Imidazopyranotacrines as Non-Hepatotoxic, Selective Acetylcholinesterase Inhibitors, and Antioxidant Agents for Alzheimer's Disease Therapy
Abstract
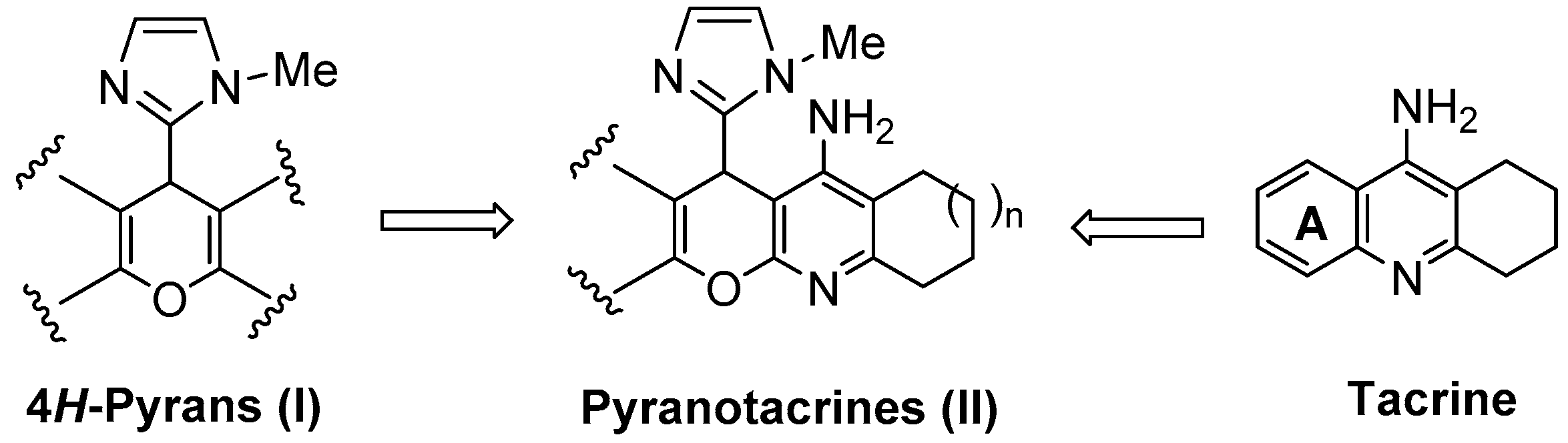
:1. Introduction
2. Results and Discussion
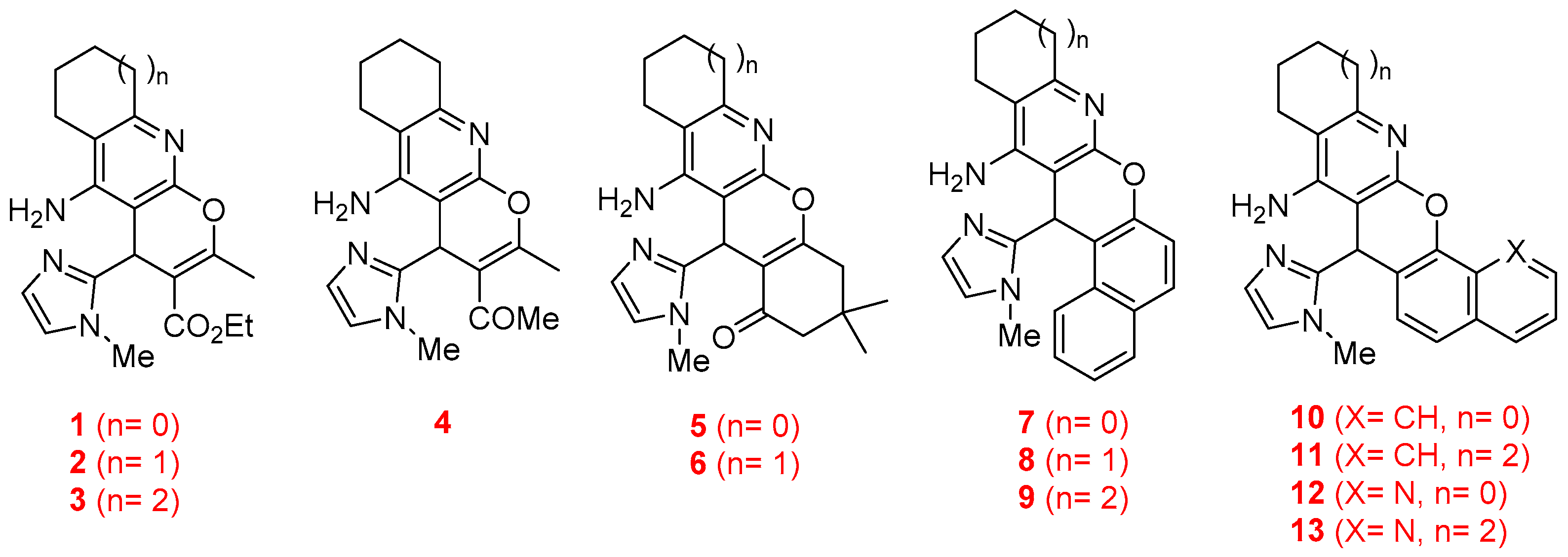
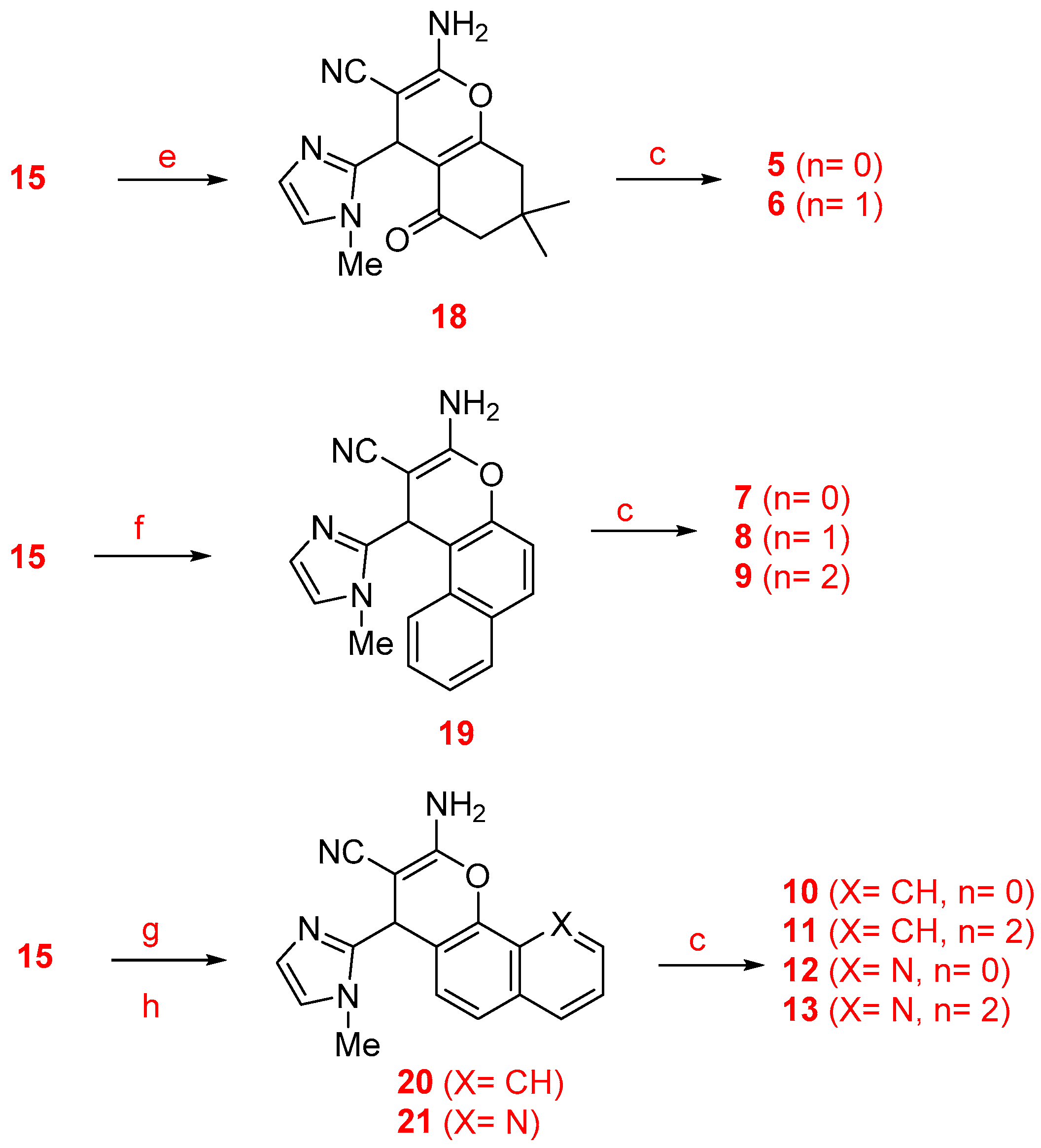
2.1. Chemistry
2.2. Biological Assays
2.2.1. Inhibition of the Cholinesterase Enzymes and Kinetic Analysis
2.2.2. Antioxidant Power
2.2.3. Hepatotoxicity Analysis on HepG2 Cells
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Preparation of 1-Methylimidazole-4H-pyran Derivatives
3.3. General Procedure for the Preparation of 1-Methylimidazole-4H-pyran Derivatives from 1- or 2-Naphthol
3.4. General Method for the Friedländer Reaction
3.5. In Vitro Toxicity in HepG2 Cells
3.5.1. Cell Culture and Treatment
3.5.2. Measurement of Cell Viability
3.6. Measurement of the Inhibitory Potency against EeAChE
3.7. Measurement of the Inhibitory Potency against eqBuChE
3.8. Kinetic Analysis of the Inhibition of EeAChE by Compound 8
3.9. Oxygen Radical Absorbance Capacity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Soler-López, M.; Badiola, N.; Zanzoni, A.; Aloy, P. Towards Alzheimer′s root cause: ECSIT as an integrating hub between oxidative stress, inflammation and mitochondrial dysfunction. Hypothetical role of the adapter protein ECSIT in familial and sporadic Alzheimer′s disease pathogenesis. Bioessays 2012, 34, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C.; Brayne, C.; Mayeux, R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011, 7, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.S.; Mangialasche, F.; Andreasen, N.; Feldman, H.; Giacobini, E.; Jones, R.; Mantua, V.; Mecocci, P.; Pani, L.; Winblad, B.; et al. Clinical trials and late-stage drug development for Alzheimer′s disease: An appraisal from 1984 to 2014. J. Intern. Med. 2014, 275, 251–283. [Google Scholar] [CrossRef] [PubMed]
- Ittner, L.M.; Götz, J. Amyloid-β and tau–a toxic pas de deux in Alzheimer′s disease. Nat. Rev. Neurosci. 2011, 12, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Ávila, J. Tau phosphorylation and aggregation in Alzheimer′s disease pathology. FEBS Lett. 2006, 580, 2922–2927. [Google Scholar] [CrossRef] [PubMed]
- Rosini, M.; Simoni, E.; Milelli, A.; Minarini, A.; Melchiorre, C. Oxidative stress in Alzheimer′s disease: Are we connecting the dots? J. Med. Chem. 2014, 57, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.L.; Xu, J.Q.; Kochanek, K.D. Deaths: Final Data for 2010; National Center for Health Statistics: Hyattsville, MD, USA, 2013. [Google Scholar]
- Chacón, M.A.; Reyes, A.E.; Inestrosa, N.C. Acetylcholinesterase induces neuronal cell loss, astrocyte hypertrophy and behavioral deficits in mammalian hippocampus. J. Neurochem. 2003, 87, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.V.; Buccafusco, J.J.; Wilson, C. Cognitive dysfunction in neuropsychiatric disorders: selected serotonin receptor subtypes as therapeutic targets. Behav. Brain Res. 2008, 195, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Gura, T. Hope in Alzheimer′s fight emerges from unexpected places. Nat. Med. 2008, 14, 894. [Google Scholar] [CrossRef] [PubMed]
- Racchi, M.; Mazzucchelli, M.; Porrello, E.; Lanni, C.; Govoni, S. Acetylcholinesterase inhibitors: Novel activities of old molecules. Pharmacol. Res. Off. J. Ital. Pharmacol. Soc. 2004, 50, 441–451. [Google Scholar]
- Castro, A.; Martínez, A. Targeting Beta-Amyloid Pathogenesis Through Acetylcholinesterase Inhibitors. Curr. Pharm. Des. 2006, 12, 4377–4387. [Google Scholar] [CrossRef] [PubMed]
- Rosini, M. Polypharmacology: The rise of multitarget drugs over combination therapies. Future Med. Chem. 2014, 6, 485–487. [Google Scholar] [CrossRef] [PubMed]
- León, R.; García, A.G.; Marco-Contelles, J. Recent advances in the multitarget-directed ligands approach for the treatment of Alzheimer′s disease. Med. Res. Rev. 2013, 33, 139–189. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Cacabelos, R.; Oset-Gasque, M.J.; Samadi, A.; Marco-Contelles, J. Novel tacrine-related drugs as potential candidates for the treatment of Alzheimer′s disease. Bioorg. Med. Chem. Lett. 2013, 23, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Reale, M.; Di Nicola, M.; Velluto, L.; D′Angelo, C.; Costantini, E.; Lahiri, D.K.; Kamal, M.A.; Yu, Q.S.; Greig, N.H. Selective acetyl and butyrylcholinesterase inhibitors reduce amyloid-beta ex vivo activation of peripheral chemo-cytokines from Alzheimer′s disease subjects: Exploring the cholinergic anti-inflammatory pathway. Curr. Alzheimer Res. 2014, 11, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Watkins, P.B.; Zimmerman, H.J.; Knapp, M.J.; Gracon, S.I.; Lewis, K.W. Hepatotoxic effects of tacrine administration in patients with Alzheimer′s disease. JAMA 1994, 271, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Soukup, O.; Jun, D.; Zdarova-Karasova, J.; Patocka, J.; Musilek, K.; Korabecny, J.; Krusek, J.; Kaniakova, M.; Sepsova, V.; Mandikova, J.; et al. A resurrection of 7-MEOTA: A comparison with tacrine. Curr. Alzheimer Res. 2013, 10, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.; Lamba, D.; Zhang, L.; Lou, Y.; Xu, C.; Kang, D.; Chen, L.; Xu, Y.; Zhang, L.; De Simone, A.; et al. Novel tacrine-benzofuran hybrids as potent multitarget-directed ligands for the treatment of Alzheimer′s disease: Design, synthesis, biological evaluation, and X‑ray crystallography. J. Med. Chem. 2016, 59, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Grau, A.; Marco, J.L. Friedländer reaction on 2-amino-3-cyano-4H-pyrans: Synthesis of derivatives of 4H-pyran[2,3-b]quinoline, new tacrine analogues. Bioorg. Med. Chem. Lett. 1997, 7, 3165–3170. [Google Scholar] [CrossRef]
- Marco, J.L.; de los Ríos, C.; Carreiras, M.C.; Baños, J.E.; Badía, A.; Vivas, N.M. Synthesis and acetylcholinesterase/butyrylcholinesterase inhibition activity of new tacrine-like analogues. Bioorg. Med. Chem. 2001, 9, 727–732. [Google Scholar] [CrossRef]
- León, R.; Marco-Contelles, J.; García, A.G.; Villarroya, M. Synthesis, acetylcholinesterase inhibition and neuroprotective activity of new tacrine analogues. Bioorg. Med. Chem. 2005, 13, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Marco-Contelles, J.; León, R.; López, M.G.; García, A.G.; Villarroya, M. Synthesis and biological evaluation of new 4H-pyrano[2,3-b]quinoline derivatives that block acetylcholinesterase and cell calcium signals, and cause neuroprotection against calcium overload and free radicals. Eur. J. Med. Chem. 2006, 41, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Kaptein, B.; Kellogg, R.M.; van Bolhuis, F. Synthesis, molecular structure, and complexation of 1,4-dihydropyridines containing ligands for intramolecular complexation of metal electrophiles. Recl. Trav. Chim. Pays-Bas 1990, 109, 388–395. [Google Scholar] [CrossRef]
- Okubo, T.; Yoshikawa, R.; Chaki, S.; Okuyama, S.; Nakazato, A. Design, synthesis and structure-affinity relationships of aryloxyanilide derivatives as novel peripheral benzodiazepine receptor ligands. Bioorg. Med. Chem. 2004, 12, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Marco-Contelles, J.; Pérez-Mayoral, E.; Samadi, A.; Carreiras, M.C.; Soriano, E. Recent Advances in the Friedländer Reaction. Chem. Rev. 2009, 109, 2652–2671. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Fang, L.; Kraus, B.; Lehmann, J.; Heilmann, J.; Zhang, Y.; Decker, M. Design and synthesis of tacrine–ferulic acid hybrids as multi-potent anti-Alzheimer drug candidates. Bioorg. Med. Chem. Lett. 2008, 18, 2905–2909. [Google Scholar] [CrossRef] [PubMed]
- Cornish-Bowden, A. A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors (Short Communication). Biochem. J. 1974, 137, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]
- O′Brien, P.J.; Irwin, W.; Diaz, D.; Howard-Cofield, E.; Krejsa, C.M.; Slaughter, M.R.; Gao, B.; Kaludercic, N.; Angeline, A.; Bernardi, P.; et al. High concordance of drug-induced human hepatotoxicity with in vitro cytotoxicity measured in a novel cell-based model using high content screening. Arch. Toxicol. 2006, 80, 580–604. [Google Scholar] [CrossRef] [PubMed]
- Schoonen, W.G.E.J.; Westerink, W.M.A.; de Roos, J.A.D.M.; Débiton, E. Cytotoxic effects of 100 reference compounds on Hep G2 and HeLa cells and of 60 compounds on ECC-1 and CHO cells. I mechanistic assays on ROS, glutathione depletion and calcein uptake. Toxicol. In Vitro 2005, 19, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Dixon, M.; Webb, E.C. Enzymes, 2nd ed.; Longmans, Green and Co.: London, UK, 1964; pp. 54–166. [Google Scholar]
- Martínez, A. Emerging Drugs and Targets for Alzheimer′s Disease: Neuronal Plasticity, Neuronal Protection and Other Miscellaneous Strategies; Royal Society of Chemistry: London, UK, 2010; Volume 2, pp. 1–24. [Google Scholar]
- Grill, J.D.; Cummings, J.L. Current therapeutic targets for the treatment of Alzheimer′s disease. Expert Rev. Neurother. 2010, 10, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Haass, C. Semagacestat′s fall: Where next for AD therapies? Nat. Med. 2013, 19, 1214–1215. [Google Scholar]
- Lovestone, S.; Boada, M.; Dubois, B.; Hull, M.; Rinne, J.O.; Huppertz, H.-J.; Calero, M.; Andrés, M.V.; Gómez-Carrillo, B.; León, T.; et al. A phase II trial of tideglusib in Alzheimer′s disease. J. Alzheimer′s Dis. 2015, 45, 75–88. [Google Scholar]
- Benchekroun, M.; Bartolini, M.; Egea, J.; Romero, A.; Soriano, E.; Pudlo, M.; Luzet, V.; Andrisano, V.; Jimeno, M.L.; López, M.G.; et al. Novel tacrine-grafted Ugi adducts as multipotent anti-Alzheimer drugs: a synthetic renewal in tacrine-ferulic acid hybrids. Chem. Med. Chem. 2015, 10, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Nepovimova, E.; Uliassi, E.; Korabecny, J.; Pena-Altamira, L.E.; Samez, S.; Pesaresi, A.; Garcia, G.E.; Bartolini, M.; Andrisano, V.; Bergamini, C.; et al. Multitarget drug design strategy: quinone-tacrine hybrids designed to block amyloid-beta aggregation and to exert anticholinesterase and antioxidant effects. J. Med. Chem. 2014, 57, 8576–8589. [Google Scholar] [CrossRef] [PubMed]
- Esquivias-Pérez, M.; Maalej, E.; Romero, A.; Chabchoub, F.; Samadi, A.; Marco-Contelles, J.; Oset-Gasque, M.J. Nontoxic and neuroprotective β-naphthotacrines for Alzheimer′s disease. Chem. Res. Toxicol. 2013, 26, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, J.; Fang, L.; Liu, M.; Peng, S.; Liao, H.; Lehmann, J.; Zhang, Y. Tacrine-ferulic acid-nitric oxide (NO) donor trihybrids as potent, multifunctional acetyl- and butyrylcholinesterase inhibitors. J. Med. Chem. 2012, 55, 4309–4321. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bachiller, M.I.; Pérez, C.; González-Muñoz, G.C.; Conde, S.; López, M.G.; Villarroya, M.; García, A.G.; Rodríguez-Franco, M.I. Novel tacrine-8-hydroxyquinoline hybrids as multifunctional agents for the treatment of Alzheimer′s disease, with neuroprotective, cholinergic, antioxidant, and copper-complexing properties. J. Med. Chem. 2010, 53, 4927–4937. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of all compounds (1)–(21) are available from the authors.


| Compound | EeAChE a IC50 Values (μM) | eqBuChE b % Inhibition at 10 μM | ORAC d |
|---|---|---|---|
| 1 | 12.9 ± 0.3 | 25.6 ± 0.7 | 0.34 ± 0.03 |
| 2 | 11.5 ± 0.4 | 34.0 ± 0.5 | 1.79 ± 0.17 |
| 3 | 432 ± 84 | 32.2 ± 0.9 | 1.99 ± 0.23 |
| 4 | 38.7 ± 1.7 | 24.9 ± 0.9 | 2.31 ± 0.29 |
| 5 | 158 ± 33 | 12.9 ± 0.5 | 1.70 ± 0.33 |
| 6 | 37.8 ± 1.2 | 33.5 ± 1.7 | 2.34 ± 0.31 |
| 7 | 8.41 ± 0.14 | 50.5 ± 7.3 | 1.47 ± 0.13 |
| 8 | 6.73 ± 0.52 | 51.6 ± 0.6 | 1.88 ± 0.01 |
| 9 | na | 15.1 ± 4.7 | 1.35 ± 0.13 |
| 10 | 10.7 ± 0.2 | 25.0 ± 0.2 | 2.75 ± 0.09 |
| 11 | na | 20.3 ± 3.7 | 2.33 ± 0.13 |
| 12 | 15.3 ± 0.6 | 27.5 ± 1.1 | 1.88 ± 0.07 |
| 13 | 30.0 ± 2.0 | 75.7 ± 2.3 | 2.25 ± 0.29 |
| Tacrine | 0.0898 ± 0.0022 | 0.005 ± 0.001 a,c | 0.20 ± 0.04 [28] |
| Compounds | 1 µM | 3 µM | 10 µM | 30 µM | 100 µM | 300 µM | 1000 µM |
|---|---|---|---|---|---|---|---|
| Tacrine | 95.8 ± 2.2 | 100.4 ± 2.2 | 96.9 ± 3.9 | 97.7 ± 3.9 | 98.6 ± 3.8 | 39.2 ± 4.7 *** | 20.0 ± 2.1 *** |
| 10 | 101.8 ± 4.2 | 100.7 ± 2.6 | 100.5 ± 1.9 | 71.4 ± 7.4 * | 63.5 ± 6.2 * | 64.8 ± 5.7 * | 66.8 ± 4.1 * |
| 8 | 108.1 ± 5.4 | 101.3 ± 10.8 | 79.0 ± 9.1 | 57.6 ± 4.2 ** | 52.7 ± 3.3 ** | 51.2 ± 2.8 ** | 66.2 ± 6.8 * |
| 2 | 102.7 ± 3.7 | 103.2 ± 4.4 | 101.3 ± 3.9 | 99.2 ± 2.6 | 93.0 ± 4.7 | 87.5 ± 3.2 | 58.8 ± 2.1 * |
| 4 | 107.7 ± 5.1 | 105.3 ± 2.9 | 102.1 ± 2.9 | 98.0 ± 3.1 | 104.6 ± 4.5 | 97.1 ± 3.0 | 96.8 ± 1.5 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boulebd, H.; Ismaili, L.; Bartolini, M.; Bouraiou, A.; Andrisano, V.; Martin, H.; Bonet, A.; Moraleda, I.; Iriepa, I.; Chioua, M.; et al. Imidazopyranotacrines as Non-Hepatotoxic, Selective Acetylcholinesterase Inhibitors, and Antioxidant Agents for Alzheimer's Disease Therapy. Molecules 2016, 21, 400. https://doi.org/10.3390/molecules21040400
Boulebd H, Ismaili L, Bartolini M, Bouraiou A, Andrisano V, Martin H, Bonet A, Moraleda I, Iriepa I, Chioua M, et al. Imidazopyranotacrines as Non-Hepatotoxic, Selective Acetylcholinesterase Inhibitors, and Antioxidant Agents for Alzheimer's Disease Therapy. Molecules. 2016; 21(4):400. https://doi.org/10.3390/molecules21040400
Chicago/Turabian StyleBoulebd, Houssem, Lhassane Ismaili, Manuela Bartolini, Abdelmalek Bouraiou, Vincenza Andrisano, Helene Martin, Alexandre Bonet, Ignacio Moraleda, Isabel Iriepa, Mourad Chioua, and et al. 2016. "Imidazopyranotacrines as Non-Hepatotoxic, Selective Acetylcholinesterase Inhibitors, and Antioxidant Agents for Alzheimer's Disease Therapy" Molecules 21, no. 4: 400. https://doi.org/10.3390/molecules21040400







