Five 2-(2-Phenylethyl)chromones from Sodium Chloride-Elicited Aquilaria sinensis Cell Suspension Cultures
Abstract
:1. Introduction
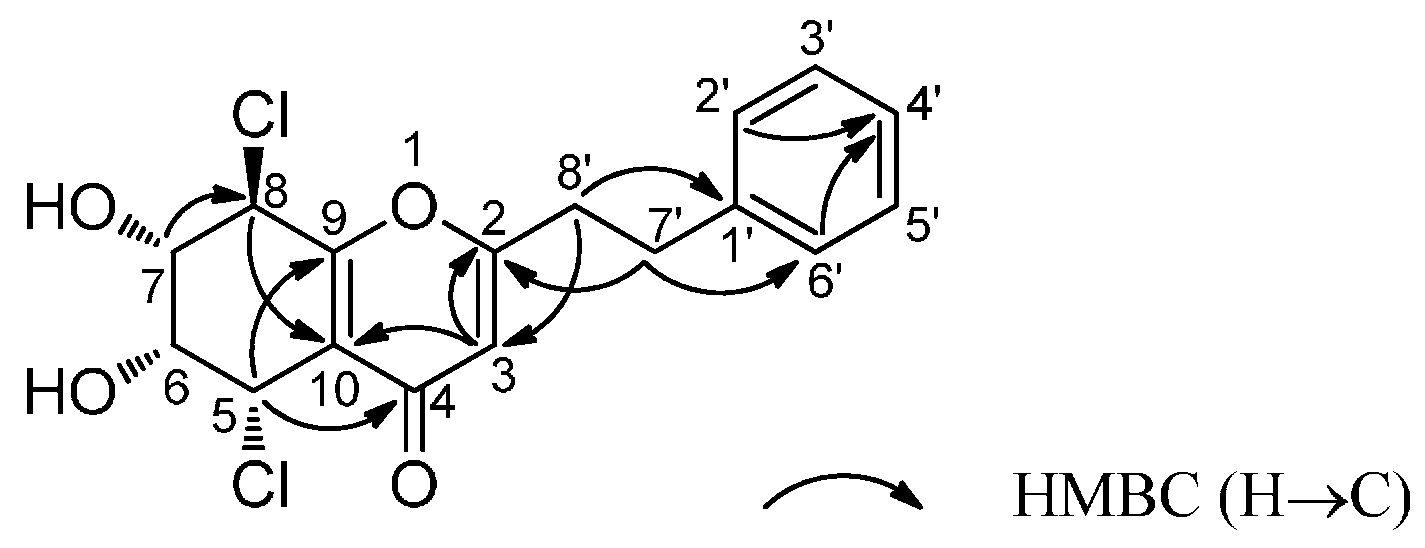
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Cell Culture and Viability
3.3. Elicitation and Harvesting Cells
3.4. Extraction and Isolation
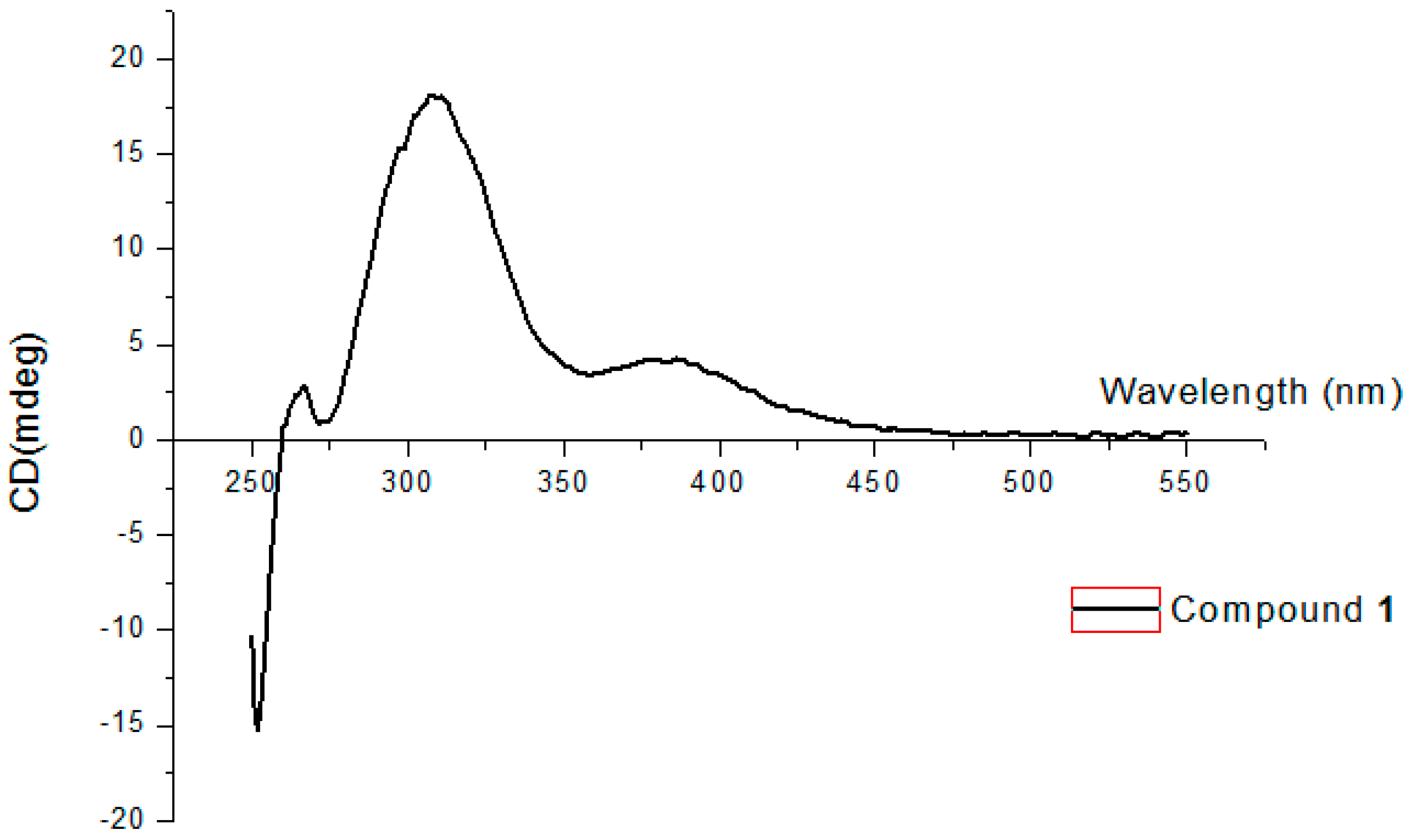
3.5. Absolute Configuration of Compound 1
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, D.; Xu, Z.R.; Chai, X.Y.; Zeng, K.W.; Jia, Y.X.; Bi, D.; Ma, Z.Z.; Tu, P.F. Nine 2-(2-phenylethyl)chromone derivatives from the resinous wood of Aquilaria sinensis and their inhibition of LPS-induced NO production in RAW 264.7 cells. Eur. J. Org. Chem. 2012, 5389–5397. [Google Scholar] [CrossRef]
- Yang, L.; Qiao, L.; Xie, D.; Yuan, Y.H.; Chen, N.H.; Dai, J.G.; Guo, S.X. 2-(2-Phenylethyl)chromones from Chinese eaglewood. Phytochemistry 2012, 76, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, J.; Zhao, Y.X.; Deng, Y.Y.; Mei, W.L.; Dai, H.F. A new cytotoxic 2-(2-phenylethyl)chromone from Chinese eaglewood. Chin. Chem. Lett. 2008, 19, 934–936. [Google Scholar] [CrossRef]
- Li, W.; Cai, C.H.; Dong, W.H.; Guo, Z.K.; Wang, H.; Mei, W.L.; Dai, H.F. 2-(2-Phenylethyl)chromone derivatives from Chinese agarwood induced by artificial holing. Fitoterapia 2014, 98, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.L.; Wang, H.; Guo, Z.K.; Dong, W.H.; Mei, W.L.; Dai, H.F. A new 2-(2-phenylethyl)chromone derivative in Chinese agarwood “Qi-Nan” from Aquilaria sinensis. J. Asian Nat. Prod. Res. 2014, 16, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Q.; Wei, J.H.; Yang, J.S.; Zhang, Z.; Yang, Y.; Gao, Z.H.; Sui, C.; Gong, B. Chemical constituents of agarwood originating from the endemic genus Aquilaria plants. Chem. Biodivers. 2012, 9, 236–250. [Google Scholar] [CrossRef] [PubMed]
- The Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES). Amendments to appendix I and II of CITES. In Proceedings of the Thirteenth Meeting of the Conference of the Parties, Bangkok, Thailand, 2–14 October 2004.
- Liu, Y.Y.; Chen, H.Q.; Yang, Y.; Zhang, Z.; Wei, J.H.; Meng, H.; Chen, W.P.; Feng, J.D.; Gan, B.C.; Chen, X.Y.; et al. Whole-tree agarwood-inducing technique: An efficient novel technique for producing high-quality agarwood in cultivated Aquilaria sinensis trees. Molecules 2013, 18, 3086–3106. [Google Scholar] [CrossRef] [PubMed]
- Yagura, T.; Ito, M.; Kiuchi, F.; Honda, G.; Shimada, Y. Four new 2-(2-phenylethyl)chromone derivatives from withered wood of Aquilaria sinensis. Chem. Pharm. Bull. 2003, 51, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Di Bari, L.; Pescitelli, G.; Pratelli, C.; Pini, D.; Salvadori, P. Determination of absolute configuration of acyclic 1, 2-diols with Mo2(OAc)4. 1. Snatzke’s method revisited. J. Org. Chem. 2001, 66, 4819–4825. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Kwon, S.W.; Hwang, G.S.; Park, J.H. Eight new 2-(2-phenylethyl)chromone (=2-(2-phenylethyl)-4H-1-benzopyran-4-one) derivatives from Aquilaria malaccensis agarwood. Helv. Chim. Acta 2012, 95, 1657–1665. [Google Scholar] [CrossRef]
- Shimada, Y.; Tominaga, T.; Konishi, T.; Kiyosawa, S. Studies on the agarwood (Jinko). I. Structures of 2-(2-phenylethyl)chromone derivatives. Biol. Pharm. Bull. 1982, 30, 3791–3795. [Google Scholar] [CrossRef]
- Iwagoe, K.; Konishi, T.; Kiyosawa, S.; Shimada, Y. Studies on the agalwood (Jinko). VII. Structures of phenylethylchromone derivatives AH7, AH8, AH9. Chem. Pharm. Bull. 1988, 36, 2417–2422. [Google Scholar] [CrossRef]
- King, R.R.; Calhoun, L.A. Characterization of cross-linked hydroxycinnamic acid amides isolated from potato common scab lesions. Phytochemistry 2005, 66, 2468–2473. [Google Scholar] [CrossRef] [PubMed]
- Moreau, C.; Kirchberger, T.; Thomas, M.P.; Zhang, B.; Weber, K.; Andreas, H. An aberrant cyclization affords a C-6 modified cyclic adenosine 5’-diphosphoribose analog with biological activity in jurkat T cells. J. Med. Chem. 2012, 55, 1478–1489. [Google Scholar] [CrossRef] [PubMed]
- Chenon, M.T.; Pugmire, R.J.; Grant, D.M.; Panzica, R.P.; Townsend, L.B. A basic set of parameters for the investigation of tautomerism in purines established from Carbon-13 magnetic resonance. J. Am. Chem. Soc. 1975, 97, 4627–4636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Xu, Y.Z. NMR and UV studies of 4-thio-2′-deoxyuridine and its derivatives. Molecules 2011, 16, 5655–5664. [Google Scholar] [CrossRef] [PubMed]
- Downey, A.M.; Richter, C.; Pohl, R.; Mahrwald, R.; Hocek, M. Direct one-pot synthesis of nucleosides from unprotected or 5-O-monoprotected d-ribose. Org. Lett. 2015, 17, 4604–4607. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.W.; Chai, H.B.; Keller, W.J.; Kinghorn, A.D. Lignans and other constituents of the fruits of Euterpe oleracea (Açai) with antioxidant and cytoprotective activities. J. Agric. Food Chem. 2008, 56, 7759–7764. [Google Scholar] [CrossRef] [PubMed]
- Ai, W.; Lin, X.P.; Tu, Z.C.; Tian, X.P.; Lu, X.; Mangaladoss, F.; Zhong, Z.L.; Liu, Y.H. Axinelline A, a new COX-2 inhibitor from Streptomyces axinellae SCSIO02208. Nat. Prod. Res. 2014, 28, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, L.X.; Zhao, Y.; Huang, G.D. Unusual sesquiterpene lactones with a new carbon skeleton and new acetylenes from Ajania przewalskii. Food Chem. 2010, 118, 228–238. [Google Scholar] [CrossRef]
- Brecker, L.; Mahut, M.; Schwarz, A.; Nidetzky, B. In situ proton NMR study of acetyl and formyl group migration in mono-O-acyl-d-glucose. Magn. Reson. Chem. 2009, 47, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Okudera, Y.; Ito, M. Production of agarwood fragrant constituents in Aquilaria calli and cell suspension cultures. Plant Biotechnol. 2009, 26, 307–315. [Google Scholar] [CrossRef]
- Qi, S.Y.; He, M.L.; Lin, L.D.; Zhang, C.H.; Hu, L.J.; Zhang, H.Z. Production of 2-(2-phenylethyl)chromones in cell suspension cultures of Aquilaria sinensis. Plant Cell Tiss. Org. 2005, 83, 217–221. [Google Scholar] [CrossRef]
- Ishihara, A.; Kawata, N.; Matsukawa, T.; Iwamura, H. Induction of N-hydroxycinnamoyltyramine synthesis and tyramine N-hydroxycinnamoyltransferase (THT) activity by wounding in maize leaves. Biosci. Biotech. Bioch. 2000, 64, 1025–1031. [Google Scholar] [CrossRef]
- Negrel, J.; Pollet, B.; Lapierre, C. Ether-linked ferulic acid amides in natural and wound periderms of potato tuber. Phytochemistry 1996, 43, 1195–1199. [Google Scholar] [CrossRef]
- König, S.; Feussner, K.; Kaever, A.; Landesfeind, M.; Thurow, C.; Karlovsky, P.; Gatz, C.; Polle, A.; Feussner, I. Soluble phenylpropanoids are involved in the defense response of Arabidopsis against Verticillium longisporum. New Phytol. 2014, 202, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Ashihara, H. Long-term effect of NaCl on the activity of uridine and urabil salvage for nucleotide synthesis in cultured mangrove (Bruguiera sexangula) cells. Plant Sci. 2009, 176, 383–389. [Google Scholar] [CrossRef]
- Sample Availability: Samples of 1, 10, 12, and 15 are available from the authors.
| No. | 1H-NMR | 13C-NMR |
|---|---|---|
| 2 | 171.4 | |
| 3 | 6.17 (1H, s) | 114.2 |
| 4 | 179.4 | |
| 5 | 4.94 (1H, br.s) | 52.0 |
| 6 | 4.19 (1H,br.s) | 74.3 |
| 7 | 4.36 (1H, br.d, J = 8.0 Hz) | 72.8 |
| 8 | 4.95 (1H, d, J = 8.0 Hz) | 58.3 |
| 9 | 161.7 | |
| 10 | 121.8 | |
| 1' | 141.0 | |
| 2', 6' | 7.21 (2H, m, overlapped) | 129.6 |
| 3', 5' | 7.27 (2H, m, overlapped) | 129.4 |
| 4' | 7.19 (1H, m, overlapped) | 127.6 |
| 7' | 2.98 (2H, m, overlapped) | 33.8 |
| 8' | 3.03 (2H, m, overlapped) | 36.2 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wang, X.; Yang, W.; Wang, J.; Su, C.; Liu, X.; Li, J.; Zhao, Y.; Shi, S.; Tu, P. Five 2-(2-Phenylethyl)chromones from Sodium Chloride-Elicited Aquilaria sinensis Cell Suspension Cultures. Molecules 2016, 21, 555. https://doi.org/10.3390/molecules21050555
Zhang Z, Wang X, Yang W, Wang J, Su C, Liu X, Li J, Zhao Y, Shi S, Tu P. Five 2-(2-Phenylethyl)chromones from Sodium Chloride-Elicited Aquilaria sinensis Cell Suspension Cultures. Molecules. 2016; 21(5):555. https://doi.org/10.3390/molecules21050555
Chicago/Turabian StyleZhang, Zhongxiu, Xiaohui Wang, Wanqing Yang, Juan Wang, Cong Su, Xiao Liu, Jun Li, Yunfang Zhao, Shepo Shi, and Pengfei Tu. 2016. "Five 2-(2-Phenylethyl)chromones from Sodium Chloride-Elicited Aquilaria sinensis Cell Suspension Cultures" Molecules 21, no. 5: 555. https://doi.org/10.3390/molecules21050555






