MP-V1 from the Venom of Social Wasp Vespula vulgaris Is a de Novo Type of Mastoparan that Displays Superior Antimicrobial Activities
Abstract
:1. Introduction
2. Results
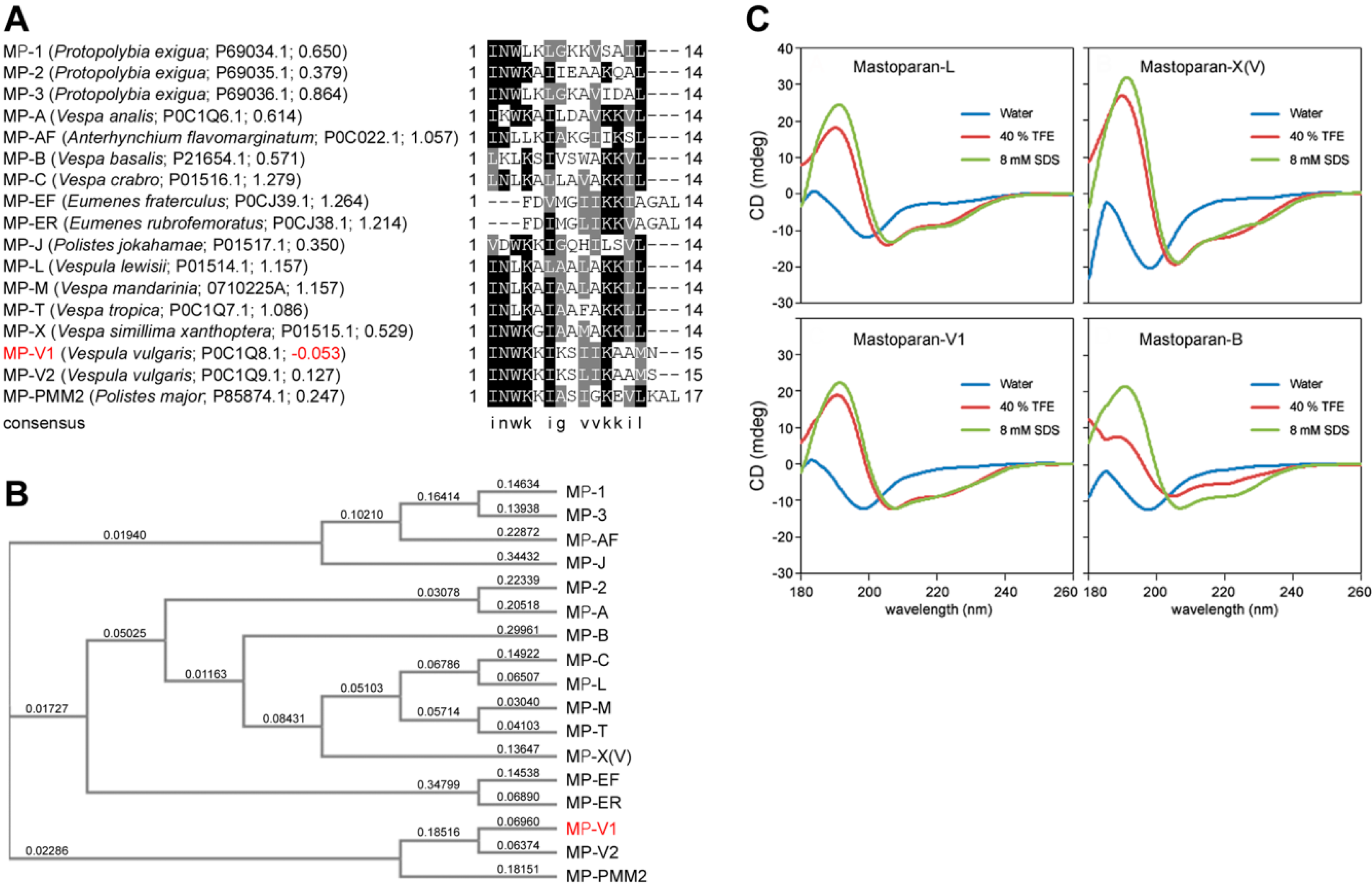
2.1. Identification of MP-V1 as a de Novo Type of Mastoparan
2.2. Comparison of the Secondary Structure of MP-V1 with Those of Other Mastoparans
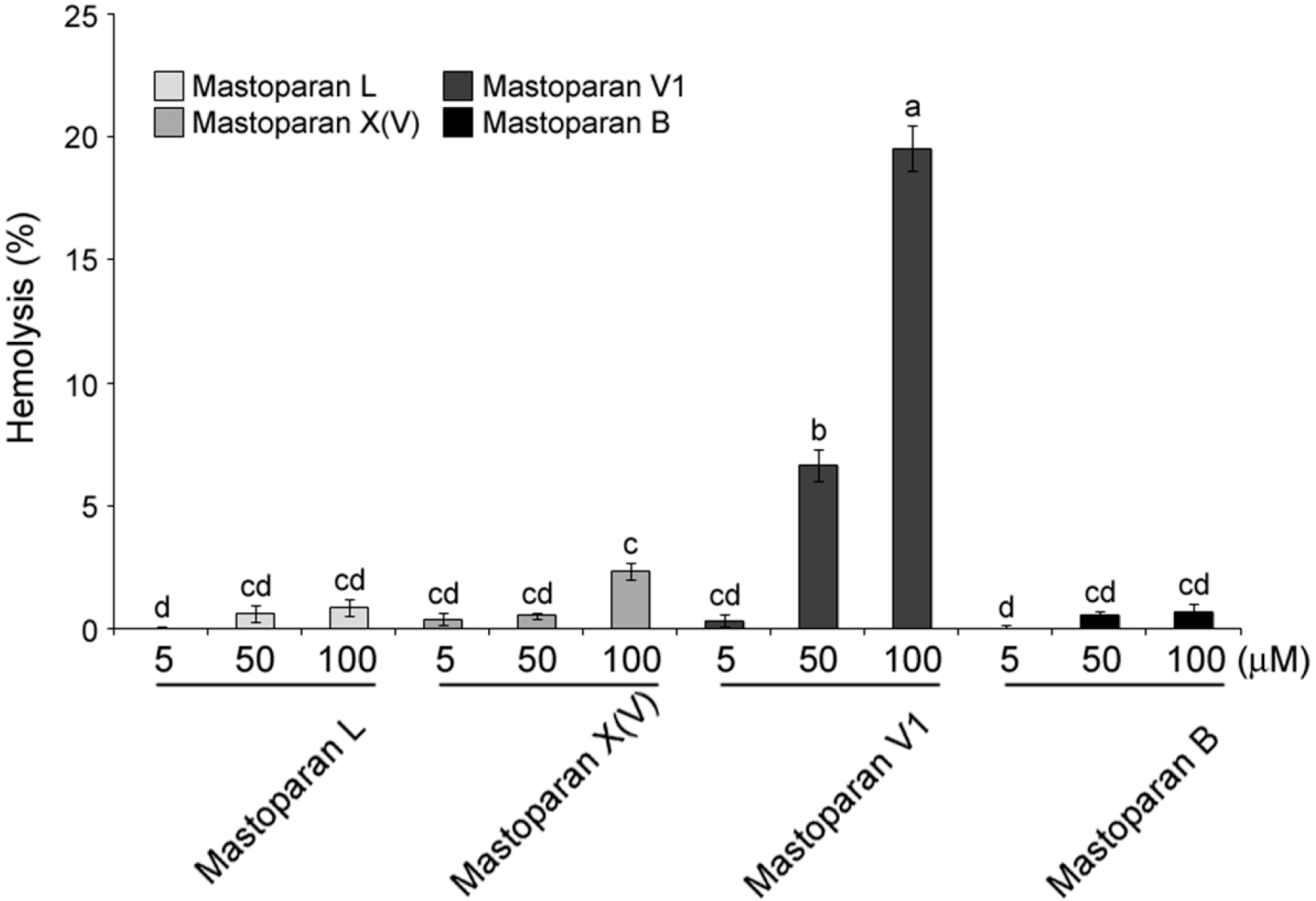
2.3. Comparison of Hemolytic Activity of MP-V1 with the One of Other Mastoparans
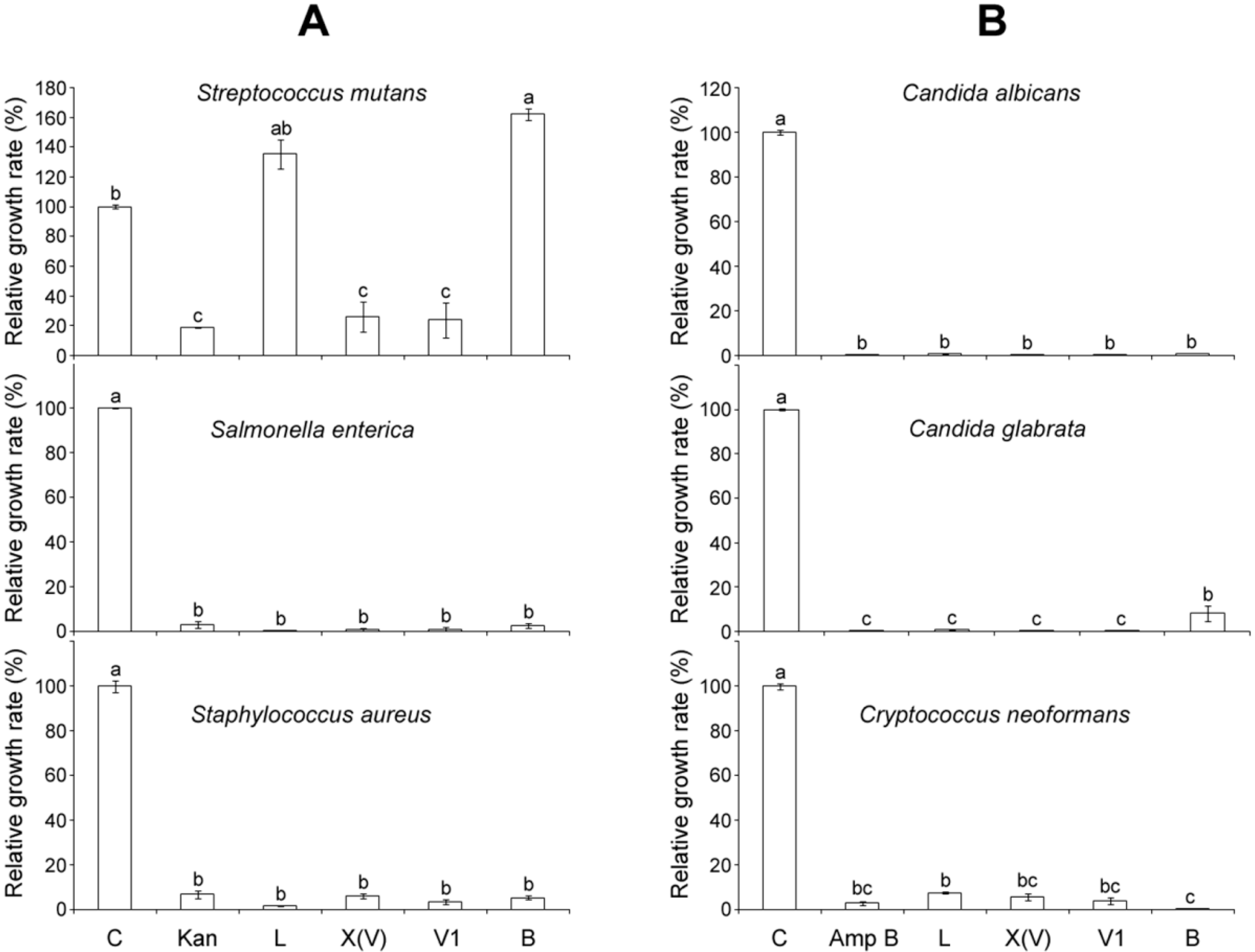
2.4. Comparison of Antimicrobial Activities of MP-V1 with Other Mastoparans
3. Discussion
3.1. The Mechanism of Action of Typical Mastoparans on Microbial Membranes
3.2. Unusual MP-V1 Peptide Amino Acid Sequence Responsible for High-Efficiency Antimicrobial Activity
3.3. Identification of C-Terminal Acidic Mastoparans as Multipurpose Antibiotics
4. Materials and Methods
4.1. Biological Materials
4.2. Bioinformatic Analyses
4.3. Peptide Synthesis and Purification
4.4. Circular Dichroism Spectroscopy
4.5. Pathogen Growth and Antimicrobial Test
4.6. Hemolytic Activity
4.7. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AMP | antimicrobial peptide |
| CD | circular dichroism |
| MP | mastoparan |
| TFE | 2,2,2-trifluoroethanol |
References
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2012, 11, 37–51. [Google Scholar] [CrossRef]
- Bechinger, B. The SMART model: Soft Membranes Adapt and Respond, also Transiently, in the presence of antimicrobial peptides. J. Pept. Sci. 2015, 21, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Deslouches, B.; Steckbeck, J.D.; Craigo, J.K.; Doi, Y.; Burns, J.L.; Montelaro, R.C. Engineered Cationic Antimicrobial Peptides To Overcome Multidrug Resistance by ESKAPE Pathogens. Antimicrob. Agents Chemother. 2015, 59, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.S.; Silva, C.S.; de Souza, V.C.; da Silva, V.L.; Diniz, C.G.; Santos, M.O. Strategies and molecular tools to fight antimicrobial resistance: Resistome, transcriptome, and antimicrobial peptides. Front. Microbiol. 2013, 4, 412. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-C.; Park, Y.; Hahm, K.-S. The role of antimicrobial peptides in preventing multidrug-resistant bacterial infections and biofilm formation. Int. J. Mol. Sci. 2011, 12, 5971–5992. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Baek, J.H.; Yoon, K.A. Differential Properties of Venom Peptides and Proteins in Solitary vs. Social Hunting Wasps. Toxins 2016, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, J.R.; Etzerodt, T.; Gjetting, T.; Andresen, T.L. Side chain hydrophobicity modulates therapeutic activity and membrane selectivity of antimicrobial peptide mastoparan-X. PLoS ONE 2014, 9, e91007. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Giralt, E. Three Valuable Peptides from Bee and Wasp Venoms for Therapeutic and Biotechnological Use: Melittin, Apamin and Mastoparan. Toxins 2015, 7, 1126–1150. [Google Scholar] [CrossRef] [PubMed]
- Wanandy, T.; Gueven, N.; Davies, N.W.; Brown, S.G.; Wiese, M.D. Pilosulins: A review of the structure and mode of action of venom peptides from an Australian ant Myrmecia pilosula. Toxicon 2015, 98, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Sample, C.J.; Hudak, K.E.; Barefoot, B.E.; Koci, M.D.; Wanyonyi, M.S.; Abraham, S.; Staats, H.F.; Ramsburg, E.A. A mastoparan-derived peptide has broad-spectrum antiviral activity against enveloped viruses. Peptides 2013, 48, 96–105. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo, R.A.; Figueiredo, C.R.; Ferreira, A.K.; Matsuo, A.L.; Massaoka, M.H.; Girola, N.; Auada, A.V.; Farias, C.F.; Pasqualoto, K.F.; Rodrigues, C.P. Mastoparan induces apoptosis in B16F10-Nex2 melanoma cells via the intrinsic mitochondrial pathway and displays antitumor activity in vivo. Peptides 2015, 68, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Giangaspero, A.; Sandri, L.; Tossi, A. Amphipathic α helical antimicrobial peptides. Eur. J. Biochem. 2001, 268, 5589–5600. [Google Scholar] [CrossRef] [PubMed]
- Etzerodt, T.; Henriksen, J.R.; Rasmussen, P.; Clausen, M.H.; Andresen, T.L. Selective acylation enhances membrane charge sensitivity of the antimicrobial peptide mastoparan-x. Biophys. J. 2011, 100, 399–409. [Google Scholar] [CrossRef] [PubMed]
- King, T.P.; Jim, S.Y.; Wittkowski, K.M. Inflammatory role of two venom components of yellow jackets (Vespula vulgaris): A mast cell degranulating peptide mastoparan and phospholipase A1. Int. Arch. Allergy Immunol. 2003, 131, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-L.; Hwang, L.-L. Structure and biological activities of a new mastoparan isolated from the venom of the hornet Vespa basalis. Biochem. J. 1991, 274, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Hirai, Y.; Yasuhara, T.; Yoshida, H.; Nakajima, T.; Fujino, M.; Kitada, C. A new mast cell degranulating peptide "mastoparan" in the venom of Vespula lewisii. Chem. Pharm. Bull. 1979, 27, 1942–1944. [Google Scholar] [CrossRef] [PubMed]
- Hirai, Y.; Kuwada, M.; Yasuhara, T.; Yoshida, H.; Nakajima, T. A new mast cell degranulating peptide homologous to mastoparan in the venom of Japanese hornet (Vespa xanthoptera). Chem. Pharm. Bull. 1979, 27, 1945–1946. [Google Scholar] [CrossRef]
- ČeŘovský, V.; Pohl, J.; Yang, Z.; Alam, N.; Attygalle, A.B. Identification of three novel peptides isolated from the venom of the neotropical social wasp Polistes major major. J. Pept. Sci. 2007, 13, 445–450. [Google Scholar] [CrossRef] [PubMed]
- The Protein Database in NCBI. Available online: http://www.ncbi.nlm.nih.gov/protein (accessed on 14 April 2016).
- Hancock, R.E. The bacterial outer membrane as a drug barrier. Trend. Microbiol. 1997, 5, 37–42. [Google Scholar] [CrossRef]
- Da Silva, A.V.; de Souza, B.M.; dos Santos Cabrera, M.P.; Dias, N.B.; Gomes, P.C.; Neto, J.R.; Stabeli, R.G.; Palma, M.S. The effects of the C-terminal amidation of mastoparans on their biological actions and interactions with membrane-mimetic systems. Biochim. Biophys. Acta 2014, 1838, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Kallenbach, N.R.; Gong, Y. C-terminal capping motifs in model helical peptides. Bioorg. Med. Chem. 1999, 7, 143–151. [Google Scholar] [CrossRef]
- Zhou, H.X.; Lyu, P.C.; Wemmer, D.E.; Kallenbach, N.R. Structure of a C-Terminal alpha-Helix Cap in a Synthetic Peptide. J. Am. Chem. Soc. 1994, 116, 1139–1140. [Google Scholar] [CrossRef]
- Yoon, K.A.; Kim, K.; Nguyen, P.; Seo, J.B.; Park, Y.H.; Kim, K.-G.; Seo, H.-Y.; Koh, Y.H.; Lee, S.H. Comparative bioactivities of mastoparans from social hornets Vespa crabro and Vespa analis. J. Asia Pac. Entomol. 2015. [Google Scholar] [CrossRef]
- ProtParam Tool. Available online: http://web.expasy.org/protparam/ (accessed on 14 April 2016).
- Lin, C.-H.; Tzen, J.T.; Shyu, C.-L.; Yang, M.J.; Tu, W.-C. Structural and biological characterization of mastoparans in the venom of Vespa species in Taiwan. Peptides 2011, 32, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Del Serrone, P.; Nicoletti, M. Antimicrobial activity of a neem cake extract in a broth model meat system. Int. J. Environ. Res. Public Health 2013, 10, 3282–3295. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds, MP-L, MP-X, MP-V1 and MP-B, are available from the authors.

| Buffers | MP-L | MP-X(V) | MP-V1 | MP-B | ||||
|---|---|---|---|---|---|---|---|---|
| [θ]222 | % α-Helix | [θ]222 | % α-Helix | [θ]222 | % α-Helix | [θ]222 | % α-Helix | |
| Water | −2792.88 | r.c. | −1828.33 | r.c. | −1480.77 | r.c. | −1477.65 | r.c. |
| 8 mM SDS | −9450.15 | 19.55 | −11459.40 | 25.63 | −9965.03 | 21.11 | −9963.52 | 21.10 |
| 40% TFE | −8896.83 | 17.87 | −12974.80 | 30.23 | −10261.60 | 22.00 | −5615.77 | 7.93 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Son, M.; Noh, E.-Y.; Kim, S.; Kim, C.; Yeo, J.-H.; Park, C.; Lee, K.W.; Bang, W.Y. MP-V1 from the Venom of Social Wasp Vespula vulgaris Is a de Novo Type of Mastoparan that Displays Superior Antimicrobial Activities. Molecules 2016, 21, 512. https://doi.org/10.3390/molecules21040512
Kim Y, Son M, Noh E-Y, Kim S, Kim C, Yeo J-H, Park C, Lee KW, Bang WY. MP-V1 from the Venom of Social Wasp Vespula vulgaris Is a de Novo Type of Mastoparan that Displays Superior Antimicrobial Activities. Molecules. 2016; 21(4):512. https://doi.org/10.3390/molecules21040512
Chicago/Turabian StyleKim, Yangseon, Minky Son, Eun-Young Noh, Soonok Kim, Changmu Kim, Joo-Hong Yeo, Chanin Park, Keun Woo Lee, and Woo Young Bang. 2016. "MP-V1 from the Venom of Social Wasp Vespula vulgaris Is a de Novo Type of Mastoparan that Displays Superior Antimicrobial Activities" Molecules 21, no. 4: 512. https://doi.org/10.3390/molecules21040512






