Ultrasound- and Molecular Sieves-Assisted Synthesis, Molecular Docking and Antifungal Evaluation of 5-(4-(Benzyloxy)-substituted phenyl)-3-((phenylamino)methyl)-1,3,4-oxadiazole-2(3H)-thiones
Abstract
:1. Introduction
2. Results and Discussion
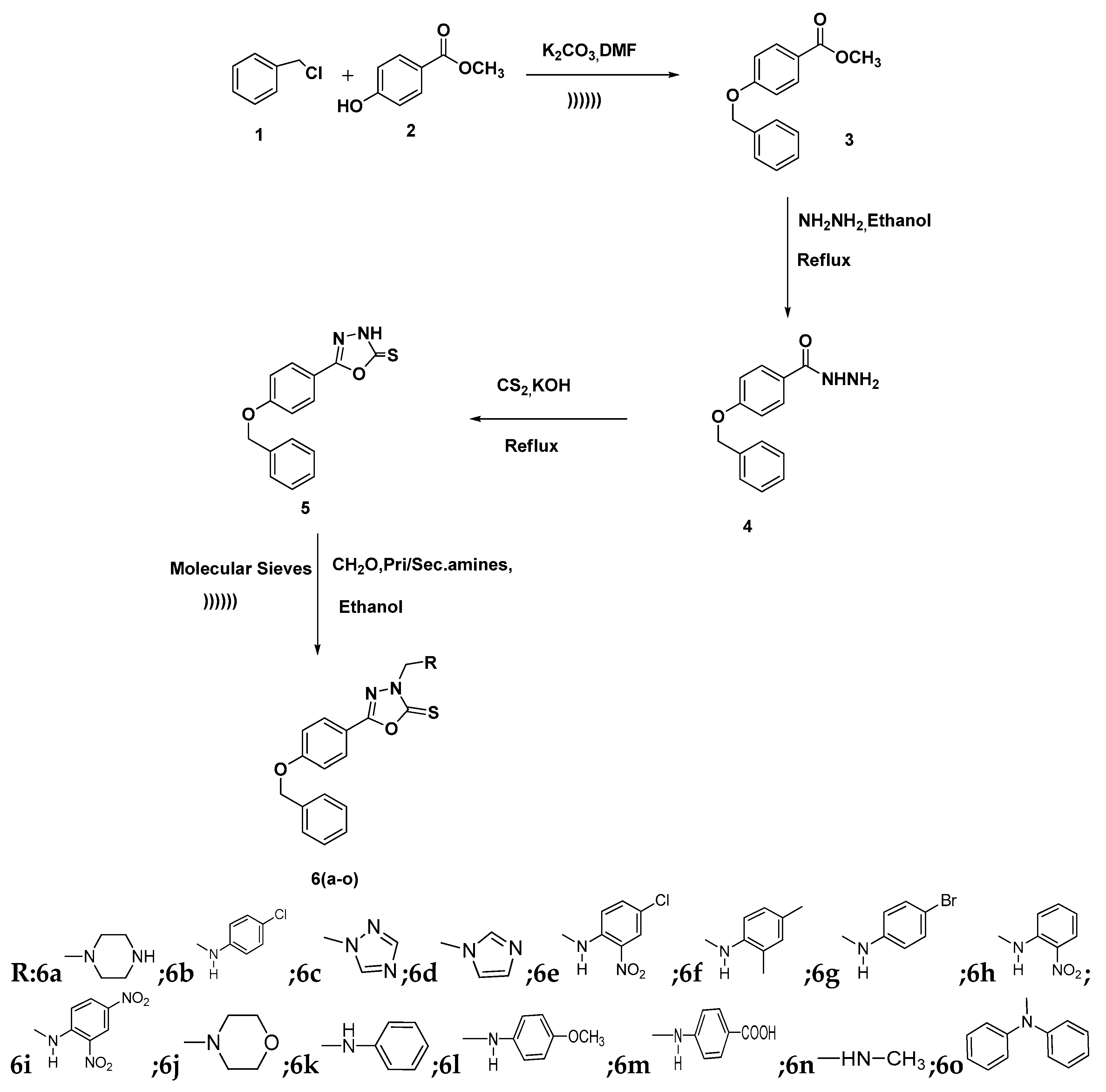
2.1. Chemistry
2.2. In Vitro Antifungal Activity
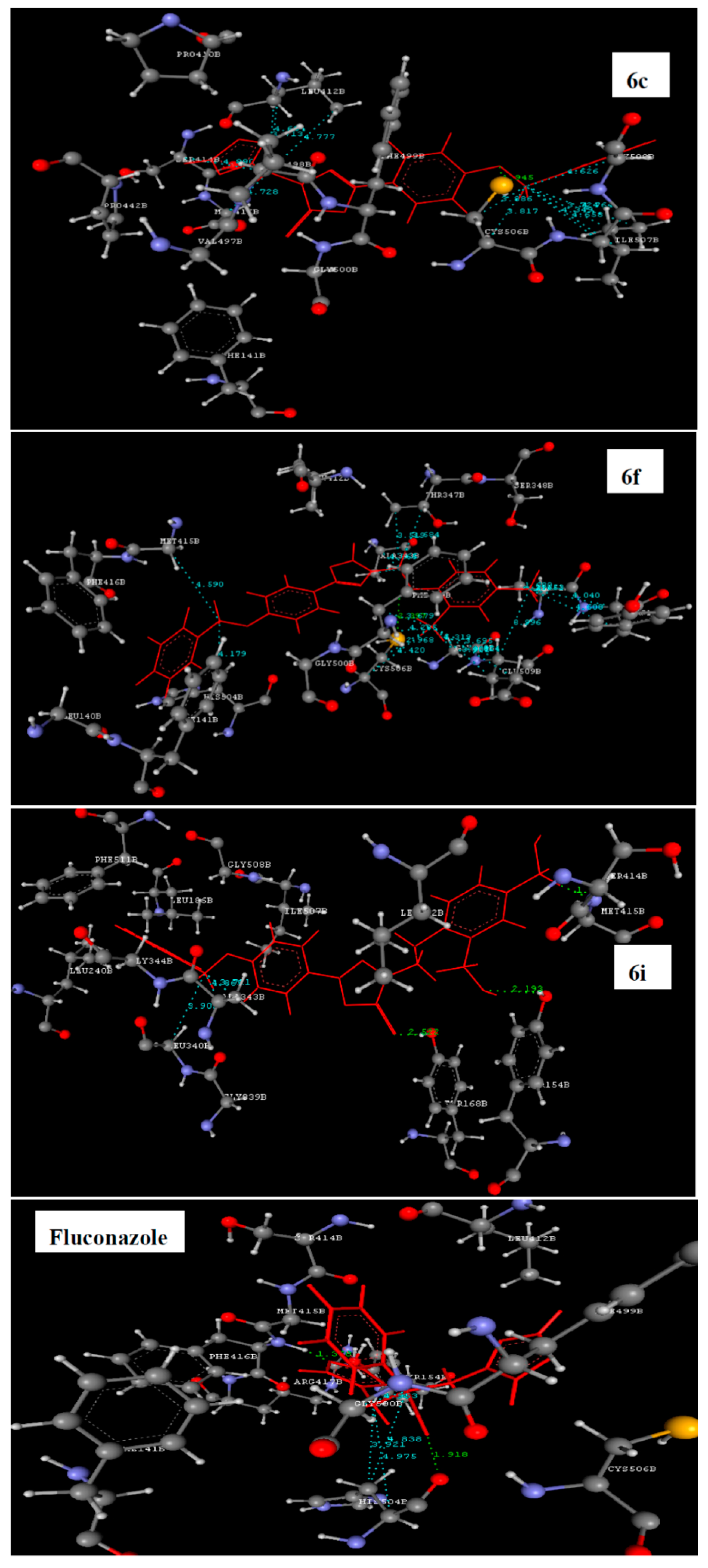
2.3. Molecular Docking Study
3. Materials and Methods
3.1. General Information
3.2. Synthesis of Methyl-4-(Benzyloxy)benzoate (3)
3.3. Synthesis of 4-(Benzyloxy)benzohydrazide (4)
3.4. Synthesis of 5-(4-(Benzyloxy)phenyl)-1,3,4-oxadiazole-2(3H)-thione
3.5. General Procedure for the Synthesis of 5-(4-(Benzyloxy)substituted phenyl)-3-((phenylamino)methyl)-1,3,4-oxadiazole-2(3H)-thiones 6a–o
3.6. Antifungal Screening
3.7. Molecular Docking Data
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Denning, D.W.; Bromley, M.J. How to bolster the antifungal pipeline. Science 2015, 347, 1414–1416. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.M.; Tupe, S.G.; Deshpande, M.V. Chitin synthase inhibitors as antifungal agents. Mini-Rev. Med. Chem. 2013, 13, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Nikalje, A.G.; Ghodke, M.S.; Khan, F.A.K.; Sangshetti, J.N. CAN catalyzed one-pot synthesis and docking study of some novel substituted imidazole coupled 1,2,4-triazole-5-carboxylic acids as antifungal agents. Chin. Chem. Lett. 2015, 26, 108–112. [Google Scholar] [CrossRef]
- Liu, F.; Luo, X.Q.; Song, B.A.; Bhadury, P.S.; Yang, S.; Jin, L.H.; Xue, W.; Hu, D.Y. Synthesis and antifungal activity of novel sulfoxide derivatives containing trimethoxyphenyl substituted 1,3,4-thiadiazole and 1,3,4-oxadiazole moiety. Bioorg. Med. Chem. Lett. 2008, 16, 3632–3640. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Song, B.A.; Yang, S.; Xu, G.F.; Bhadury, P.S.; Jin, L.H.; Hu, D.Y.; Li, Q.Z.; Liu, F.; Xue, W.; et al. Synthesis and antifungal activities of 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-thiadiazole and 5-(3,4,5-trimeth-oxyphenyl)-2-sulfonyl-1,3,4-oxadiazole derivatives. Bioorg. Med. Chem. 2007, 15, 3981–3989. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, N.; Harrison, D.A. Synthesis and antifungal activity of some new derivatives of 5-{4′-(2′′,4′′-dichlorobenzyloxy)-phenyl}-1,3,4-oxadiazole-2(3H)-thione. J. Indian Chem. Soc. 2012, 89, 1543–1550. [Google Scholar] [CrossRef]
- Jin, L.; Chen, J.; Song, B.; Chen, Z.; Yang, S.; Li, Q.; Hu, D.; Xu, R. Synthesis, structure, and bioactivity of N′-substituted benzylidene-3,4,5-trimethoxybenzohydrazide and 3-acetyl- 2-substituted phenyl-5-(3,4,5-trimethoxyphenyl)-2,3-dihydro-1,3,4-oxadiazole derivatives. Bioorg. Med. Chem. Lett. 2006, 16, 5036–5040. [Google Scholar] [CrossRef] [PubMed]
- Rastogia, N.; Kantb, P.; Sethib, R.; Shuklac, S.; Tripathi, D. Synthesis of a new series of oxadiazoles and triazoles as antimicrobial agents. J. Indian Chem. Soc. 2011, 88, 1721–1731. [Google Scholar]
- Macaev, F.; Rusu, G.; Pogrebnoi, S.; Gudima, A.; Stingaci, E.; Vlad, L.; Shvets, N.; Kandemirli, F.; Dimoglo, A.; Reynolds, R. Synthesis of novel 5-aryl-2-thio-1,3,4-oxadiazoles and the study of their structure–anti-mycobacterial activities. Bioorg. Med. Chem. 2005, 13, 4842–4850. [Google Scholar] [CrossRef] [PubMed]
- Kucukguzel, S.G.; Oruc, E.E.; Rollas, S.; Sahin, F.; Ozbek, A. Synthesis, characterization and biological activity of novel 4-thiazolidinones, 1,3,4-oxadiazoles and some related compounds. Eur. J. Med. Chem. 2002, 37, 197–206. [Google Scholar] [CrossRef]
- Tan, T.M.C.; Chen, Y.; Kong, K.H.; Jing, B.; Li, Y.; Lim, S.G.; Ang, T.H.; Lam, Y. Synthesis and the biological evaluation of 2-benzenesulfonylalkyl-5-substituted-sulfanyl-[1,3,4]-oxadiazoles as potential anti-hepatitis B virus agents. Antivir. Res. 2006, 71, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Bhat, J.J.; Shah, B.R.; Shah, H.P.; Trivedi, P.B.; Undavia, N.K.; Desai, N.C. Synthesis of anti-HIV, anticancer and antitubercular 4-oxo-thiazolidines, 2-imino-4-oxo-thiazolidines and their 5-arylidine derivatives. Indian J. Chem. 1994, 33B, 189–192. [Google Scholar]
- Zarghi, A.; Tabatabai, A.; Faizi, M.; Ahadian, A.; Navabi, P.; Zanganeh, V.; Shafiee, A. Synthesis and anticonvulsant activity of new 2-substituted-5-(2-benzyloxyphenyl)-1,3,4-oxadiazoles. Bioorg. Med. Chem. Lett. 2005, 15, 1863–1865. [Google Scholar] [CrossRef] [PubMed]
- Palaska, E.; Sahin, G.; Kelicen, P.; Durlu, N.T.; Altinok, G. Synthesis and anti-inflammatory activity of 1-acylthiosemicarbazides, 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazole-3-thiones. IIFarmaco 2002, 57, 101–107. [Google Scholar] [CrossRef]
- Kagthara, P.R.; Shah, N.S.; Doshi, R.K.; Parekh, H.H. Synthesis of 2,5-disubstituted 1,3,4-oxadiazoles as biologically active heterocycles. Indian J. Chem. 1999, 38B, 572–576. [Google Scholar]
- Amir, M.; Shikha, K. Synthesis and anti-inflammatory, analgesic, ulcerogenic and lipid peroxidation activities of some new 2-[(2,6-dichloroanilino) phenyl]acetic acid derivatives. Eur. J. Med. Chem. 2004, 39, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Pandey, A.K.; Butcher, R.J.; Singh, N.K. Syntheses and X-ray crystallographic studies of {[Ni(en)2(pot)2]0.5CHCl3} and [Ni(en)2](3-pytol)2. Polyhedron 2009, 28, 461–466. [Google Scholar] [CrossRef]
- Soni, N.; Barthwal, J.P.; Saxena, A.K.; Bhargava, K.P.; Parmar, S.S. Monoamine oxidase and succinate dehydrogenase inhibitory properties of substituted 1,3,4-oxadiazole- 2-thiones. J. Heterocycl. Chem. 2009, 19, 29–32. [Google Scholar] [CrossRef]
- Yamamoto, S. Jpn. Patent 05124948. Chem. Abstr. 1993, 119, 146370. [Google Scholar]
- Tramontini, M.; Angiolini, L. Mannich Bases—Chemistry and Uses; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Ram, V.J.; Pandey, H.N. Synthesis of 5-membered heterocycles and related compounds. Chem. Pharm. Bull. 1974, 22, 2778–2783. [Google Scholar] [CrossRef] [PubMed]
- Sasse, A.; Ligneau, X.; Sadek, B.; Elz, S.; Pertz, H.H.; Ganellin, C.R.; Arrang, J.M.; Schwartz, J.C.; Schunack, W.; Stark, H. Benzophenone derivatives and related compounds as potent histamine H3-receptor antagonists and potential PET/SPECT ligands. Arch. Pharm. 2001, 334, 45–52. [Google Scholar] [CrossRef]
- Mitsch, A.; Wibner, P.; Sattler, I.; Schlitzer, M. Non-Thiol Farnesyltransferase Inhibitors: Structure-Activity Relationships of Aralkyl subsituted Benzophenones. Arch. Pharm. Med. Chem. 2001, 334, 40–44. [Google Scholar] [CrossRef]
- Lindley, J.; Lorimer, J.P.; Mason, T.J. Enhancement of an ullman coupling reaction induced by ultrasound. Ultrasonics 1986, 24, 292–293. [Google Scholar] [CrossRef]
- Bang, K.; Lee, K.; Park, Y.K.; Lee, P.H. Sonochemical Reformatsky reaction using indium. Bull. Korean Chem. Soc. 2002, 23, 1272–1276. [Google Scholar] [CrossRef]
- Park, S.Y.; Han, H.J.; Yoo, B.; Choi, K.; Yoon, H.J.; Yoo, M.C.; Woo, B. Facile synthesis of β-ketoesters by indium-mediated reaction of acyl cyanides with ethyl bromoacetate under ultrasonication. Bull. Korean Chem. Soc. 2005, 26, 878–879. [Google Scholar] [CrossRef]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.S.; Abd EL-Rahman, N.M. Ultrasound promoted synthesis of substituted pyrazoles and isoxazoles containing sulphonemoiety. Ultrason. Sonochem. 2009, 16, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Corma, A. From Microporous to mesoporous molecular sieve materials and their use in catalysis. Chem Rev. 1997, 97, 2373–2420. [Google Scholar] [CrossRef] [PubMed]
- Chindananda, N.; Poojary, B.; Sumangala, V.; Sucheta, N.; Shetty, P.; Arulmoli, T. Facile synthesis, characterization and pharmacological activities of 3,6-disubstituted 1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles and 5,6-dihydro-3,6-disubstituted-1,2,4-triazolo[3,4-b][1,3,4] thiadiazoles. Eur. J. Med. Chem. 2012, 51, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Pulya, S.; Kommagalla, Y.; Sant, D.; Jorwekar, S.; Tupe, S.G.; Deshpande, M.V.; Ramana, C. Re-engineering of PIP3-antagonist triazole PITENIN’s chemical scaffold: Development of novel antifungal leads. RSC Adv. 2016, 6, 11691–11701. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. M27-A3, Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute. M38-A2, Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. Approved Standard, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Sangshetti, J.N.; Khan, F.A.K.; Chouthe, R.S.; Damale, M.G.; Shinde, D.B. Synthesis, docking and ADMET prediction of novel 5-((5-substituted-1-H-1,2,4-triazol-3-yl)methyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine as antifungal agents. Chin. Chem. Lett. 2014, 25, 1033–1038. [Google Scholar] [CrossRef]
- Halgren, T.A. Merckmolecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Hooft, R.W.; Vriend, G.; Sander, C.; Abola, E.E. Errors in protein structures. Nature 1996, 381, 272. [Google Scholar] [CrossRef] [PubMed]
- VLife Molecular Design Suite 4.3, VLife Sciences Technologies Pvt. Ltd. Available online: http://www.Vlifesciences.com (accessed on 23 December 2015).
- Sample Availability: Samples of the compounds are not available from the authors.
| Entry | Conventional | Ultrasonic Irradiation | ||
|---|---|---|---|---|
| Time (h) | Yield (%) | Time (h) | Yield (%) | |
| 6a | 15 | 68 | 1.5 | 80 |
| 6b | 16 | 63 | 1.0 | 81 |
| 6c | 15 | 62 | 1.5 | 78 |
| 6d | 17 | 58 | 2.0 | 79 |
| 6e | 18 | 45 | 1.0 | 81 |
| 6f | 18 | 56 | 1.0 | 79 |
| 6g | 19 | 57 | 2.0 | 81 |
| 6h | 17 | 61 | 1.5 | 90 |
| 6i | 18 | 53 | 1.0 | 82 |
| 6j | 17 | 63 | 1.5 | 85 |
| 6k | 15 | 58 | 1.5 | 80 |
| 6l | 17 | 57 | 1.0 | 81 |
| 6m | 15 | 61 | 2.0 | 79 |
| 6n | 16 | 58 | 2.0 | 79 |
| 6o | 15 | 45 | 2.0 | 78 |
| Compound | Candida albicans ATCC 24433 | Candida albicans ATCC 10231 | Candida glabrata NCYC 388 | Cryptococcus neoformans ATCC 34664 | Cryptococcus neoformans PRL 518 | Aspergillus fumigatus NCIM 902 | Aspergillus niger ATCC 10578 |
|---|---|---|---|---|---|---|---|
| 6a | 64 | 49.6 | 64 | 64 | 64 | >256 | 27.7 |
| 6b | 128 | 67.12 | 59.6 | 77.5 | >128 | 24.2 | 24.1 |
| 6c | 21.6 | 108 | 53.6 | 108.9 | 196.5 | 11.2 | 93 |
| 6d | 68 | 159 | 65.6 | 128 | >128 | 6 | 130 |
| 6e | 54.4 | 44.6 | 24.5 | 64 | 14.21 | 25.8 | 47 |
| 6f | 16 | 84.9 | 5.4 | >256 | 90 | 256 | 256 |
| 6g | 62 | 107.6 | 256 | 76.8 | 94.7 | 4 | >256 |
| 6h | 55.3 | 57.4 | 64 | 47.6 | 66.6 | 16 | 18.61 |
| 6i | 23.3 | 80.6 | 40.3 | 256 | 93.5 | 24.6 | >256 |
| 6j | 38.4 | 31.5 | 56.6 | 192 | 39 | 8 | 128 |
| 6k | 150 | 53.3 | 36.2 | 64.2 | 146.6 | 35.1 | 22.9 |
| 6l | 115 | 95.3 | 42.5 | 49.7 | 145.8 | 81 | 73.2 |
| 6m | 91.5 | 49.7 | 78.6 | 62.5 | 54.6 | 87.4 | 84.8 |
| 6n | 30.3 | 43 | 48.8 | 47.5 | 105.6 | 81.5 | 56.9 |
| 6o | 95 | 28.1 | 50.7 | 42.7 | 156.5 | 76.1 | 70.5 |
| Fluconazole | 0.12 | 0.11 | 9.4 | 16 | 4 | 64 | 46 |
| Compound | Binding Energy | Hydrogen Bonds | Hydrophobic Bonds |
|---|---|---|---|
| 6a | −41.69 | LEU412-O=C | ALA343, GLY344, LEU412, MET415, PHE499, GLY508 and ALA512 |
| 6b | −49.33 | LEU412-O=C | ALA343, THR347, MET415, GLY500 and HIS504 |
| 6c | −57.85 | CYS506-O-CH2 | LEU412, SER414, MET415, CYC506, ILE507 and GLY508 |
| 6d | −45.23 | CYS506-O-CH2 | ALA343, LEU412, MET415, CYC506, ILE507 and GLY508 |
| 6e | −35.50 | LEU412-O=C; GLY500-N of nitro and GLY500-O-N of NO2 | ALA343, LEU412, ILE507 and GLY508 |
| 6f | −65.25 | CYS506-NH | ALA343, THR347, MET415, PHE499, HIS504, CYC506, GLY508, GLU509, ALA512 and TYR513 |
| 6g | −52.37 | LEU412-O=C | THR347, LEU412, MET415, PHE499 and HIS504 |
| 6h | −47.18 | VAL497 and GLY500-N of NO2 | ALA343, GLY344, PHE499, GLY500 and GLY508 |
| 6i | −61.03 | TYR154-O-N of 2-nitro; TYR168- O=C and MET415-N of 4-nitro | LEU340 and ALA343 |
| 6j | −40.29 | LEU412-O-CH2 | ALA343, GLY344, LEU412, MET415, PHE499, GLY508 and ALA512 |
| 6k | −37.85 | - | ALA343, GLY344, THR347, PHE499, GLY500 and GLY508 |
| 6l | −42.91 | - | LEU186, LEU240, GLY344, THR347, MET415, PHE499, GLY500, CYS506, GLY508 and PHE511 |
| 6m | −36.83 | - | ALA343, LEU412 and PHE499 |
| 6n | −40.09 | CYS506-O-CH2 | ALA343, VAL440, PRO442, VAL497, GLY500 and CYC506 |
| 6o | −38.97 | TYR168-O=C | LEU340, ALA343, ILE507 and GLY508 |
| Fluconazole | −67.29 | TYR168-F of phenyl | GLY500 and HIS504 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nimbalkar, U.D.; Tupe, S.G.; Seijas Vazquez, J.A.; Khan, F.A.K.; Sangshetti, J.N.; Nikalje, A.P.G. Ultrasound- and Molecular Sieves-Assisted Synthesis, Molecular Docking and Antifungal Evaluation of 5-(4-(Benzyloxy)-substituted phenyl)-3-((phenylamino)methyl)-1,3,4-oxadiazole-2(3H)-thiones. Molecules 2016, 21, 484. https://doi.org/10.3390/molecules21050484
Nimbalkar UD, Tupe SG, Seijas Vazquez JA, Khan FAK, Sangshetti JN, Nikalje APG. Ultrasound- and Molecular Sieves-Assisted Synthesis, Molecular Docking and Antifungal Evaluation of 5-(4-(Benzyloxy)-substituted phenyl)-3-((phenylamino)methyl)-1,3,4-oxadiazole-2(3H)-thiones. Molecules. 2016; 21(5):484. https://doi.org/10.3390/molecules21050484
Chicago/Turabian StyleNimbalkar, Urja D., Santosh G. Tupe, Julio A. Seijas Vazquez, Firoz A. Kalam Khan, Jaiprakash N. Sangshetti, and Anna Pratima G. Nikalje. 2016. "Ultrasound- and Molecular Sieves-Assisted Synthesis, Molecular Docking and Antifungal Evaluation of 5-(4-(Benzyloxy)-substituted phenyl)-3-((phenylamino)methyl)-1,3,4-oxadiazole-2(3H)-thiones" Molecules 21, no. 5: 484. https://doi.org/10.3390/molecules21050484









