The Unusual Acid-Accumulating Behavior during Ripening of Cherimoya (Annona cherimola Mill.) is Linked to Changes in Transcription and Enzyme Activity Related to Citric and Malic Acid Metabolism
Abstract
:1. Introduction
2. Results and Discussion
2.1. Changes in Organic Acid Content during Growth of Cherimoya
2.2. Sequence Analysis of Organic Acid-Related Genes from Cherimoya
2.3. Expression of Citric and Malic Acid-Related Genes during A. cherimola Fruit Growth
2.4. Changes In Quality Parameters and Organic Acid Patterns during Cherimoya cv. “Concha Lisa” Ripening
2.5. Gene Expression and Activity Analysis of Key Enzymes Related to Malic and Citric Metabolism during Cherimoya Ripening
3. Materials and Methods
3.1. Plant Materials
3.2. Fruit Parameters
3.3. Organic Acid Extraction and Measurement
3.4. Crude Protein Extraction and Enzyme Assays
- Aconitase (ACON): 40 mM Tris-HCl (pH 7.5), 0.1 M NaCl and 200 µM cis-aconitate, with 100 µL from tube A; measurement at 240 nm.
- Malate dehydrogenase (MDH): 40 mM Tris-HCl (pH 8.2), 2 mM MgCl2, 10 mM KHCO3, 0.5 mM GSH, 2 mM oxaloacetate and 150 µM NADH, with 100 µL from tube B; measurement at 340 nm.
- Citrate synthase (CS): 40 mM Tris-HCl (pH 9), 40 µM DTNB, 40 µM acetyl-CoA and 4 mM oxaloacetate, with 100 µL from tube C; measurement at 412 nm.
3.5. RNA Extraction and cDNA Synthesis
3.6. Isolation of Partial cDNAs for Organic Acid-Related Genes
3.7. Cloning of Full-Length Annona Cherimola Organic Acid-Related Genes
3.8. Real-Time Quantitative PCR Assays
3.9. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| cyNAD-MDH | cytoplasmic NAD-dependent malate dehydrogenase |
| mCS | mitochondrial citrate synthase |
| TSS | total soluble solids |
| TA | titratable acidity |
| TCA | tricarboxylic acid cycle |
| PEP | phosphoenolpyruvate |
| PEPC | phosphoenolpyruvate carboxylase |
| NADP-ME | NADP-malic enzyme |
| ACON | aconitase |
| NAD-IDH | NAD-dependent isocitrate dehydrogenase |
| BBCH | Biologische Bundesanstalt, Bundessortenamt und Chemische Industrie |
| FW | fresh weight |
| WAB | weeks after blooming |
| NAD-ME | NAD-dependent malic enzyme |
| ATP-CS | ATP citrate synthase |
| aa | amino acids |
| qPCR | real-time quantitative PCR assays |
| OAA | oxaloacetate |
| SE | standard error |
| ORF | open reading frame |
References
- Giovannoni, J.J. Molecular biology of fruit maturation and ripening. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 725–749. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, J.J. Genetic regulation of fruit development and ripening. Plant Cell 2004, 16, S170–S180. [Google Scholar] [CrossRef] [PubMed]
- Kader, A.A. Flavor quality of fruits and vegetables. J. Sci. Food Agric. 2008, 88, 1863–1868. [Google Scholar] [CrossRef]
- Obenland, D.; Collin, S.; Mackey, B.; Sievert, J.; Fjeld, K.; Arpaia, M.L. Determinants of flavor acceptability during the maturation of navel oranges. Postharvest Biol. Technol. 2009, 52, 156–163. [Google Scholar] [CrossRef]
- Ferguson, I.B.; Boyd, L.M. Inorganic nutrients and fruit quality. In Fruit Quality and its Biological Basis; Knee, M., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2002; pp. 17–45. [Google Scholar]
- Pehrson, J.E.; Ivans, E.M. Variability in early season navel orange clone maturity and consumer acceptance. Proc. Int. Soc. Citric. 1988, 4, 1631–1635. [Google Scholar]
- Crisosto, C.H.; Crisosto, G.M. Understanding American and Chinese consumer acceptance of “Redglobe” table grapes. Postharvest Biol. Technol. 2002, 24, 155–162. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M.; Metheney, P. Consumer acceptance of “Brooks” and “Bing” cherries is mainly dependent on fruit SSC and visual skin color. Postharvest Biol. Technol. 2003, 28, 159–167. [Google Scholar] [CrossRef]
- Sweetman, C.; Deluc, L.G.; Cramer, G.R.; Ford, C.M.; Soole, K.L. Regulation of malate metabolism in grape berry and other developing fruits. Phytochemistry 2009, 70, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Etienne, A.; Génard, M.; Lobit, P.; Mbeguié-A-Mbéguié, D.; Bugaud, C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 2013, 64, 1451–1469. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Dougherty, L.; Cheng, L.; Xu, K. A co-expression gene network associated with developmental regulation of apple fruit acidity. Mol. Genet. Genom. 2015, 4, 1247–1463. [Google Scholar] [CrossRef] [PubMed]
- Schulze, J.; Tesfaye, M.; Litjens, R.; Bucciarelli, B.; Trepp, G.; Miller, S.; Samac, D.; Allan, D.; Vance, C.P. Malate plays a central role in plant nutrition. Plant Soil 2002, 247, 133–139. [Google Scholar] [CrossRef]
- Sadka, A.; Dahan, E.; Or, E.; Roose, M.L.; Marsh, K.B.; Cohen, L. Comparative analysis of mitochondrial citrate synthase gene structure, transcript level and enzymatic activity in acidless and acid-containing Citrus varieties. Funct. Plant Biol. 2001, 28, 383–390. [Google Scholar] [CrossRef]
- Iannetta, P.P.M.; Escobar, N.M.; Ross, H.A.; Souleyre, E.J.F.; Hancock, R.D.; Witte, C.P.; Davies, H.V. Identification, cloning and expression analysis of strawberry (Fragaria X ananassa) mitochondrial citrate synthase and mitochondrial malate dehydrogenase. Physiol. Plant. 2004, 121, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Sadka, A.; Dahan, E.; Cohen, L.; Marsh, K.B. Aconitase activity and expression during the development of lemon fruit. Physiol. Plant. 2000, 108, 255–262. [Google Scholar] [CrossRef]
- Sha, S.F.; Li, J.C.; Wu, J.; Zhang, S.L. Changes in the organic acid content and related metabolic enzyme activities in developing “Xinping” pear fruit. Afr. J. Agric. Res. 2011, 6, 3560–3567. [Google Scholar]
- Etienne, C.; Moing, A.; Dirlewanger, E.; Raymond, P.; Monet, R.; Rothan, C. Isolation and characterization of six peach cDNAs encoding key proteins in organic acid metabolism and solute accumulation: Involvement in regulating peach fruit acidity. Physiol. Plant. 2002, 114, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, J.; Fischer, M.; Amado, R. Changes in sugars, acids, and amino acids during ripening and storage of apples (cv. Glockenapfel). J. Agric. Food Chem. 1992, 40, 1131–1134. [Google Scholar] [CrossRef]
- Gutierrez-Carnelossi, M.A.; Costa de Sena, H.; Narain, N.; Yaguiu, P.; da Silva, G.F. Physico-Chemical Quality Changes in Mangaba (Hancornia speciosa gomes) Fruit Stored at Different Temperatures. Braz. Arch. Biol. Technol. 2009, 52, 985–990. [Google Scholar] [CrossRef]
- Paull, R.E.; Deputy, J.; Chen, N.J. Changes in organic acids, sugars, and headspace volatiles during fruit ripening of soursop. J. Am. Soc. Hortic. Sci. 1983, 108, 931–934. [Google Scholar]
- Palma, T.; Aguilera, J.M.; Stanley, D. A review of postharvest events in cherimoya. Postharvest Biol. Technol. 1993, 2, 187–208. [Google Scholar] [CrossRef]
- Gutierrez, M.; Lahoz, J.M.; Sola, M.M.; Pascaul, L.; Vargas, A.M. Postharvest changes in total soluble solids and tissue pH of cherimoya fruit stored at chilling and non-chilling temperatures. J. Hortic. Sci. 1994, 69, 459–463. [Google Scholar] [CrossRef]
- Manríquez, D.; Muñoz, P.; Gudenschwager, O.; Robledo, P.; Defilippi, B. Development of flavor-related metabolites in cherimoya (Annona cherimola Mill.) fruit and their relationship with ripening physiology. Postharvest Biol. Technol. 2014, 94, 58–65. [Google Scholar] [CrossRef]
- Muñoz, T.; Sanchez-Ballesta, M.T.; Ruiz-Cabello, J.; Escribano, M.I.; Merodio, C. The acid metabolism of Annona fruit during ripening. J. Hortic. Sci. Biotechnol. 2004, 79, 472–478. [Google Scholar] [CrossRef]
- Cautín, R.; Agustí, M. Phenological growth stages of the cherimoya tree (Annona cherimola Mill.). Sci. Hortic. 2005, 105, 491–497. [Google Scholar] [CrossRef]
- Higuchi, H.; Utsunomiya, N.; Sakuratani, T. High temperature effects on cherimoya fruit set, growth and development under greenhouse conditions. Sci. Hortic. 1998, 77, 23–31. [Google Scholar] [CrossRef]
- Alique, R.; Oliveira, G. Changes in sugar and organic acids in cherimoya (Annona cherimola Mill.) fruit under controlled-atmosphere storage. J. Agric. Food Chem. 1994, 42, 799–803. [Google Scholar] [CrossRef]
- Mounet, F.; Moing, A.; Garcia, V.; Petit, J.; Maucourt, M.; Deborde, C.; Bernillon, S.; le Gall, G.; Colquhoun, I.; Defernez, M.; et al. Gene and metabolite regulatory network analysis of early developing fruit tissues highlights new candidate genes for the control of tomato fruit composition and development. Plant Physiol. 2009, 149, 1505–1528. [Google Scholar] [CrossRef] [PubMed]
- Ruffner, H.P.; Hawker, J.S. Control of glycolysis in ripening berries of Vitis vinifera. Phytochemistry 1977, 16, 1171–1175. [Google Scholar] [CrossRef]
- Famiani, F.; Walker, R.P.; Tecsi, L.; Chen, Z.H.; Proietti, P.; Leegood, R.C. An immunohistochemical study of the compartmentation of metabolism during the development of grape (Vitis vinifera L.) berries. J. Exp. Bot. 2000, 51, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Fortes, A.M.; Agudelo-Romero, P.; Silva, M.S.; Ali, K.; Sousa, L.; Maltese, F.; Choi, Y.H.; Grimplet, J.; Martinez-Zapater, J.M.; Verpoorte, R.; Pais, M.S. Transcript and metabolite analysis in Trincadeira cultivar reveals novel information regarding the dynamics of grape ripening. BMC Plant Biol. 2011, 11, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeBolt, S.; Cook, D.; Ford, C. L-Tartaric acid synthesis from vitamin C in higher plants. Proc. Natl. Acad. Sci. USA 2006, 103, 5608–5613. [Google Scholar] [CrossRef] [PubMed]
- The Basic Local Alignment Search Tool. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 9 December 2015).
- Tang, M.; Bie, Z.; Wub, M.; Yi, H.; Feng, J. Changes in organic acids and acid metabolism enzymes in melon fruit during development. Sci. Hortic. 2010, 123, 360–365. [Google Scholar] [CrossRef]
- Pareek, S.; Yahia, E.M.; Pareek, O.P.; Kaushik, R.A. Postharvest physiology and technology of Annona fruits. Food Res. Int. 2011, 44, 1741–1751. [Google Scholar] [CrossRef]
- Bruinsma, J.; Paull, R.E. Respiration during postharvest development of soursop fruit, Annona muricata L. Plant Physiol. 1984, 76, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.X.; Liu, X.H.; Chen, L.S. Developmental changes in pulp organic acid concentration and activities of acid-metabolising enzymes during the fruit development of two loquat (Eriobotrya japonica L.) cultivars differing in fruit acidity. Food Chem. 2009, 114, 657–664. [Google Scholar] [CrossRef]
- Saradhuldhat, P.; Paull, R.E. Pineapple organic acid metabolism and accumulation during fruit development. Sci. Hortic. 2007, 112, 297–303. [Google Scholar] [CrossRef]
- Morgan, M.J.; Osorio, S.; Gehl, B.; Baxter, C.J.; Kruger, N.J.; Ratcliffe, R.G.; Fernie, A.R.; Sweetlove, L.J. Metabolic engineering of tomato fruit organic acid content guided by biochemical analysis of an introgression line. Plant Physiol. 2013, 161, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, A.; Modi, V. Citrate cleavage enzyme in mango fruit. Biochem. Biophys. Res. Commun. 1970, 39, 895–904. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and quantitative method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–252. [Google Scholar] [CrossRef]
- Gudenschwager, O.; González-Agüero, M.; Defilippi, B.G. A general method for high-quality RNA isolation from metabolite-rich fruits. S. Afr. J. Bot. 2012, 83, 186–192. [Google Scholar] [CrossRef]
- Yang, L.T.; Jiang, H.X.; Qi, J.P.; Chen, L.S. Differential expression of genes involved in alternative glycolytic pathways, phosphorus scavenging and recycling in response to aluminum and phosphorus interactions in Citrus roots. Mol. Biol. Rep. 2012, 39, 6353–6366. [Google Scholar] [CrossRef] [PubMed]
- Moing, A.; Rothan, C.; Svanella, L.; Just, D.; Diakou, P.; Raymond, P.; Gaudillere, J.P.; Monet, R. Role of phosphoenolpyruvate carboxylase in organic acid accumulation during peach fruit development. Physiol. Plant. 2000, 108, 1–10. [Google Scholar] [CrossRef]
- González-Agüero, M.; Cifuentes-Esquivel, N.; Ibañez-Carrasco, F.; Gudenschwager, O.; Campos-Vargas, R.; Defilippi, B.G. Identification and characterization of genes differentially expressed in cherimoya (Annona cherimola Mill.) after exposure to chilling injury conditions. J. Agric. Food Chem. 2011, 59, 13295–13299. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.L.; Church, D.M.; Federhen, S.; Lash, A.E.; Madden, T.L.; Pontius, J.U.; Schuler, G.D.; Schriml, L.M.; Sequeira, E.; Tatusova, T.A. Database resources of the National Center for Biotechnology. Nucleic Acids Res. 2003, 31, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.; Bordoli, L.; Kopp, J.; Schwede, T. The SWISS-MODEL Workspace: A web-based environment for protein structure homology modelling. Bioinformatics 2006, 22, 195–201. [Google Scholar] [CrossRef] [PubMed]
- TargetP software. Available online: http://www.cbs.dtu.dk/services/TargetP/ (accessed on 6 June 2015).
- García-Rojas, M.; Gudenschwager, O.; Defilippi, B.G.; González-Agüero, M. Identification of genes possibly related to loss of quality in late-season “Hass” avocados in Chile. Postharvest Biol. Technol. 2012, 73, 1–7. [Google Scholar] [CrossRef]
- Sample Availability: No available.
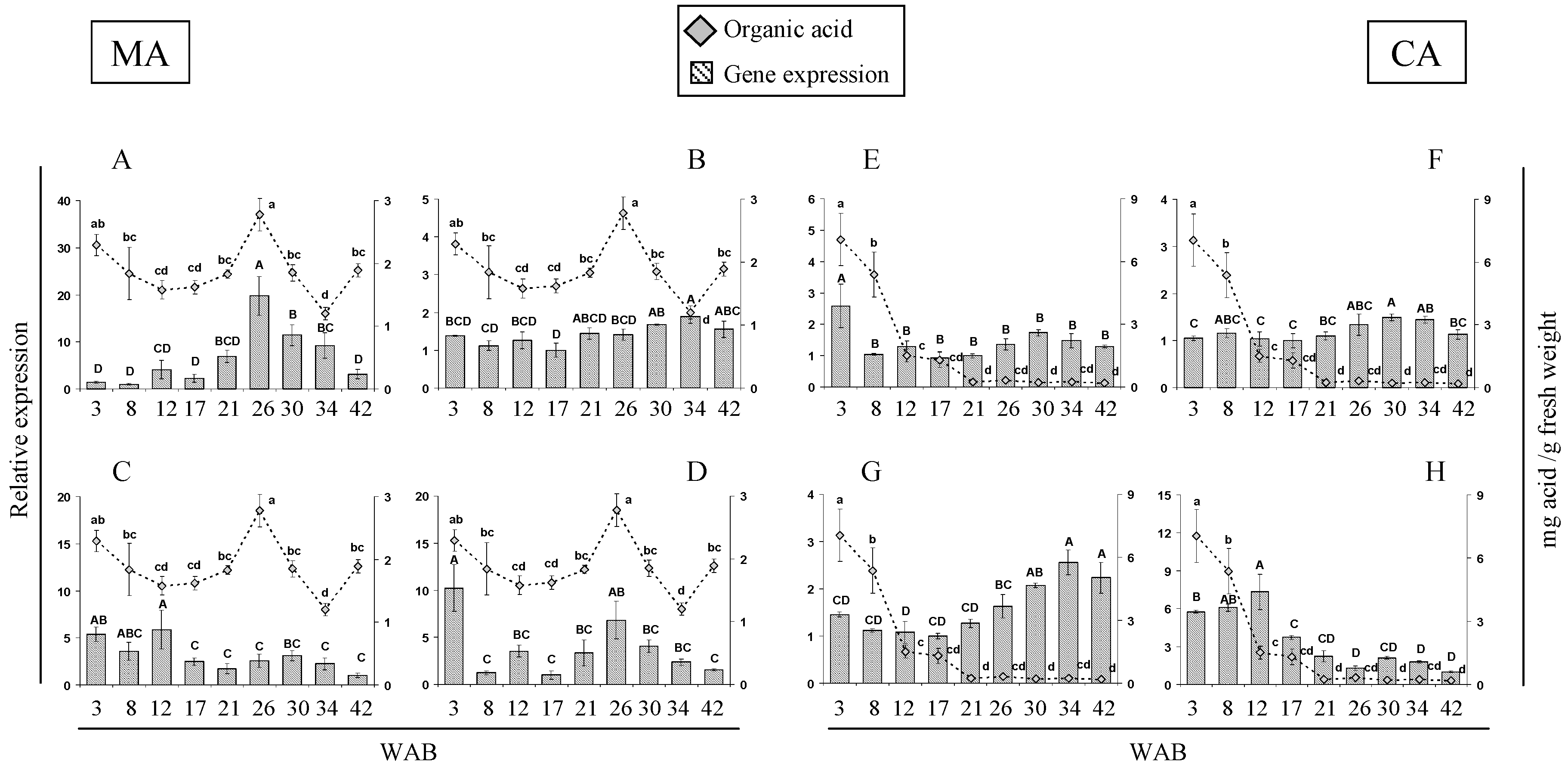

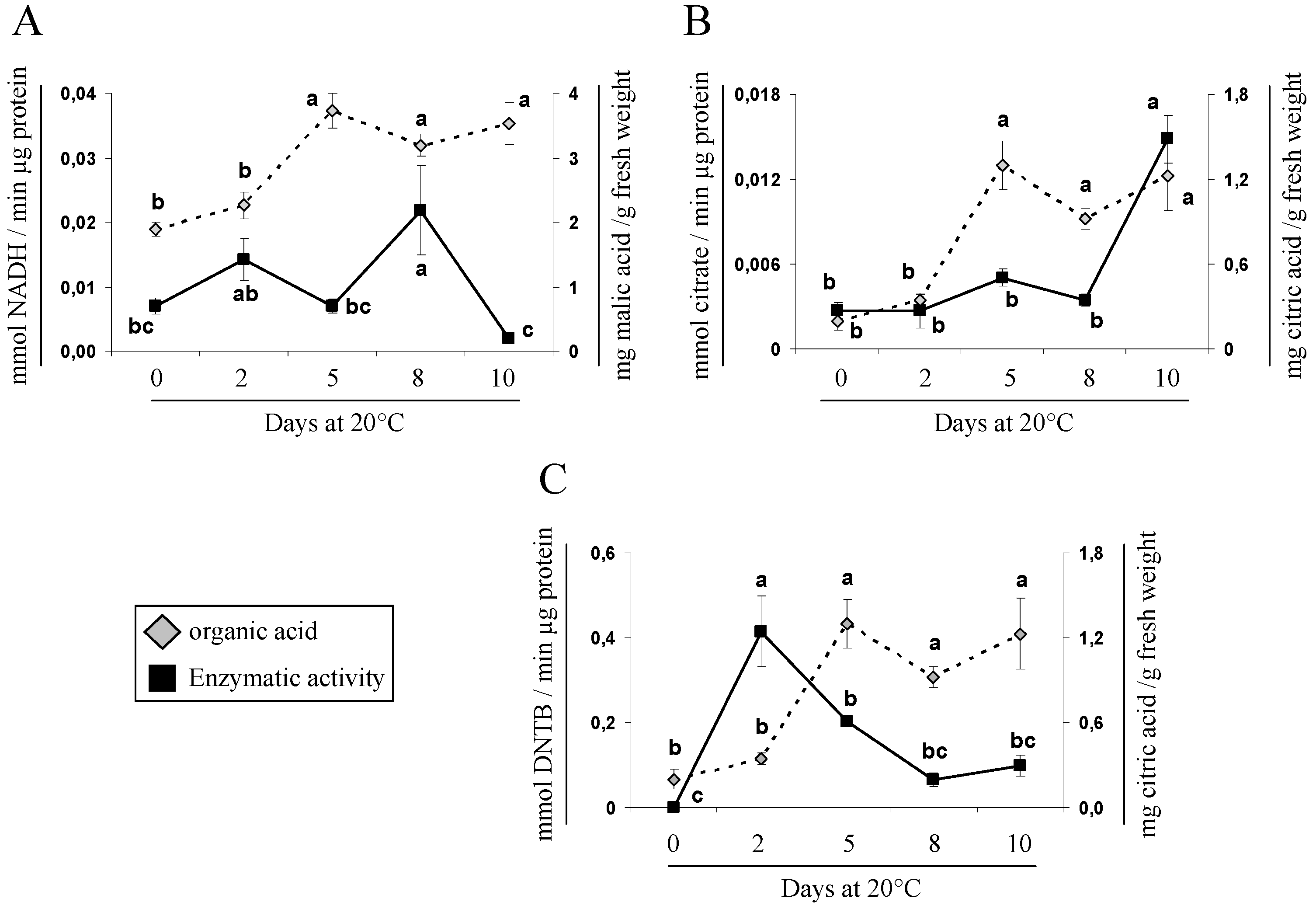
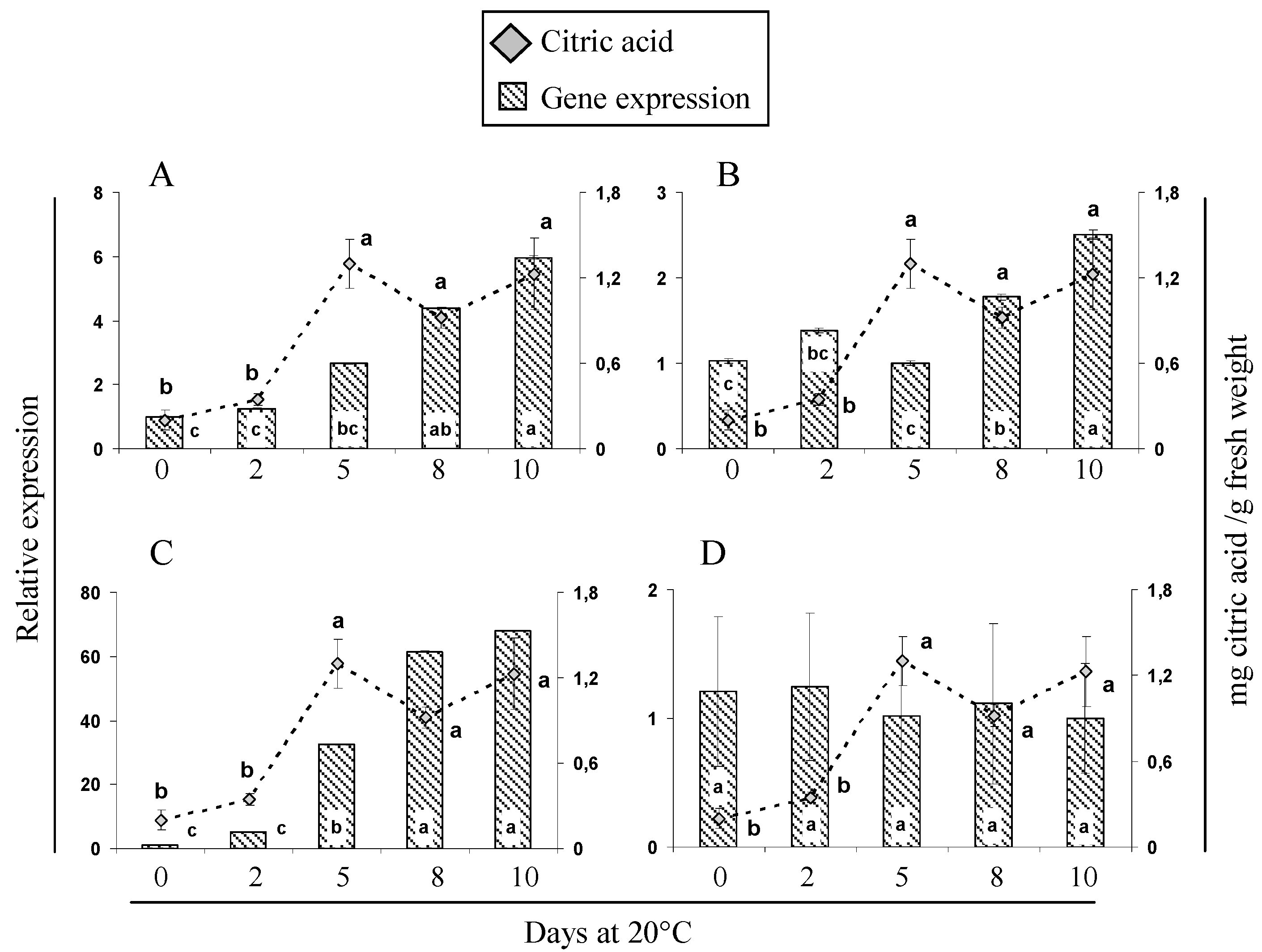
| Sample Point | Weeks after Blooming (WAB) | Phenological Stage 1 | Fruit Weight (g) | Organic Acids (mg/g Fresh Weight) | ||||
|---|---|---|---|---|---|---|---|---|
| Tartaric | Malic | Ascorbic | Citric | Succinic | ||||
| S1 | 3 | 71 | 1.1 ± 0.1 *,h | 1.12 ± 0.01 b | 2.29 ± 0.17 a,b | 0.14 ± 0.01 b | 7.05 ± 1.25 a | 2.97 ± 0.69 a |
| S2 | 8 | 72 | 3.4 ± 0.7 h | 1.34 ± 0.17 a | 1.84 ± 0.43 b,c | 0.18 ± 0.04 a | 5.37 ± 1.09 b | 0.62 ± 0.12 c |
| S3 | 12 | 73 | 32.6 ± 4.0 g | 0.57 ± 0.06 c,d | 1.58 ± 0.15 c,d | 0.03 ± 0.01 c | 1.51 ± 0.31 c | 1.75 ± 0.29 b |
| S4 | 17 | 74 | 70.5 ± 14.6 f | 0.40 ± 0.05 d,e | 1.62 ± 0.12 c,d | 0.03 ± 0.01 c | 0.97 ± 0.39 c,d | 0.62 ± 0.19 c |
| S5 | 21 | 75 | 123.0 ± 14.4 e | 0.41 ± 0.03 c,d,e | 1.83 ± 0.07 b,c | 0.03 ± 0.00 c | 0.25 ± 0.04 d | 0.61 ± 0.14 c |
| S6 | 26 | 76 | 186.8 ± 10.3 d | 0.61 ± 0.8 c | 2.78 ± 0.27 a | 0.05 ± 0.01 c | 0.29 ± 0.03 c,d | 0.85 ± 0.06 c |
| S7 | 30 | 77 | 231.1 ± 15.7 c | 0.39 ± 0.7 d,e | 1.85 ± 0.17 b,c | 0.04 ± 0.01 c | 0.23 ± 0.02 d | 0.66 ± 0.03 c |
| S8 | 34 | 78 | 328.5 ± 49.9 b | 0.25 ± 0.06 e | 1.20 ± 0.10 d | 0.04 ± 0.00 c | 0.20 ± 0.05 c,d | 0.55 ± 0.06 c |
| S9 | 42 | 79 | 475.0 ± 74.4 a,b | 0.51 ± 0.15 c,d | 1.89 ± 0.11 b,c | 0.04 ± 0.00 c | 0.20 ± 0.07 d | 0.46 ± 0.05 c |
| Gene | GenBank Access | Length (aa) | Coding Enzyme and Type of Sequence Identified | Orthologous Sequence: Specie and GenBank Access 1 | Identity (%) |
|---|---|---|---|---|---|
| Ac-cyNAD-MDH | KU524480 | 332 | Cytosolic NAD-malate dehydrogenase, complete sequence | Annona cherimola—AHY99589 | 100 |
| AcNADP-ME | KU524481 | 587 | Putative NADP-dependent malic enzyme, complete sequence | Theobroma cacao—EOY15729 | 85 |
| AcNAD-ME | KU524482 | 606 | Putative NAD-dependent malic enzyme, complete sequence | Theobroma cacao—EOX96044 | 85 |
| AcATP-CS | KU524483 | 423 | Putative ATP-citrate synthase, complete sequence | Ricinus communis—XP_002512567 | 91 |
| AcNADP-IDH | KU524484 | 416 | Putative isocitrate dehydrogenase protein, complete sequence | Prunus persica—AAL11503 | 90 |
| Ac-mCS | KU524485 | 473 | Putative mitochondrial citrate synthase, complete sequence | Cucumis sativus—XP_004135902 | 88 |
| AcPEPC | KU524486 | 164 | Putative phosphoenolpyruvate carboxylase, partial sequence | Elaeis guineensis—AEQ94112 | 86 |
| AcACON | KU524487 | 473 | Putative aconitate hydratase 1, partial sequence | Citrus clementina—CBE71056 | 91 |
| Days at 20 °C | ||||||
|---|---|---|---|---|---|---|
| 0 | 2 | 5 | 8 | 10 | ||
| Quality and physiological parameters | Firmness (kgf) | 18.2 ± 2.2 *,a | 18.1 ± 1.6 a | 1.6 ± 0.8 b | 0.5 ± 0.2 b | 0.6 ± 0.2 b |
| Respiratory rate (mL CO2 Kg−1·h−1) | 17.3 ± 0.8 d | 67.6 ± 3.7 b | 54.7 ± 3.0 c | 121.7 ± 9.5 a | 138.8 ± 8.3 a | |
| Ethylene rate (µl·Kg−1·h−1) | 0.0 ± 0.0 c | 1.0 ± 0.3 c | 68.6 ± 13.3 b | 327.2 ± 38.0 a | 266.7 ± 49.6 a | |
| TA (% malic acid) | 0.09 ± 0.01 c | 0.10 ± 0.01 c | 0.22 ± 0.01 b | 0.30 ± 0.01 a | 0.25 ± 0.01 b | |
| TSS (%) | 6.8 ± 0.2 d | 10.2 ± 0.2 c | 15.5 ± 0.3 b | 16.2 ± 0.5 b | 18.2 ± 0.9 a | |
| Organic acids (mg/g fresh weight) | Tartaric | 0.51 ± 0.15 a,b | 0.65 ± 0.07 a | 0.30 ± 0.04 b,c | 0.26 ± 0.05 c | 0.31 ± 0.06 b,c |
| Malic | 1.89 ± 0.11 b | 2.26 ± 0.21 b | 3.73 ± 0.27 a | 3.19 ± 0.18 a | 3.53 ± 0.32 a | |
| Ascorbic | 0.04 ± 0.00 a | 0.04 ± 0.00 a | 0.05 ± 0.01 a | 0.05 ± 0.00 a | 0.05 ± 0.00 a | |
| Citric | 0.20 ± 0.07 b | 0.35 ± 0.04 b | 1.30 ± 0.17 a | 0.92 ± 0.08 a | 1.23 ± 0.25 a | |
| Succinic | 0.46 ± 0.05 a,b | 0.63 ± 0.06 a,b | 0.85 ± 0.27 a | 0.75 ± 0.20 a,b | 0.44 ± 0.07 b | |
| Primer Name | Forward (F) and Reverse (R) Primers (5′→3′) | Tm (°C) | Reference |
|---|---|---|---|
| PpNADP-IDH heterologous primers (Prunus persica) | F: GGCCATGTACAACACTGATGAG | 58 | Yang et al. [43] |
| R: CATTGTCATCCAACTTTGCCCTG | |||
| AcNADP-IDH | F: TCCAAATGGAAGTCAAAGTTCGAG | 64 | N/A 1 |
| R: CCTTTCTGGTGAACACGGTAATG | |||
| CmACON heterologous primers (Citrus maxima) | F: GAAGCAATCACCAAAGGGAATC | 52 | Yang et al. [43] |
| R: TACTACGGTGAATTCGCTCAAAG | |||
| AcACON RACE-PCR primers | F: ACCATGTCCCCTCCTGGCCC | 64 | N/A |
| R: TCCACATTGGATTTCCTTTCGTG | |||
| AcACON qPCR primers | F: GCAGGCACGGTTGACATTGATT | 64 | N/A |
| R: GCCTGATGGCACGGATAATTGG | |||
| VvNADP-ME heterologous primers (Vitis vinifera) | F: GGAGGAGTTCGTCCTTCAGCCTG | 58 | Yang et al. [43] |
| R: CCTTTGAGTCCACAAGCCAAATC | |||
| AcNADP-ME RACE-PCR primers | F: GCACAATCTCCGCCAATATGAAG | 64 | N/A |
| F: AAGCCTCGCCAACGGTCGGT | |||
| AcNADP-ME qPCR primers | F: AGAACGACTGGTCAGGAGTATG | 64 | N/A |
| R: CCTCGCCTGCGCCAAGGAATA | |||
| VvNAD-ME heterologous primers (Vitis vinifera) | F: TGATCTTGGAGTTCAGGGAATTGGA | 58 | Yang et al. [43] |
| R: GCACTTCCTGCTCCAACTACAACTA | |||
| AcNAD-ME | F: GCTGCTGGCATGAATCCACAAA | 64 | N/A 1 |
| R: GCGTGTAAAAACAGCTTCCATGA | |||
| PEPC degenerate primers | F: CCITGGATHTTYGCITGGAC | 54 | Moing et al. [44] |
| R: GCIGCDATICCYTTCATIG | |||
| AcPEPC RACE-PCR primers | F: GAGACCCTGGTATTGCTGCTTT | 64 | N/A |
| R: ACATTTTCTTGAGCATTTGGAGC | |||
| AcPEPC qPCR primers | F: TGGACCCAGACCAGATTTCACC | 66 | N/A |
| R: AATTGCTTCTCAGCCGCTCACC | |||
| mCS degenerate primers | F: GGTTTRGCTGGRCCACTYC | 46 | Iannetta et al. [14] |
| R: GRGCATCAACATTWGGC | |||
| Ac-mCS | F: ATCAAGTCTGTTGTAGAAGAATGT | 62 | N/A 1 |
| R: CTCTGGCATGTGTATCGTGGA | |||
| AcATP-CS | F: AAGTTCACCGTCCTCAACCCTA | 62 | González-Agüero et al. [45] |
| R: GCATAACCCAAATCTCCAACAG | |||
| DEGcyNAD-MDH degenerate primers | F: TTTCYATYTACAAGTCMCARGC | 46 | N/A 2 |
| R: CAGCTTWCGRGCCTTGATRATT | |||
| Ac-cyNAD-MDH | F: AGAGTTTGCTCCATCCATTCCT | 62 | N/A 1 |
| R: CTCGTTGTTGGACGGTTGTAAT | |||
| AcUbiq (Genbank FJ664263) | F: TCCTGCAGAATCAGTGGAGTC | 64 | González-Agüero et al. [45] |
| R: AGGAACCAAATCCGCAAACAGC |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Agüero, M.; Tejerina Pardo, L.; Zamudio, M.S.; Contreras, C.; Undurraga, P.; Defilippi, B.G. The Unusual Acid-Accumulating Behavior during Ripening of Cherimoya (Annona cherimola Mill.) is Linked to Changes in Transcription and Enzyme Activity Related to Citric and Malic Acid Metabolism. Molecules 2016, 21, 398. https://doi.org/10.3390/molecules21050398
González-Agüero M, Tejerina Pardo L, Zamudio MS, Contreras C, Undurraga P, Defilippi BG. The Unusual Acid-Accumulating Behavior during Ripening of Cherimoya (Annona cherimola Mill.) is Linked to Changes in Transcription and Enzyme Activity Related to Citric and Malic Acid Metabolism. Molecules. 2016; 21(5):398. https://doi.org/10.3390/molecules21050398
Chicago/Turabian StyleGonzález-Agüero, Mauricio, Luis Tejerina Pardo, María Sofía Zamudio, Carolina Contreras, Pedro Undurraga, and Bruno G. Defilippi. 2016. "The Unusual Acid-Accumulating Behavior during Ripening of Cherimoya (Annona cherimola Mill.) is Linked to Changes in Transcription and Enzyme Activity Related to Citric and Malic Acid Metabolism" Molecules 21, no. 5: 398. https://doi.org/10.3390/molecules21050398






