. Nutr. 2005, 135, 33–41. [Google Scholar] [PubMed]
- Yang, H.; Li, F.; Xiong, X.; Kong, X.; Zhang, B.; Yuan, X.; Fan, J.; Duan, Y.; Geng, M.; Li, L.; Yin, Y. Soy isoflavones modulate adipokines and myokines to regulate lipid metabolism in adipose tissue, skeletal muscle and liver of male Huanjiang mini-pigs. Mol. Cell. Endocrinol. 2013, 365, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Mezei, O.; Li, Y.; Mullen, E.; Ross-Viola, J.S.; Shay, N.F. Dietary isoflavone supplementation modulates lipid metabolism via PPARalpha-dependent and -independent mechanisms. Physiol. Genom. 2006, 26, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Brown, M.S.; Goldstein, J.L. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 3441–3446. [Google Scholar] [CrossRef] [PubMed]
- Porstmann, T.; Santos, C.R.; Griffiths, B.; Cully, M.; Wu, M.; Leevers, S.; Griffiths, J.R.; Chung, Y.L.; Schulze, A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008, 8, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Peterson, T.R.; Laplante, M.; Oh, S.; Sabatini, D.M. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature 2010, 468, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Chen, Z.; Zhang, J.; Zhang, L.; Ke, H.; Huang, L.; Peng, Y.; Zhang, X.; Li, S.; Lahn, B.T.; Xiang, A.P. Critical role of phosphoinositide 3-kinase cascade in adipogenesis of human mesenchymal stem cells. Mol. Cell. Biochem. 2008, 310, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Chen, J. regulation of peroxisome proliferator-activated receptor-gamma activity by mammalian target of rapamycin and amino acids in adipogenesis. Diabetes 2004, 53, 2748–2756. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lovekamp-Swan, T.; Bekele, W.; Dohi, A.; Schreihofer, D.A. Hypoxia-inducible factor and vascular endothelial growth factor are targets of dietary soy during acute stroke in female rats. Endocrinology 2013, 154, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Cousin, S.P.; Hugl, S.R.; Wrede, C.E.; Kajio, H.; Myers, M.G., Jr.; Rhodes, C.J. Free fatty acid-induced inhibition of glucose and insulin-like growth factor I-induced deoxyribonucleic acid synthesis in the pancreatic beta-cell line INS-1. Endocrinology 2001, 142, 229–240. [Google Scholar] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.

| Ingredients | Content |
|---|---|
| Corn | 54.0% |
| Fish meal | 6.0% |
| Wheat bran | 14.0% |
| Alfalfa meal | 13.0% |
| Cotton meal | 10.0% |
| Limestone | 1.00% |
| Dicalcium phosphate | 0.2% |
| Dodium chloride | 0.3% |
| Vitamin & mineral | 1.5% |
| Groups | Control Groups (Ctr, n = 10) | Obesity Groups (OB, n = 15) | Low-Dose Soy Isoflavones (LSI, n = 9) | Middle-Dose Soy Isoflavones (MSI, n = 15) | High-Dose Soy Isoflavones (HIS, n = 15) |
|---|---|---|---|---|---|
| Diets | Basal diets | Basal diets | Basal diets + 50 mg/kg soy isoflavones | Basal diets + 150 mg/kg soy isoflavones | Basal diets + 400 mg/kg soy isoflavones |
| Compounds | Content |
|---|---|
| Daidzin | 50.98% |
| Glycitin | 30.36% |
| Genistein | 8.80% |
| Daidzein | 1.24% |
| Genistin | 0.06% |
| Total isoflavones | 91.64% |
| Gene | Primers |
|---|---|
| SREBP-1c | Fr 5’-TGGACTACTAGTGTTGGCCTGCTT-3’ |
| Rv 5’-ATCCAGGTCAGCTTGTTTGCGATG-3’ | |
| ACC-1 | Fr 5’-ATTGTGGCTCAAACTGCAGGT-3’ |
| Rv 5’-GCCAATCCACTCGAAGACCA-3’ | |
| ACC-2 | Fr 5’-CAACATCCGTCAGACGACCTC-3’ |
| Rv 5’-CGGACTCGTTGGTGATGAAGA-3’ | |
| FASN | Fr 5’-TCCCAGGTCTTGCCGTGC-3’ |
| Rv 5’-GCGGATGCCTAGGATGTGTGC-3’ | |
| ACSL1 | Fr 5’-GGTGCTTCAGCCTACCATCTTCC-3’ |
| Rv 5’-AATCCAACAGCCATCGCTTCACT-3’ | |
| ACSL4 | Fr 5’-TATGGGCTGACAGAATCATG-3’ |
| Rv 5’-CAACTCTTCCAGTAGTGTAG-3’ | |
| PPARα | Fr 5’-ACTCGCAGGAAAGACTAGCA-3’ |
| Rv 5’-AGCAGTGGAAGAATCGGACC-3’ | |
| HSL | Fr 5’-TCAGGTGTCTTTGCGGGTAT-3’ |
| Rv 5’-CTTGTGCGGAAGAAGATGCT-3’ | |
| ATGL | Fr 5’-TCACCAACACCAGCATCCA-3’ |
| Rv 5’-GCACATCTCTCGAAGCACCA-3’ | |
| PPARγ | Fr 5’-CATTCGCATCTTTCAGGG-3’ |
| Rv 5’-GGACGCCATACTTTAGGA-3’ | |
| ACL | Fr 5’-GCAGCACGTGATCCATGAAT-3’ |
| Rv 5’-GTGGGATGCTGGACAACATC-3’ | |
| β-Actin | Fr 5’-GTACCACTGGCATTGTGATG-3’ |
| Rv 5’-ATCTTCATGGTGCTAGGAGC-3’ |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Pang, D.; Luo, Q.; Chen, X.; Gao, Q.; Shi, L.; Liu, W.; Zou, Y.; Li, L.; Chen, Z. Soy Isoflavones Regulate Lipid Metabolism through an AKT/mTORC1 Pathway in Diet-Induced Obesity (DIO) Male Rats. Molecules 2016, 21, 586. https://doi.org/10.3390/molecules21050586
Huang C, Pang D, Luo Q, Chen X, Gao Q, Shi L, Liu W, Zou Y, Li L, Chen Z. Soy Isoflavones Regulate Lipid Metabolism through an AKT/mTORC1 Pathway in Diet-Induced Obesity (DIO) Male Rats. Molecules. 2016; 21(5):586. https://doi.org/10.3390/molecules21050586
Chicago/Turabian StyleHuang, Chao, Dejiang Pang, Qihui Luo, Xiaolin Chen, Qi Gao, Liangqin Shi, Wentao Liu, Yuanfeng Zou, Lixia Li, and Zhengli Chen. 2016. "Soy Isoflavones Regulate Lipid Metabolism through an AKT/mTORC1 Pathway in Diet-Induced Obesity (DIO) Male Rats" Molecules 21, no. 5: 586. https://doi.org/10.3390/molecules21050586