Extraction Optimization, Purification and Physicochemical Properties of Polysaccharides from Gynura medica
Abstract
:1. Introduction
2. Results and Discussion
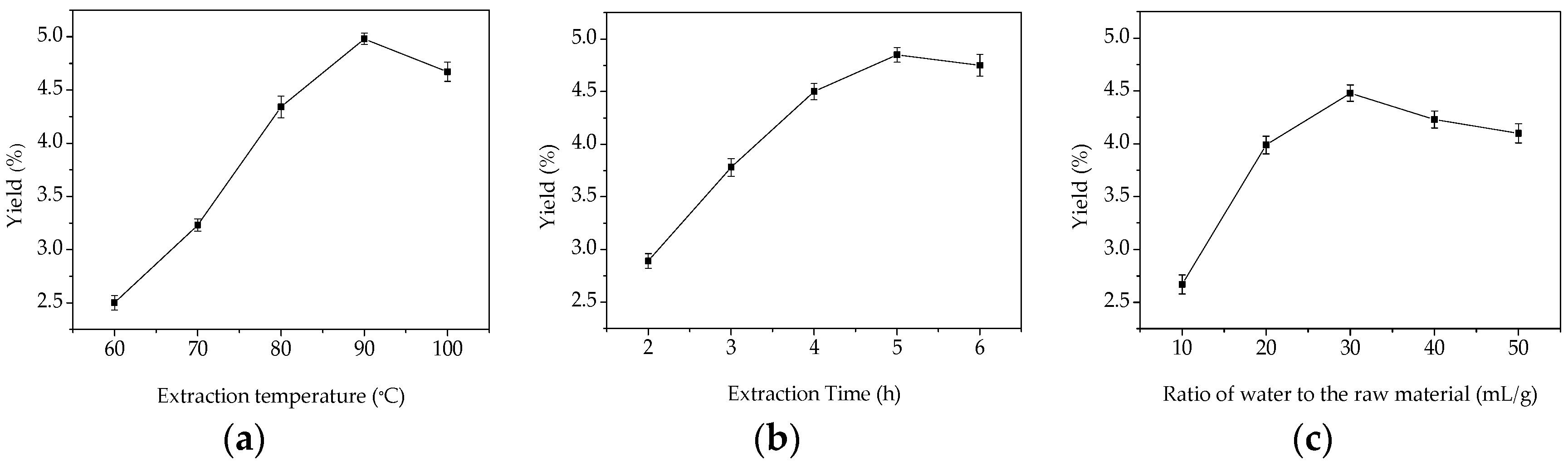
2.1. Single-Factor Experimental Analysis
2.2. Optimization of the GMPs Extraction Conditions
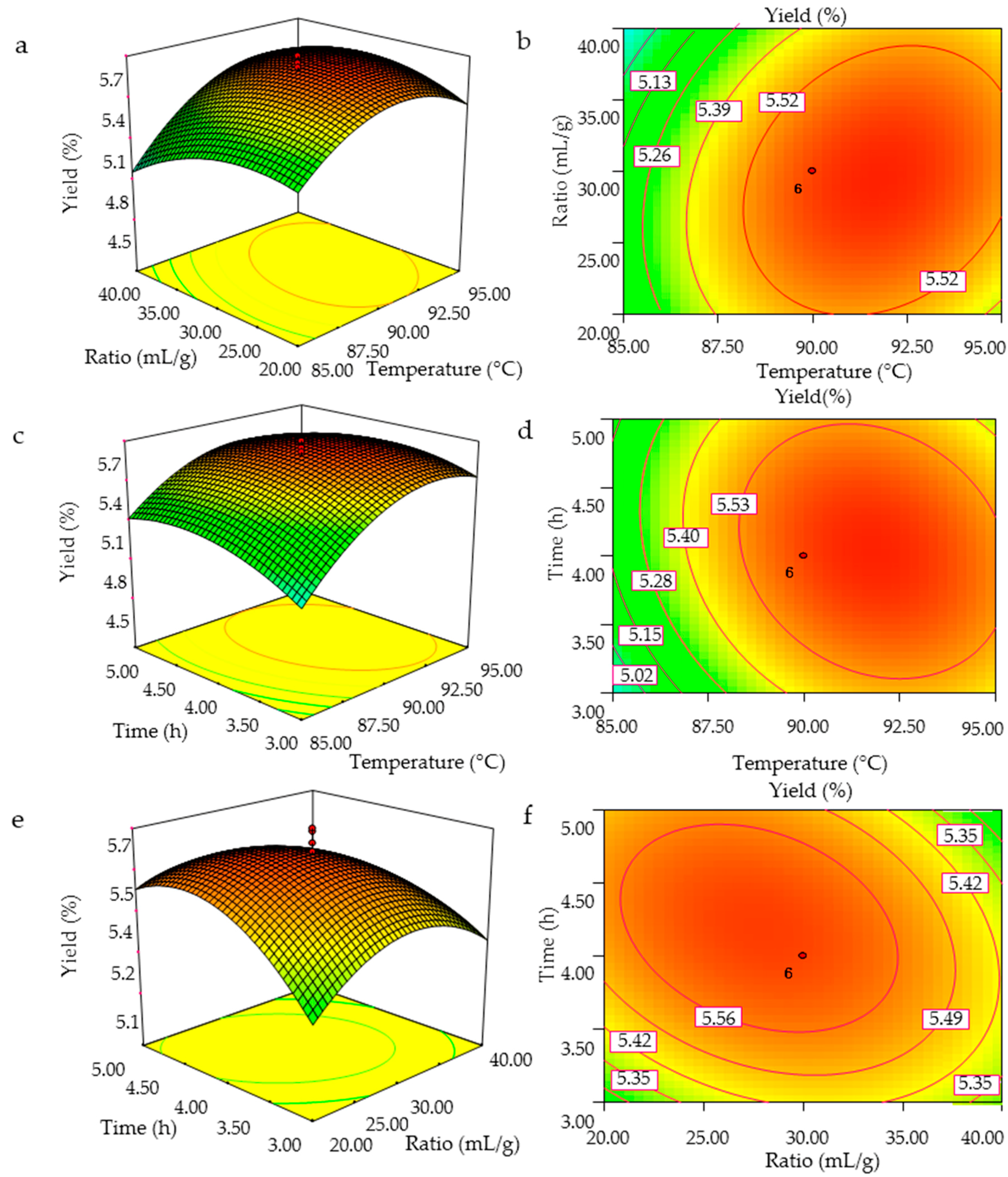
2.2.1. Predicted Model and Statistical Analysis
2.2.2. Optimization of Extraction Procedure
2.2.3. Verification of the Model
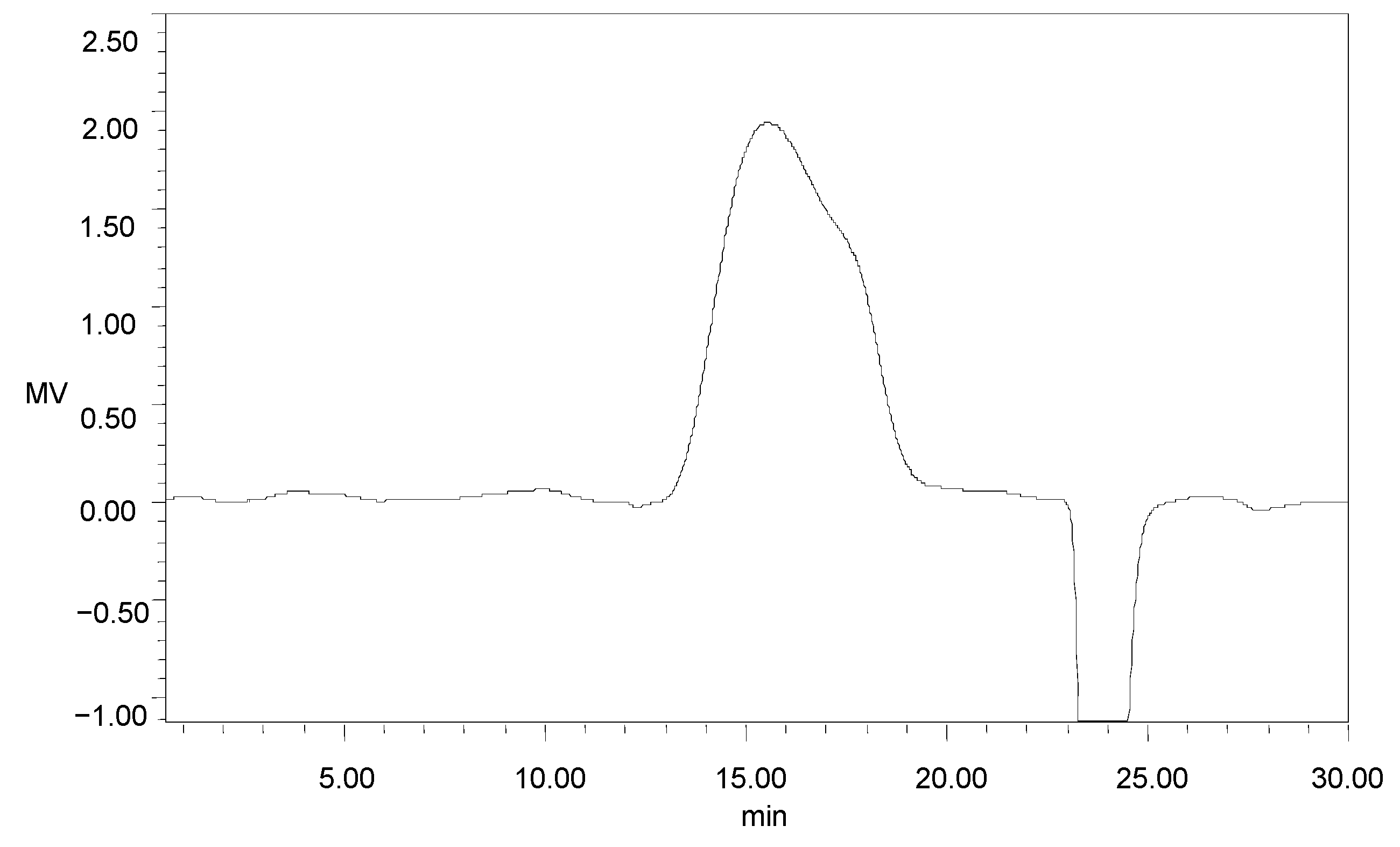
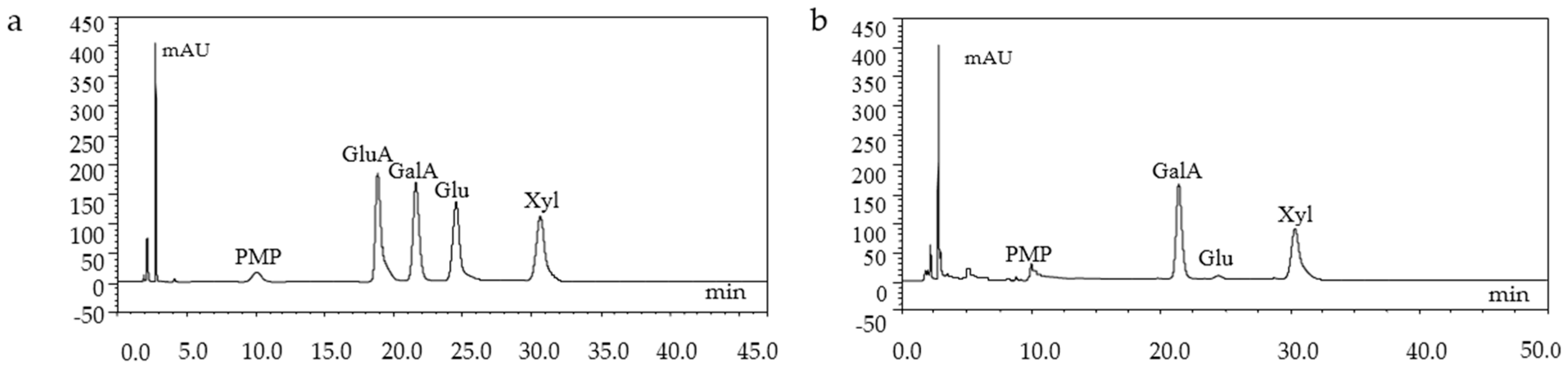
2.3. Chemical Characterization of GMP-1
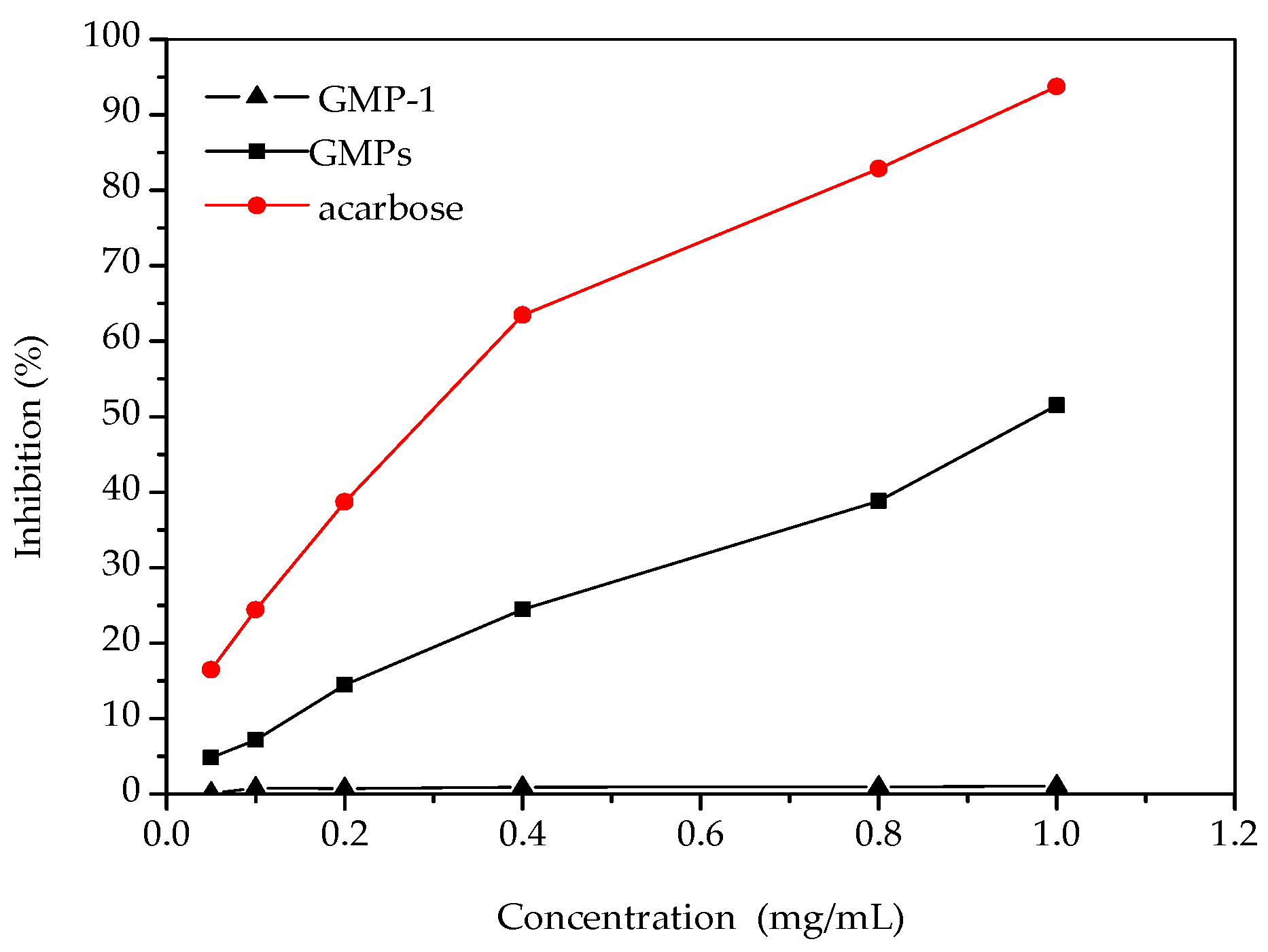
2.4. Results of α-Glycosidase Inhibitory Activities
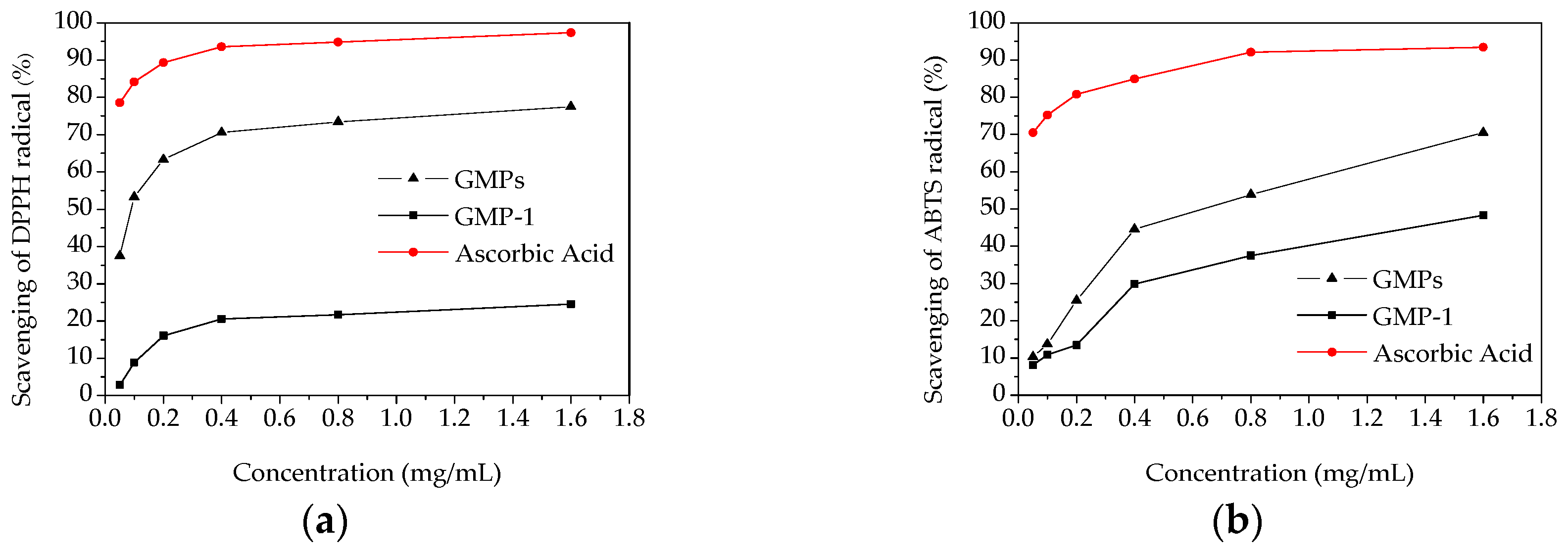
2.5. In Vitro Antioxidant Activity
3. Materials and Methods
3.1. Materials and Reagents
3.2. Extraction of Polysaccharides
3.3. Experimental Design
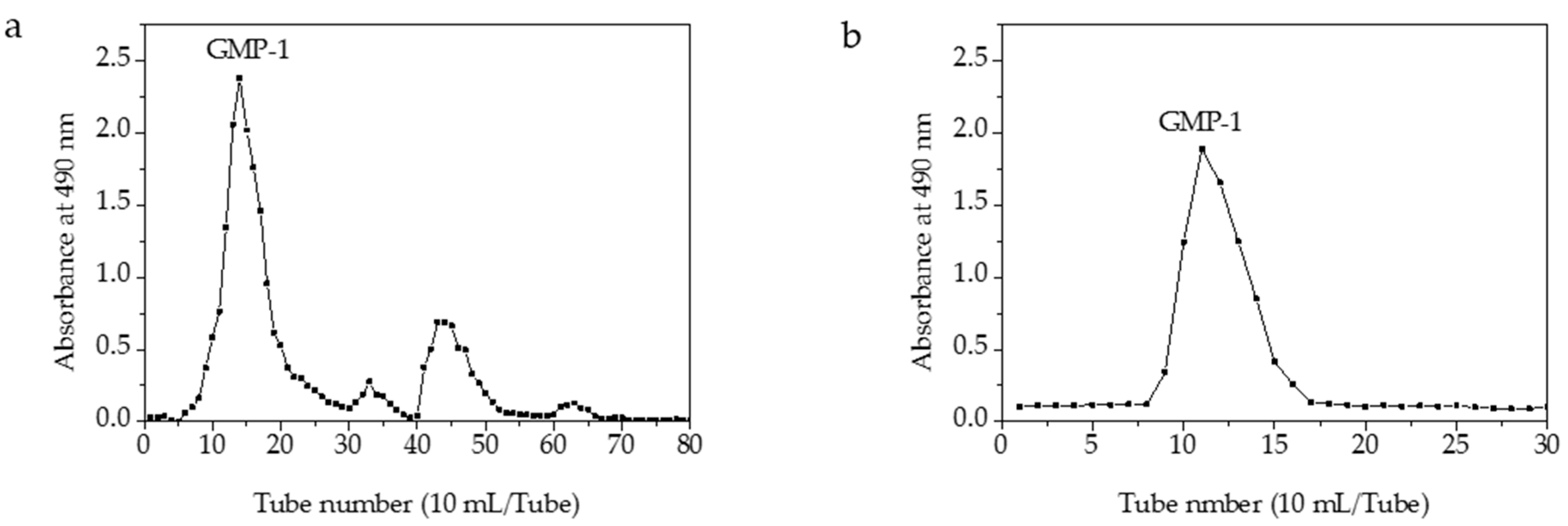
3.4. Purification of Crude Polysaccharides
3.5. Chemical Characterization of GMP-1
3.6. α-Glucosidase Inhibitory Activity
3.7. In Vitro Antioxidant Activity
3.7.1. DPPH Radical Scavenging Assay
3.7.2. ABTS Radical Scavenging Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
References
- Chen, L.; Li, H.Q.; Song, H.T.; Zhang, G.G. A new cerebroside from Gynura divaricata. Fitoterapia 2009, 80, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yu, Y.Y.; Yang, R.Z.; Wan, C.P.; Xu, B.B.; Cao, S.W. Optimization of total flavonoid compound extraction from Gynura. medica leaf using response surface methodology and chemical composition analysis. Int. J. Mol. Sci. 2010, 11, 4750–4763. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.Z.; Tan, L.; Jin, C.G.; Lu, J.; Tian, L.; Chang, Q.Q.; Wang, K. Extraction, isolation, characterization and antioxidant activity of polysaccharides from Astragalus membranaceus. Ind. Crops Prod. 2015, 77, 434–443. [Google Scholar] [CrossRef]
- Cui, J.; Gu, X.; Wang, F.J.; Yang, J.O.; Wang, J.Z. Purification and structural characterization of an α-glucosidase inhibitory polysaccharide from apricot (Armeniaca sibirica L. Lam.) pulp. Carbohydr. Polym. 2015, 121, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Fatimawati, S.; Shimizu, K.; Kondo, R. Ganoderol B: A potent α-glucosidase inhibitor isolated from the fruiting body of Ganoderma lucidum. Phytomedicine 2011, 18, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.K.; Hsu, T.H.; Lin, F.Y.; Cheng, Y.K.; Yang, J.P. Separation, purification, and α-glucosidase inhibition of polysaccharides from Coriolus versicolor LH1 mycelia. Carbohydr. Polym. 2013, 92, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Kant, R.; Kumar, V. α-D-Glucosidase inhibitory activity of polysaccharide isolated from Acacia tortilis gum exudates. Int. J. Biol. Macromol. 2013, 59, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, G.L.; Zhang, J.; Jia, S.Y.; Li, F.; Wang, Y.Y.; Wu, S.H. Response surface optimization of ultrasound-assisted enzymatic extraction polysaccharides from Lycium barbarum. Carbohydr. Polym. 2014, 110, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.R.; Gu, X.H.; Tang, H. Extraction purification and characterization of the polysaccharides from Opuntia milpa alta. Carbohydr. Polym. 2008, 71, 403–410. [Google Scholar] [CrossRef]
- Gan, C.Y.; Manaf, N.H.A.; Latiff, A.A. Optimization of alcohol insoluble polysaccharides (AIPS) extraction from the Parkia speciosa pod using response surface methodology (RSM). Carbohydr. Polym. 2010, 79, 825–831. [Google Scholar] [CrossRef]
- Tahmouzi, S. Optimization of polysaccharides from Zagros oak leaf using RSM: Antioxidant and antimicrobial activities. Carbohydr. Polym. 2014, 106, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, H.; Tu, D.W.; Yang, Y.; Zhan, Y. Extraction optimization, preliminary characterization, and in vitro antioxidant activities of crude polysaccharides from finger citron. Ind. Crops Prod. 2013, 44, 145–151. [Google Scholar] [CrossRef]
- Ye, C.L.; Jiang, C.J. Optimization of extraction process of crude polysaccharides from Plantago asiatica L. by response surface methodology. Carbohydr. Polym. 2011, 84, 495–502. [Google Scholar] [CrossRef]
- Liu, J.L.; Zheng, S.L.; Fan, Q.J.; Yuan, J.C.; Yang, S.M.; Kong, F.L. Optimisation of high-pressure ultrasonic-assisted extraction and antioxidant capacity of polysaccharides from the rhizome of Ligusticum chuanxiong. Int. J. Biol. Macromol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Zhang, Q.; Li, Y.F.; Dong, L.L.; Liu, S.L. Optimization of ultrasound extraction of Alisma orientalis polysaccharides by response surface methodology and their antioxidant activities. Carbohydr. Polym. 2015, 119, 101–109. [Google Scholar]
- Thangam, R.; Suresh, V.; Kannan, S. Optimized extraction of polysaccharides from Cymbopogon citratus and its biological activities. Int. J. Biol. Macromol. 2014, 65, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.P.; Zhai, X.C.; Li, L.Q.; Wu, X.X.; Li, B. Response surface optimization of ultrasound-assisted polysaccharides extraction from pomegranate peel. Food Chem. 2015, 177, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.Z.; Li, H.P.; Li, S.Z.; Jin, C.G.; Lu, J. Extraction optimization, preliminary characterization and immunological activity of polysaccharides from figs. Int. J. Biol. Macromol. 2015, 72, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.F.; Zhou, X.; Chen, H.G. Optimization of enzyme assisted extraction of Fructus mori polysaccharides and its activities on antioxidant and alcohol dehydrogenase. Carbohydr. Polym. 2014, 111, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Wang, F.; Ma, X.Q.; Sun, S.C.; Leng, F.F.; Zhang, W.J.; Wang, X.L. Extraction, purification, characterization and antioxidant activity of polysaccharides from Piteguo fruit. Ind. Crops Prod. 2015, 77, 467–475. [Google Scholar] [CrossRef]
- Ren, A.N.; Lu, Y.; Zou, Y.F.; Shen, H. Separation, purification and preliminary structure analysis of acidic polysaccharides from safflower. Eur. Food Res. Technol. 2013, 237, 449–455. [Google Scholar] [CrossRef]
- You, Q.H.; Yin, X.L.; Zhang, S.N. Extraction, purification, and antioxidant activities of polysaccharides from Tricholoma mongolicum Imai. Carbohydr. Polym. 2014, 99, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wu, J.; Zhang, Y.; Chen, H.; Wang, Y.F. Physicochemical characterization of puerh tea polysaccharides and their antioxidant and α-glycosidase inhibition. J. Funct. Foods 2014, 545–554. [Google Scholar] [CrossRef]
- Satoh, T.; Igarashi, M.; Yamada, S.; Takahashi, N.; Watanabe, K. Inhibitory effect of black tea and its combination with acarbose on small intestinal α-glucosidase activity. J. Ethnopharmacol. 2015, 161, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Q.; Zhang, L.; Yang, Y.; Song, X.M.; Yu, Z.Y. Optimization of ultrasound-assisted compound enzymatic extraction and characterization of polysaccharides from blackcurrant. Carbohydr. Polym. 2015, 117, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Kong, L.C.; Yin, C.P.; Jiang, D.H.; Jiang, J.Q.; He, J.; Xiao, W.X. Extraction optimization by response surface methodology, purification and principal antioxidant metabolites of red pigments extracted from bayberry (Myrica rubra) pomace. LWT Food Sci. Technol. 2013, 51, 343–347. [Google Scholar] [CrossRef]
- Wang, B.S.; Li, B.S.; Zeng, Q.X.; Liu, H.X. Antioxidant and free radical scavenging activities of pigments extracted from molasses alcohol wastewater. Food Chem. 2008, 107, 1198–1204. [Google Scholar] [CrossRef]
- Cheng, Z.Y.; Song, H.Y.; Yang, Y.J.; Liu, Y.; Liu, Z.G.; Hu, H.B.; Zhang, Y. Optimization of microwave-assisted enzymatic extraction of polysaccharides from the fruit of Schisandra chinensis Baill. Ind. Crops Prod. 2015, 76, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ghavi, P.P. The extraction process optimization of antioxidant polysaccharides from Marshmallow (Althaea officinalis L.) roots. Int. J. Biol. Macromol. 2015, 75, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Yang, Y.N.; Hu, X.S.; Zhang, Z.Y. Effect of ultrasonic extraction conditions on antioxidative and immunomodulatory activities of a Ganoderma lucidum polysaccharide originated from fermented soybean curd residue. Food Chem. 2014, 155, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; Lv, G.Y.; He, W.Q.; Shi, L.G.; Pan, H.J.; Fan, L.F. Effects of extraction methods on the antioxidant activities of polysaccharides obtained from Flammulina velutipes. Carbohydr. Polym. 2013, 98, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Shen, S.A.; Chen, D.J.; Li, X.; Li, T.; Yuan, M.; Zhou, Y.H.; Ding, C.B. Optimization of extraction process and antioxidant activity of polysaccharides from leaves of Paris polyphylla. Carbohydr. Polym. 2014, 104, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Fu, X.; Huang, Q.; Luo, F.X.; You, L.J. Ultrasonic extraction and structural identification of polysaccharides from Prunella vulgaris and its antioxidant and antiproliferative activities. Eur. Food. Res. Technol. 2015, 240, 49–60. [Google Scholar] [CrossRef]
- Fang, X.B.; Yin, X.X.; Yuan, G.F.; Chen, X. Chemical and biological characterization of polysaccharides from the bark of Avicennia marina. Eur. Food. Res. Technol. 2015, 241, 17–25. [Google Scholar] [CrossRef]
- Zhang, T.; Tian, Y.; Jiang, B.; Miao, M.; Mu, W.M. Purification, preliminary structural characterization and in vitro antioxidant activity of polysaccharides from Acanthus ilicifolius. LWT Food Sci. Technol. 2014, 56, 9–14. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Yin, Q.S.; Han, T.; Zhao, Y.X.; Su, J.J.; Li, M.Z.; Ling, J.Y. Purification and antioxidant effect of novel fungal polysaccharides from the stroma of Cordyceps kyushuensis. Ind. Crops Prod. 2015, 69, 485–491. [Google Scholar] [CrossRef]
- Ren, S.; Xu, D.D.; Pan, Z.; Gao, Y.; Jiang, Z.G.; Gao, Q.P. Two flavanone compounds from litchi (Litchi chinensis Sonn.) seeds, one previously unreported, and appraisal of their α-glucosidase inhibitory activities. Food Chem. 2011, 127, 1760–1763. [Google Scholar] [CrossRef]
- Ganesh, C.; Mongolla, P.; Pombala, S.; Kamle, A.; Joseph, J. Physicochemical characterization and antioxidant activity of melanin from a novel strain of Aspergillus bridgeri ICTF-201. Lett. Appl. Microbiol. 2011, 53, 350–358. [Google Scholar]
- Smith, H.; Doyle, S.; Murphy, R. Filamentous fungi as a source of natural antioxidants. Food Chem. 2015, 185, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds GMPs and GMP-1 are available from the authors.

| Run | X1 | X2 | X3 | Yield (%) |
|---|---|---|---|---|
| 1 | 90.0 | 30.0 | 4.0 | 5.62 |
| 2 | 95.0 | 40.0 | 3.0 | 5.33 |
| 3 | 81.59 | 30.0 | 4.0 | 4.52 |
| 4 | 90.0 | 30.0 | 4.0 | 5.65 |
| 5 | 90.0 | 30.0 | 4.0 | 5.49 |
| 6 | 85.0 | 20.0 | 5.0 | 5.30 |
| 7 | 95.0 | 20.0 | 5.0 | 5.29 |
| 8 | 95.0 | 20.0 | 3.0 | 5.25 |
| 9 | 85.0 | 20.0 | 3.0 | 4.83 |
| 10 | 85.0 | 40.0 | 5.0 | 4.67 |
| 11 | 90.0 | 30.0 | 4.0 | 5.70 |
| 12 | 90.0 | 30.0 | 2.32 | 5.11 |
| 13 | 90.0 | 13.18 | 4.0 | 5.12 |
| 14 | 90.0 | 30.0 | 5.68 | 5.23 |
| 15 | 85.0 | 40.0 | 3.0 | 4.68 |
| 16 | 98.41 | 30.0 | 4.0 | 5.19 |
| 17 | 90.0 | 46.82 | 4.0 | 5.48 |
| 18 | 90.0 | 30.0 | 4.0 | 5.57 |
| 19 | 95.0 | 40.0 | 5.0 | 5.15 |
| 20 | 90.0 | 30.0 | 4.0 | 5.69 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | f Value | p Value (Prob. > F) |
|---|---|---|---|---|---|
| Model | 2.18 | 9 | 0.24 | 25.01 | <0.0001 a |
| X1 | 0.52 | 1 | 0.52 | 53.01 | <0.0001 a |
| X2 | 0.038 | 1 | 0.038 | 3.94 | 0.0753 |
| X3 | 0.020 | 1 | 0.020 | 2.06 | 0.1821 |
| X1X2 | 0.065 | 1 | 0.065 | 6.68 | 0.0272 b |
| X1X3 | 0.045 | 1 | 0.045 | 4.64 | 0.0566 |
| X2X3 | 0.061 | 1 | 0.061 | 6.32 | 0.0307 b |
| X12 | 1.00 | 1 | 1.00 | 102.63 | <0.0001 a |
| X22 | 0.35 | 1 | 0.35 | 36.50 | 0.0001 a |
| X32 | 0.33 | 1 | 0.33 | 34.07 | 0.0002 a |
| Residual | 0.097 | 10 | 9.696 × 10−3 | ||
| Lack of Fit | 0.065 | 5 | 0.013 | 2.07 | 0.2220 ns |
| Pure Error | 0.032 | 5 | 6.320 × 10−3 | ||
| Cor. Total | 2.28 | 19 | |||
| R2 | 0.9575 | ||||
| Adj. R2 | 0.9192 | ||||
| Pred. R2 | 0.7565 | ||||
| Adeq. precision | 15.391 | ||||
| C.V.% | 1.88 |
| Independent Variables | Level | ||||
|---|---|---|---|---|---|
| −1.682 | −1 | 0 | 1 | 1.682 | |
| Extraction temperature (X1, °C) | 81.59 | 85 | 90 | 95 | 98.41 |
| Ratio of water to the raw material (X2, mL/g) | 13.18 | 20 | 30 | 40 | 46.82 |
| Extraction time (X3, h) | 2.318 | 3 | 4 | 5 | 5.682 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Gao, J.; Xue, F.; Yu, X.; Shao, T. Extraction Optimization, Purification and Physicochemical Properties of Polysaccharides from Gynura medica. Molecules 2016, 21, 397. https://doi.org/10.3390/molecules21040397
Li F, Gao J, Xue F, Yu X, Shao T. Extraction Optimization, Purification and Physicochemical Properties of Polysaccharides from Gynura medica. Molecules. 2016; 21(4):397. https://doi.org/10.3390/molecules21040397
Chicago/Turabian StyleLi, Fengwei, Jian Gao, Feng Xue, Xiaohong Yu, and Tao Shao. 2016. "Extraction Optimization, Purification and Physicochemical Properties of Polysaccharides from Gynura medica" Molecules 21, no. 4: 397. https://doi.org/10.3390/molecules21040397





