Fruitful Decades for Canthin-6-ones from 1952 to 2015: Biosynthesis, Chemistry, and Biological Activities
Abstract
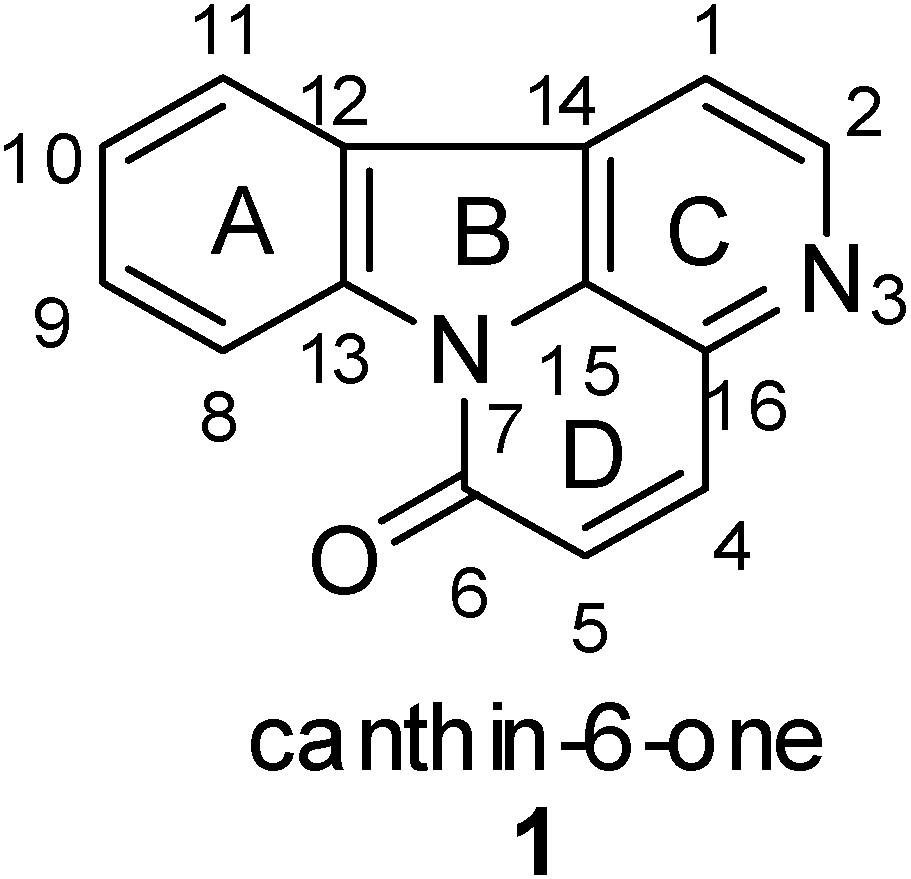
:1. Introduction
2. Biosynthesis
2.1. Biosynthetic Pathway
2.2. Biosynthetic Assembly Line
3. Chemistry
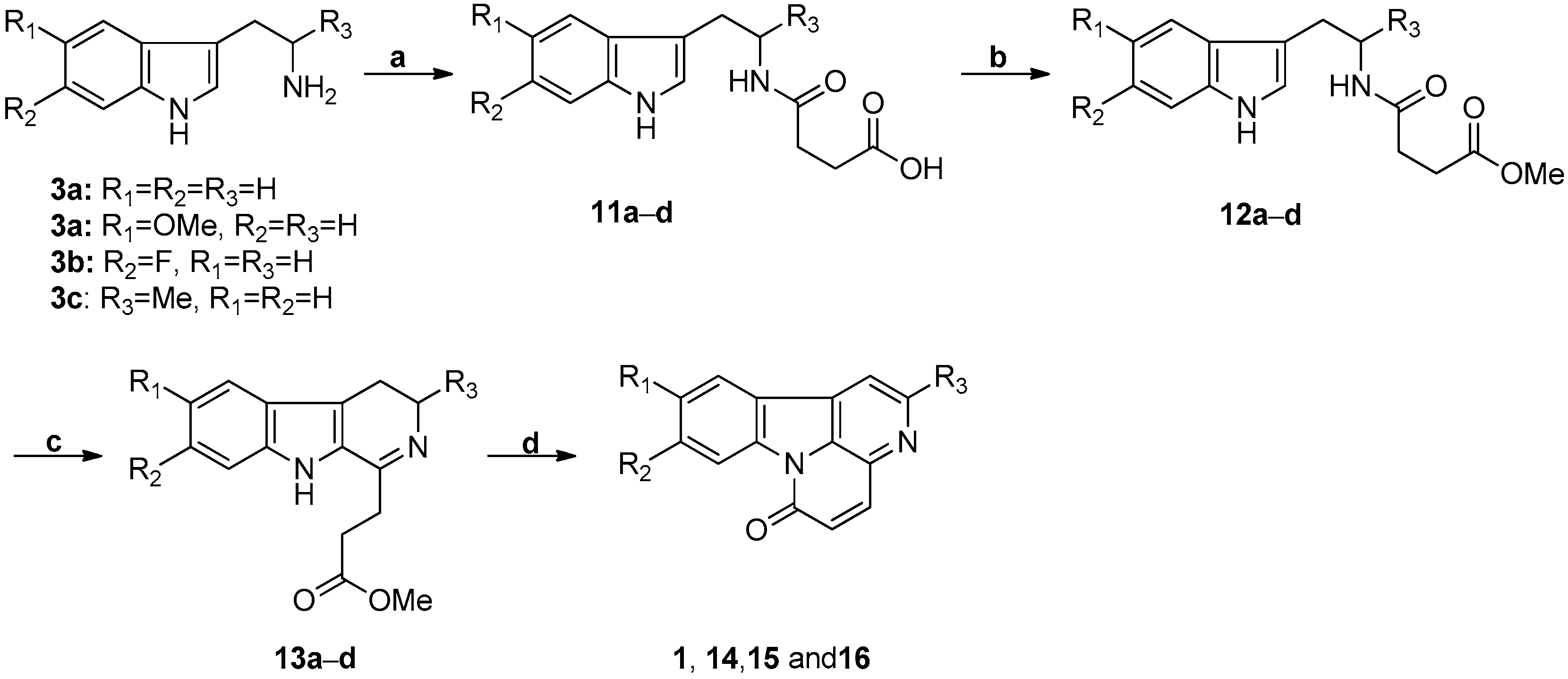
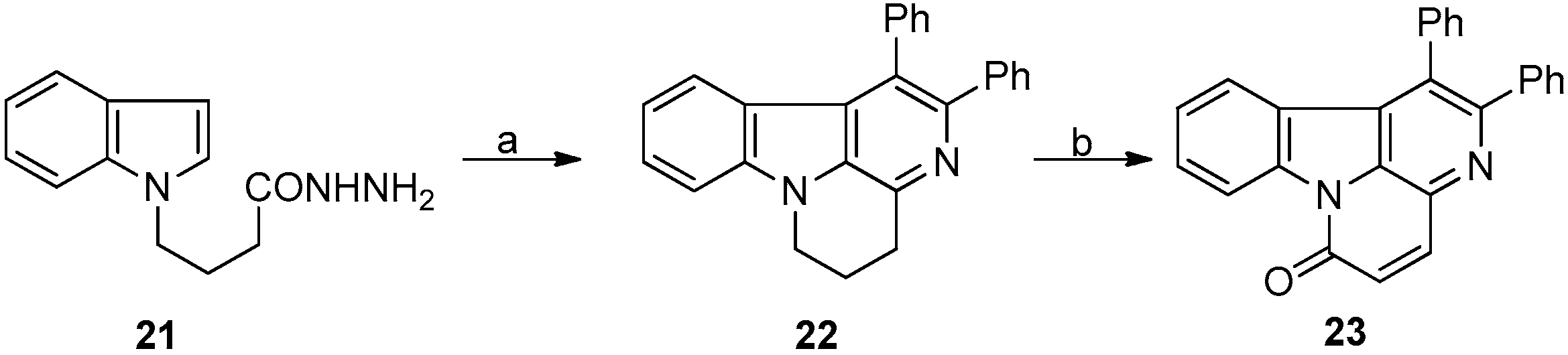
3.1. Bischer-Napieralski Reaction
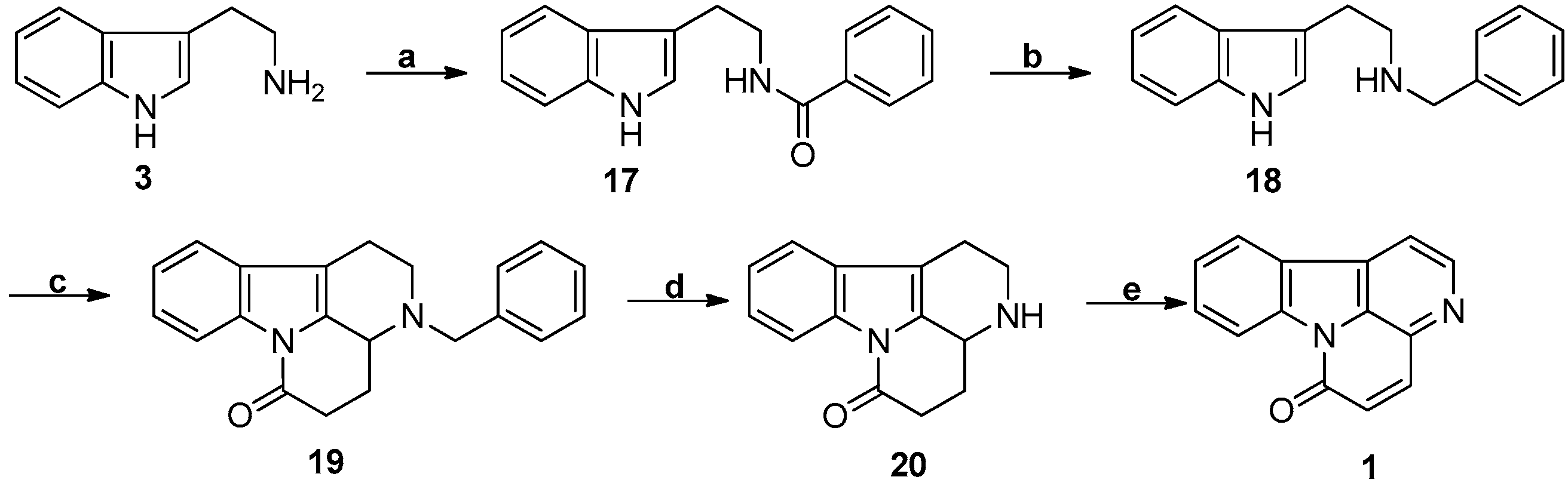
3.2. Pictet-Spengler Reaction
3.3. Diels-Alder Reaction
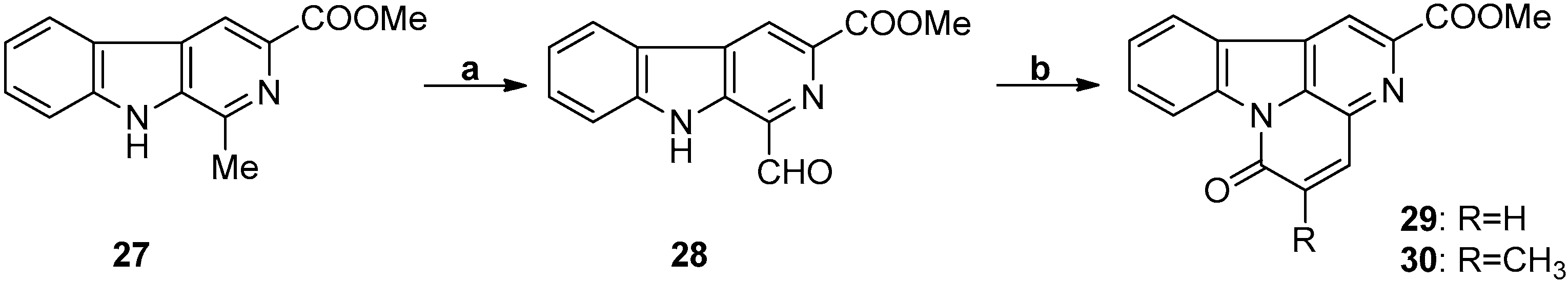
3.4. Aldol Reaction
3.5. Perkin Reaction
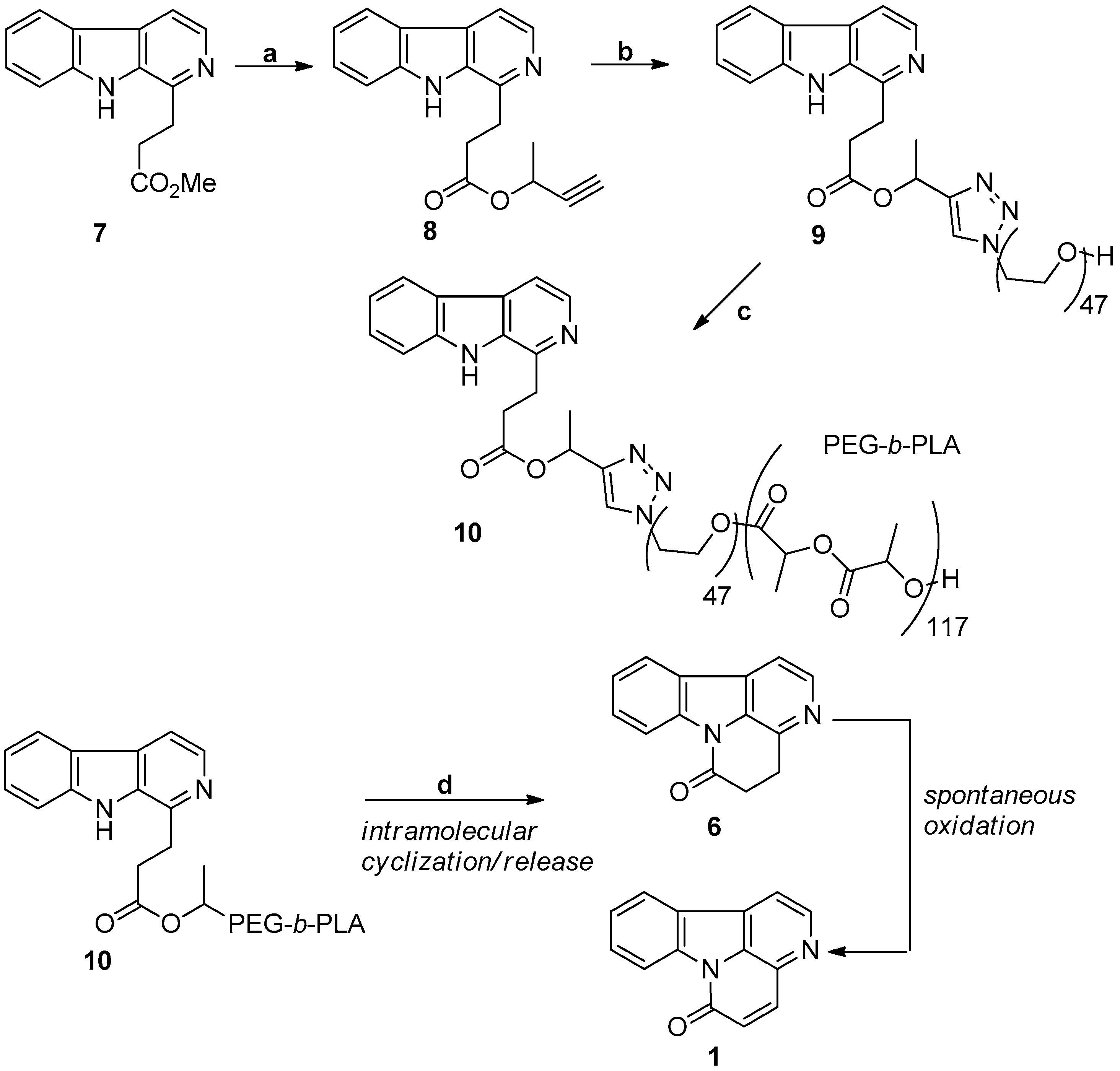
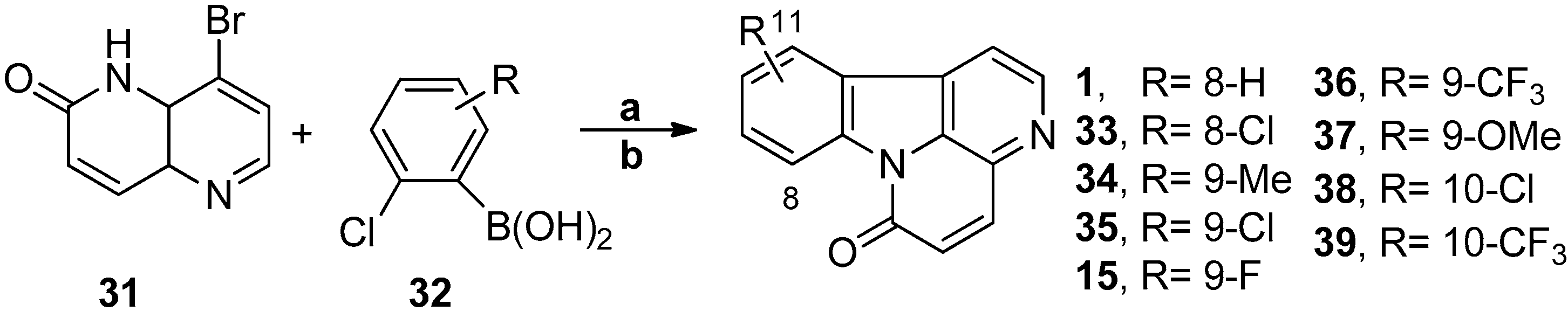
3.6. Non-Classic Strategy
4. Biological Activity
4.1. Antibacterial
4.2. Antitumor
4.3. Antifungal
4.4. Anti-Inflammatory
4.5. Wnt Signaling Inhibitors
4.6. Protein Tyrosine Phosphatase 1B Inhibitors
5. Conclusions and Future Prospects
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tessier, A.; Campbell, P.G.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Soriano-Agatón, F.; Lagoutte, D.; Poupon, E.; Roblot, F.; Fournet, A.; Gantier, J.C.; Hocquemiller, R. Extraction, hemisynthesis, and synthesis of canthin-6-one analogues. Evaluation of their antifungal activities. J. Nat. Prod. 2005, 68, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Mukhlesur, R.M.; Anwarul, I.M.; Khondkar, P.; Gray, A.I. Alkaloids and lignans from Zanthoxylum budrunga (Rutaceae). Biochem. Syst. Ecol. 2005, 33, 91–96. [Google Scholar] [CrossRef]
- He, W.; Puyvelde, L.V.; Kimpe, N.D.; Verbruggen, L.; Anthonissen, K.; Flaas, M.V.; Bosselaers, J.; Mathenge, S.G.; Mudida, F.P. Chemical constituents and biological activities of Zanthoxylum usambarense. Phytother. Res. 2002, 16, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Cebrián-Torrejón, G.; Kablan, L.; Ferreira, M.E.; Cruz, D.R.; Doménech-Carbó, A.; Bilbao, N.V.; Arias, A.R.; Figadère, B.; Poupon, E.; Fournet, A. Harvesting canthinones: Identification of the optimal seasonal point of harvest of Zanthoxylum chiloperone leaves as a source of 5-methoxycanthin-6-one. Nat. Prod. Res. 2015, 29, 2054–2058. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.D.; Kwon, H.C.; Yang, M.C.; Lee, K.H.; Choi, S.U.; Lee, K.R. Isolation of limonoids and alkaloids from Phellodendron amurense and their multidrug resistance (MDR) reversal activity. Arch. Pharm. Res. 2007, 30, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.J.; Liu, W.W.; Wang, H.Y.; Cheng, H.; Hu, L. Effect of canthin-6-one experimental arrhythmia. Chin. Tradit. Herba. Drugs 2000, 31, 37–39. [Google Scholar]
- Ferreira, M.E.; Cebrián-Torrejón, G.; Corrales, A.S.; de Bilbao, N.V.; Rolón, M.; Gomez, C.V.; Leblanc, K.; Yaluf, G.; Schinini, A.; Torres, S.; et al. Zanthoxylum chiloperone leaves extract: First sustainable Chagas disease treatment. J. Ethnopharmacol. 2011, 133, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Cruz, J.F.; Lezutekong, R.; Lobo-Echeverri, T.; Ito, A.; Mi, Q.; Chai, H.B.; Soejarto, D.D.; Cordell, G.A.; Pezzuto, J.M.; Swanson, S.M.; et al. Cytotoxic constituents of the twigs of Simarouba glauca collected from a plot in southern florida. Phytother. Res. 2005, 19, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.V.A.; Malainer, C.; Schwaiger, S.; Atanasov, A.G.; Heiss, E.H.; Dirsch, V.M.; Stuppner, H. NF-κB inhibitors from Eurycoma longifolia. J. Nat. Prod. 2014, 77, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Devkota, K.P.; Wilson, J.A.; Henrich, C.J.; McMahon, J.B.; Reilly, K.M.; Beutler, J.A. Compounds from Simarouba berteroana which inhibit proliferation of NF1-defective cancer cells. Phytochem. Lett. 2014, 7, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yan, X.H.; Dong, J.H.; Sang, P.; Fang, X.; Di, Y.T.; Zhang, Z.K.; Hao, X.J. Tobacco mosaic virus (TMV) inhibitors from Picrasma quassioides Benn. J. Agric. Food Chem. 2009, 57, 6590–6595. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.C.; Shi, L.S.; Damu, A.G.; Su, C.R.; Huang, C.H.; Ke, C.H.; Wu, .B.; Lin, A.J.; Bastow, K.F.; Lee, K.H.; et al. Cytotoxic and antimalarial β-carboline alkaloids from the roots of Eurycoma longifolia. J. Nat. Prod. 2003, 66, 1324–1327. [Google Scholar] [CrossRef] [PubMed]
- Handa, S.S.; Kinghorn, A.D.; Cordell, G.A.; Farnsworth, N.R. Plant anticancer agents XXV. Constituents of Soulamea soulameoides. J. Nat. Prod. 1983, 46, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Takizawa, S.; Hirano, T.; Murata, S.; Kagechika, H.; Kishida, A.; Ohsaki, A. Amarastelline A: A fluorescent alkaloid from Quassia amara and its properties in living cells. ChemPlusChem 2012, 77, 427–431. [Google Scholar] [CrossRef]
- Kardono, L.B.; Angerhofer, C.K.; Tsaur, S.; Padmawinata, K.; Pezzuto, J.M.; Kinghorn, A.D. Cytotoxic and antimalarial constituents of the roots of Eurycoma longifolia. J. Nat. Prod. 1991, 54, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Kanchanapoom, T.; Kasai, R.; Chumsri, P.; Hiraga, Y.; Yamasaki, K. Canthin-6-one and β-carboline alkaloids from Eurycoma harmandiana. Phytochemistry 2001, 56, 383–386. [Google Scholar] [CrossRef]
- Bharitkar, Y.P.; Hazra, A.; Apoorva Poduri, N.S.; Ash, A.; Maulik, P.R.; Mondal, N.B. Isolation, structural elucidation and cytotoxicity evaluation of a new pentahydroxy-pimarane diterpenoid along with other chemical constituents from Aerva lanata. Nat. Prod. Res. 2015, 29, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.W.; Chang, F.R.; Lee, K.H.; Hwang, T.L.; Chang, S.M.; Wu, Y.C. A new anti-HIV alkaloid, drymaritin, and a new c-glycoside flavonoid, diandraflavone, from Drymaria diandra. J. Nat. Prod. 2004, 67, 1175–1177. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.Z.; Hano, Y.; Nomura, T.; Chen, Y.J. Alkaloids and phenylpropanoids from Peganum nigellastrum. Phytochemistry 2000, 53, 1075–1078. [Google Scholar] [CrossRef]
- Bröckelmann, M.G.; Dasenbrock, J.; Steffan, B.; Steglich, W.; Wang, Y.; Raabe, G.; Fleischhauer, J. An unusual series of thiomethylated canthin-6-ones from the North American mushroom Boletus curtisii. Eur. J. Org. Chem. 2004, 4856–4863. [Google Scholar] [CrossRef]
- Tanaka, N.; Momose, R.; Takahashi, Y.; Kubota, T.; Takahashi-Nakaguchi, A.; Gonoi, T.; Fromont, J.; Kobayashi, J. Hyrtimomines D and E, bisindole alkaloids from a marine sponge Hyrtios sp. Tetrahedron Lett. 2013, 54, 4038–4040. [Google Scholar] [CrossRef]
- Haynes, H.F.; Nelson, E.R.; Price, J.R. Alkaloids of the Australian Rutaceae: Pentaceras australis Hook. F.I. Isolation of the Alkaloids and Identification of Canthin-6-one. Aust. J. Sci. Res. Ser. A 1952, 5, 387–400. [Google Scholar]
- Crespi-Perellino, N.; Guicciardi, A.; Malyszko, G.; Minghetti, A. Biosynthetic relationship between indole alkaloids produced by cell cultures of Ailanthus altissima. J. Nat. Prod. 1986, 49, 814–822. [Google Scholar] [CrossRef]
- Aragozzini, F.; Maconi, E.; Gualandris, R. Evidence for involvement of ketoglutarate in the biosynthesis of canthin-6-one from cell cultures of Ailanthus altissima. Plant Cell Rep. 1988, 7, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Cebrián-Torrejón, G.; Mackiewicz, N.; Vázquez-Manrique, R.P.; Fournet, A.; Figadère, B.; Nicolas, J.; Poupon, E. Solution phase and nanoparticular biosynthetically inspired interconnections in the canthi-6-one β-carboline series and study of phenotypic properties on C. elegans. Eur. J. Org. Chem. 2013, 2013, 5821–5828. [Google Scholar] [CrossRef]
- Rosenkranz, H.J.; Botyos, G.; Schmid, H. Synthese von tuboflavin, 4-Äthyl-canthin-6-one and canthin-6-one. Justus Liebigs Ann. Chem. 1966, 691, 159–164. [Google Scholar] [CrossRef]
- Hollis Showalter, H.D. Progress in the synthesis of canthine alkaloids and ring-truncated congeners. J. Nat. Prod. 2013, 76, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Peduto, A.; More, V.; de Caprariis, P.; Festa, M.; Capasso, A.; Piacente, S.; de Martino, L.; de Feo, V.; Filosa, R. Synthesis and cytotoxic activity of new β-carboline derivatives. Mini-Rev. Med. Chem. 2011, 11, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Miyake, K.; Tezuka, Y.; Awale, S.; Li, F.; Kadota, S. Canthin-6-one alkaloids and a tirucallanoid from Eurycoma longifolia and their cytotoxic activity against a human HT-1080 fibrosarcoma cell line. Nat. Prod. Commun. 2010, 5, 17–22. [Google Scholar] [PubMed]
- Ammirante, M.; di Giacomo, R.; de Martino, L.; Rosati, A.; Festa, M.; Gentilella, A.; Pascale, M.C.; Belisario, M.A.; Leone, A.; Turco, M.C.; et al. 1-Methoxy-canthin-6-one induces c-Jun NH2-terminal kinase-dependent apoptosis and synergizes with tumor necrosis factor-related apoptosis-inducing ligand activity in human neoplastic cells of hematopoietic or endodermal origin. Cancer Res. 2006, 66, 4385–4393. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.C.; Damu, A.G.; Lee, K.H.; Wu, T.S. Cytotoxic and antimalarial constituents from the roots of Eurycoma longifolia. Bioorg. Med. Chem. 2004, 12, 537–544. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, G.; Gibbons, S. Antibacterial activity of two canthin-6-one alkaloids from Allium neapolitanum. Phytother. Res. 2007, 21, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Ostrov, D.A.; Prada, J.A.H.; Corsino, P.E.; Finton, K.A.; Le, N.; Rowe, T.C. Discovery of novel DNA gyrase inhibitors by high-throughput virtual screening. Antimicrob. Agents Chemother. 2007, 51, 3688–3698. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Fan, H.Y.; Dai, S.J.; Liu, K. Alkaloids from twigs and leaves of Picrasma quassioides. Chin. Tradit. Herbal Drugs 2007, 38, 807–810. [Google Scholar]
- Thouvenel, C.; Gantier, J.C.; Duret, P.; Fourneau, C.; Hocquemiller, R.; Ferreira, M.E.; de Arias, A.R.; Fournet, A. Antifungal compounds from Zanthoxylum chiloperone var. angustifolium. Phytother. Res. 2003, 17, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Koike, K.; Ohmoto, T. New alkaloids, picrasidines W, X and Y, from Picrasma quassioides and X-ray crystallographic analysis of picrasidine Q. Chem. Pharm. Bull. 1993, 41, 1807–1811. [Google Scholar] [CrossRef]
- Dejos, C.; Régnacq, M.; Bernard, M.; Voisin, P.; Bergès, T. The MFS-type efflux pump Flr1 induced by Yap1 promotes canthin-6-one resistance in yeast. Fed. Eur. Biochem. Soc. Lett. 2013, 587, 3045–3051. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.E.; Nakayama, H.; de Arias, A.R.; Schinini, A.; de Bilbao, N.V.; Serna, E.; Lagoutte, D.; Soriano-Agatón, F.; Poupon, E.; Hocquemiller, R.; et al. Effects of canthin-6-one alkaloids from Zanthoxylum chiloperone on trypanosoma cruzi-infected mice. J. Ethnopharmacol. 2007, 109, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Takasu, K.; Shimogama, T.; Saiin, C.; Kim, H.S.; Wataya, Y.; Brun, R.; Ihara, M. Synthesis and evaluation of β-carbolinium cations as new antimalarial agents based on π-delocalized lipophilic cation (DLC) hypothesis. Chem. Pharm. Bull. 2005, 53, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.E.; de Arias, A.R.; de Ortiz, S.T.; Inchausti, A.; Nakayama, H.; Thouvenel, C.; Hocquemiller, R.; Fournet, A. Leishmanicidal activity of two canthin-6-one alkaloids, two major constituents of Zanthoxylum chiloperone var. angustifolium. J. Ethnopharmacol. 2002, 80, 199–202. [Google Scholar] [CrossRef]
- Brahmbhatt, K.G.; Ahmed, N.; Sabde, S.; Mitra, D.; Singh, I.P.; Bhutani, K.K. Synthesis and evaluation of β-carboline derivatives as inhibitors of human immunodeficiency virus. Bioorg. Med. Chem. Lett. 2010, 20, 4416–4419. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chang, F.R.; Wang, H.K.; Kashiwada, Y.; McPhail, A.T.; Bastow, K.F.; Tachibana, Y.; Cosentino, M.; Lee, K.H. Anti-HIV agents 451 and antitumor agents 205.2 two new sesquiterpenes, Leitneridanins A and B, and the cytotoxic and anti-HIV principles from Leitneria floridana. J. Nat. Prod. 2000, 63, 1712–1715. [Google Scholar] [CrossRef] [PubMed]
- Ohomoto, T.; Koike, K. Studies on the constituents of Ailanthus altissima Swingle. III. The alkaloidal constituents. Chem. Pharm. Bull. 1984, 32, 170–173. [Google Scholar] [CrossRef]
- Dejos, C.; Voisin, P.; Bernard, M.; Régnacq, M.; Bergès, T. Canthin-6-one displays antiproliferative activity and causes accumulation of cancer cells in the G2/M phase. J. Nat. Prod. 2014, 77, 2481–2487. [Google Scholar] [CrossRef] [PubMed]
- De Feo, V.; Martino, L.D.; Santoro, A.; Leone, A.; Pizza, C.; Franceschelli, S.; Pascale, M. Antiproliferative effects of tree of heaven (Ailanthus altissima Swingle). Phytother. Res. 2005, 19, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.F.; Wu, T.S. 9-Hydroxycanthin-6-one induces penile erection and delays ejaculation. J. Sex. Med. 2012, 9, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Murakami, C.; Fukamiya, N.; Tamura, S.; Okano, M.; Bastow, K.F.; Tokuda, H.; Mukainaka, T.; Nishino, H.; Lee, K.H. Multidrug-resistant cancer cell susceptibility to cytotoxic quassinoids, and cancer chemopreventive effects of quassinoids and canthine alkaloids. Bioorg. Med. Chem. 2004, 12, 4963–4968. [Google Scholar] [CrossRef] [PubMed]
- Doménech-Carbó, A.; Cebrián-Torrejón, G.; de Miguel, L.; Tordera, V.; Rodrigues-Furtado, D.; Assad-Kahn, S.; Fournet, A.; Figadère, B.; Vázquez-Manrique, R.P.; Poupon, E. DsDNA, ssDNA, G-quadruplex DNA, and nucleosomal DNA electrochemical screening using canthin-6-one alkaloid-modified electrodes. Electrochim. Acta 2014, 115, 546–552. [Google Scholar] [CrossRef]
- Siveen, K.; Kuttan, G. Modulation of humoral immune responses and inhibition of proinflammatory cytokines and nitric oxide production by 10-methoxycanthin-6-one. Immunopharmacol. Immunotoxicol. 2012, 34, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Irikawa, H.; Okumura, Y. Autoxidation of indolo [3,2,1-de][1,5]naphthyridines. Bull. Chem. Soc. Jpn. 1987, 60, 3797–3798. [Google Scholar] [CrossRef]
- Hagen, T.J.; Cook, J.M. Synthesis of 1-methoxycanthine-6-one. Tetrahedron Lett. 1988, 29, 2421–2424. [Google Scholar] [CrossRef]
- Hagen, T.J.; Narayanan, K.; Names, J.; Cook, J.M. DDQ oxidations in the indole area. Synthesis of 4-alkoxy-β-carbolines including the natural products crenatine and 1-methoxycanthin-6-one. J. Org. Chem. 1989, 54, 2170–2178. [Google Scholar] [CrossRef]
- Czerwinski, K.M.; Zificsak, C.A.; Stevens, J.; Oberbeck, M.; Randlett, C.; King, M.; Mennen, S. An improved synthesis of canthin-6-one. Synth. Commun. 2003, 33, 1225–1231. [Google Scholar] [CrossRef]
- Rößler, U.; Blechert, S.; Steckhan, E. Single electron transfer induced total synthesis of canthin-6-one. Tetrahedron Lett. 1999, 40, 7075–7078. [Google Scholar] [CrossRef]
- Suzuki, H.; Adachi, M.; Ebihara, Y.; Gyoutoku, H.; Furuya, H.; Murakami, Y.; Okuno, H. A total synthesis of 1-methoxycanthin-6-one: An efficient one-pot synthesis of the canthin-6-one skeleton from β-Carboline-1-carbaldehyde. Synthesis 2005, 1, 28–32. [Google Scholar] [CrossRef]
- Markgraf, J.H.; Dowst, A.A.; Hensley, L.A.; Jakobsche, C.E.; Kaltner, C.J.; We, P.J.; Zimmerman, P.W. A versatile route to benzocanthinones. Tetrahedron 2005, 61, 9102–9110. [Google Scholar] [CrossRef]
- Bergman, J.; Lidgren, G.; Gogoll, A. Synthesis and reactions of oxazolones from l-tryptophan and α-haloacetic anhydrides. Bull. Soc. Chim. Belg. 1992, 101, 643–660. [Google Scholar] [CrossRef]
- Condie, G.C.; Bergman, J. Reactivity of β-carbolines and cyclopenta[b]indolones prepared from the intramolecular cyclization of 5(4H)-oxazolones derived from l-tryptophan. Eur. J. Org. Chem. 2004, 2004, 1286–1297. [Google Scholar] [CrossRef]
- Singh, V.; Hutait, S.; Batra, S. Baylis-Hillman reaction of 1-formyl-β-carboline: One-step synthesis of the canthin-6-one framework by an unprecedented cascade cyclization reaction. Eur. J. Org. Chem. 2009, 2009, 6211–6216. [Google Scholar] [CrossRef]
- Gollner, A.; Koutentis, P.A. Two-step total syntheses of canthin-6-one alkaloids: New one-pot sequential Pd-catalyzed Suzuki-Miyaura coupling and Cu-catalyzed amidation reaction. Org. Lett. 2010, 12, 1352–1355. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, H.A.; Martin, A.; Gollner, A.; Koutentis, P.A. Three-step synthesis of ethyl canthinone-3-carboxylates from ethyl 4-bromo-6-methoxy-1,5-naphthyridine-3-carboxylate via a Pd-catalyzed Suzuki-Miyaura coupling and a Cu-catalyzed amidation reaction. J. Org. Chem. 2011, 76, 5113–5122. [Google Scholar] [CrossRef] [PubMed]
- Markgraf, J.H.; Snyder, S.A.; Vosburg, D.A. A concise route to isocanthin-6-one. Tetrahedron Lett. 1998, 39, 1111–1112. [Google Scholar] [CrossRef]
- Snyder, S.A.; Vosburg, D.A.; Jarvis, M.G.; Markgraf, J.H. Intramolecular hetero Diels-Alder routes to γ-carboline alkaloids. Tetrahedron 2000, 56, 5329–5335. [Google Scholar]
- Hájíček, J.; Trojánek, J. Synthesis of optically active 4,4- and 5,5-disubstituted 4,5-dihydro-6H-canthin-6-ones and their CD spectra. Collect. Czechoslov. Chem. Commun. 1982, 47, 3306–3311. [Google Scholar] [CrossRef]
- Hájíček, J.; Holubek, J.; Trojánek, J. Synthesis of 4,4- and 5,5-disubstituted 4,5-dihydro-6H-canthin-6-ones. Collect. Czechoslov. Chem. Commun. 1982, 47, 2749–2762. [Google Scholar] [CrossRef]
- Chughtai, M.; Eagan, J.M.; Padwa, A. Intramolecular [4 + 2]-cycloaddition of 5-amino-substituted oxazoles as an approach toward the left-hand segment of haplophytine. Synlett 2011, 2, 215–218. [Google Scholar]
- Benson, S.C.; Li, J.H.; Snyder, J.K. Indole as a dienophile in inverse electron demand Diels-Alder reactions. intramolecular reactions with 1,2,4-triazines to access the canthine skeleton. J. Org. Chem. 1992, 57, 5285–5287. [Google Scholar] [CrossRef]
- Li, J.H.; Snyder, J.K. Selective oxidation of canthines to canthin-6-ones with triethylbenzylammonium permanganate. Tetrahedron Lett. 1994, 35, 1485–1488. [Google Scholar] [CrossRef]
- Lindsley, C.W.; Wisnoski, D.D.; Wang, Y.; Leister, W.H.; Zhao, Z. A “one pot” microwave-mediated synthesis of the basic canthine skeleton: Expedient access to unnatural β-carboline alkaloids. Tetrahedron Lett. 2003, 44, 4495–4498. [Google Scholar] [CrossRef]
- De Bruyn, A.; Eeckhaut, G.; Villaneuva, J.; Hannart, J. Synthesis and 1H-NMR study of 3,4-diethyl-1,2,3,3a,4,5-hexahydro-canthinone-6. Tetrahedron 1985, 41, 5553–5561. [Google Scholar] [CrossRef]
- Puzik, A.; Bracher, F. A convenient approach to the canthin-4-one ring system: Total synthesis of the alkaloids tuboflavine and norisotuboflavine. J. Heterocycl. Chem. 2009, 46, 770–773. [Google Scholar] [CrossRef]
- Mitscher, L.; Shipchandler, M.; Showaleter, H.; Bathala, M. Antimicrobial agents from higher-plants-synthesis in canthin-6-one (6H-indolo[3,2,1-de][1,5]naphthyridin-6-one) series. Heterocycles 1975, 3, 7–14. [Google Scholar] [CrossRef]
- Fang, H.W.; Liao, Y.R.; Hwang, T.L.; Shieh, P.C.; Lee, K.H.; Hung, H.Y.; Wu, T.S. Total synthesis of cordatanine, structural reassignment of drymaritin, and anti-inflammatory activity of synthetic precursors. Bioorg. Med. Chem. Lett. 2015, 25, 3822–3824. [Google Scholar] [CrossRef] [PubMed]
- Giudice, M.R.D.; Settimj, F.G.G. New tetracyclic compounds containing the β-carboline moiety. J. Heterocycl. Chem. 1990, 27, 967–973. [Google Scholar] [CrossRef]
- Kim, H.M.; Kim, S.J.; Kim, H.Y.; Ryu, B.; Kwak, H.; Hur, J.; Choi, J.H.; Jang, D.S. Constituents of the stem barks of Ailanthus altissima and their potential to inhibit LPS-induced nitric oxide production. Bioorg. Med. Chem. Lett. 2015, 25, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.K.; Dan, W.J.; Li, N.; Du, H.T.; Zhang, J.W.; Wang, J.R. Synthesis, in vitro antibacterial activities of a series of 3-N-substituted canthin-6-ones. Bioorg. Med. Chem. Lett. 2016, 26, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Cebrian-Torrejon, G.; Domenech-Carbo, A.; Scotti, M.T.; Fournet, A.; Figadere, B.; Poupon, E. Experimental and theoretical study of possible correlation between the electrochemistry of canthin-6-one and the anti-proliferative activity against human cancer stem cells. J. Mol. Struct. 2015, 1102, 242–246. [Google Scholar] [CrossRef]
- Lagoutte, D.; Nicolas, V.; Poupon, E.; Fournet, A.; Hocquemiller, R.; Libong, D.; Chaminade, P.; Loiseau, P.M. Antifungal canthin-6-one series accumulate in lipid droplets and affect fatty acid metabolism in Saccharomyces cerevisiae. Biomed. Pharmacother. 2008, 62, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, K.; Toume, K.; Arai, M.A.; Koyano, T.; Kowithayakorn, T.; Mizoguchi, T.; Itoh, M.; Ishibashi, M. 9-Hydroxycanthin-6-one, a β-Carboline Alkaloid from Eurycoma longifolia, Is the First Wnt Signal Inhibitor through Activation of Glycogen Synthase Kinase 3β without Depending on Casein Kinase 1α. J. Nat. Prod. 2015, 78, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Li, W.; Higai, K.; Koike, K. Canthinone alkaloids are novel protein tyrosine phosphatase 1B inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 1979–1981. [Google Scholar] [CrossRef] [PubMed]




| When Isolated | Natural Canthin-6-one Alkaloids |
|---|---|
| 1952–1999 (25) |  |
| 2000–2004 (16) |  |
| 2005–2015 (19) |  |
| Compound | Cell Viability (Untreated Controls = 100) | Inhibition of Proliferation (%) |
|---|---|---|
| Canthin-6-one-9-methoxy-5-O-β-d-glucopyranoside | 51 | 71 |
| 9-Methoxycanthin-6-one | 41 | 76 |
| 8-Hydroxy-9-methoxycanthin-6-one | 51 | 59 |
| 9-Hydroxycanthin-6-one | 56 | 48 |
| canthin-6-one-3-N-oxide | 59 | 70 |
| 9-Hydroxycanthin-6-one-3-N-oxide | 54 | 76 |
| 11-Hydroxycanthin-6-one-3-N-oxide | 54 | 70 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, J.; Li, N.; Wang, J.; Schneider, U. Fruitful Decades for Canthin-6-ones from 1952 to 2015: Biosynthesis, Chemistry, and Biological Activities. Molecules 2016, 21, 493. https://doi.org/10.3390/molecules21040493
Dai J, Li N, Wang J, Schneider U. Fruitful Decades for Canthin-6-ones from 1952 to 2015: Biosynthesis, Chemistry, and Biological Activities. Molecules. 2016; 21(4):493. https://doi.org/10.3390/molecules21040493
Chicago/Turabian StyleDai, Jiangkun, Na Li, Junru Wang, and Uwe Schneider. 2016. "Fruitful Decades for Canthin-6-ones from 1952 to 2015: Biosynthesis, Chemistry, and Biological Activities" Molecules 21, no. 4: 493. https://doi.org/10.3390/molecules21040493






