Optimization of Betulinic Acid Extraction from Tecomella undulata Bark Using a Box-Behnken Design and Its Densitometric Validation
Abstract
:1. Introduction
2. Results and Discussion
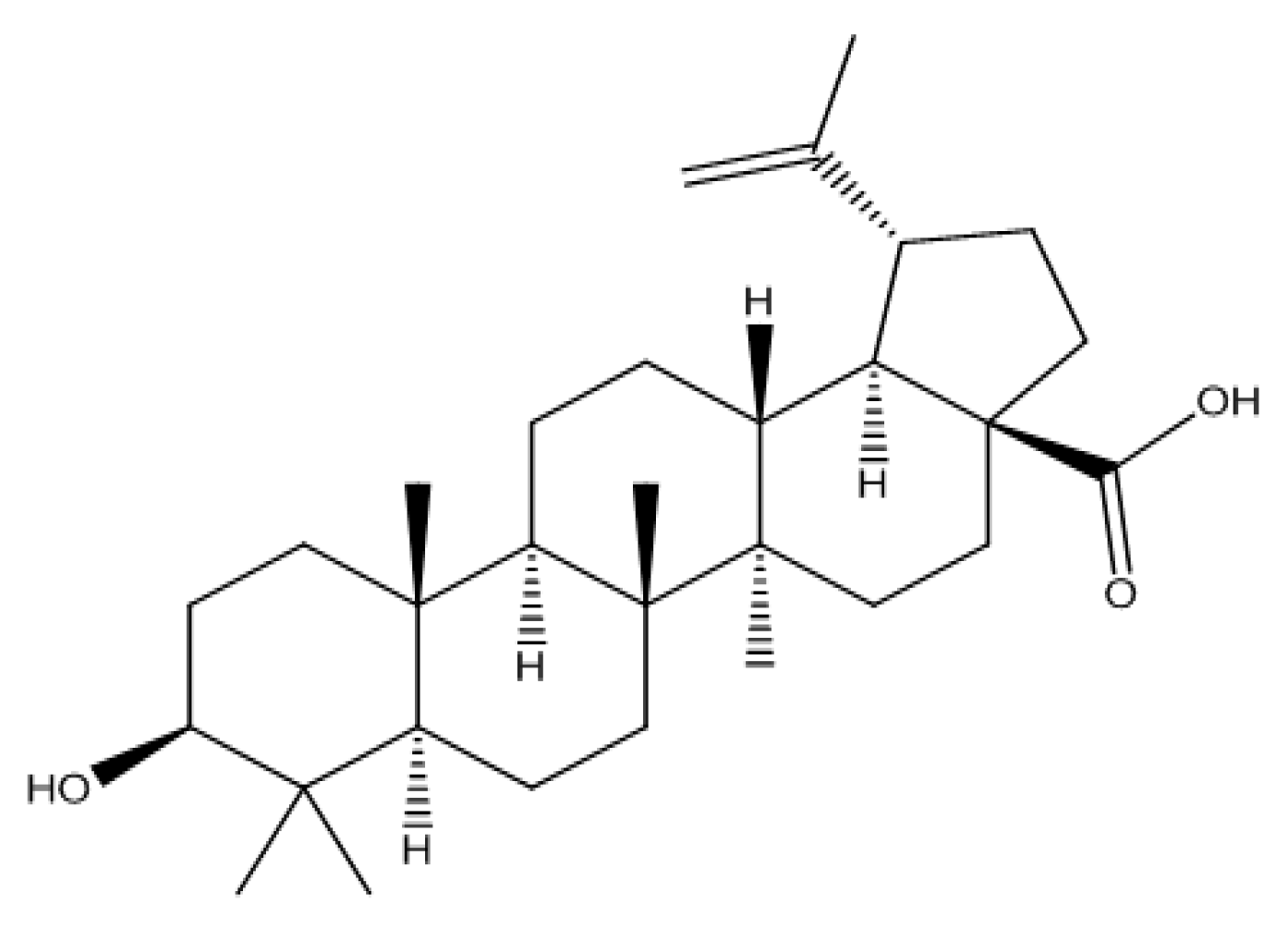
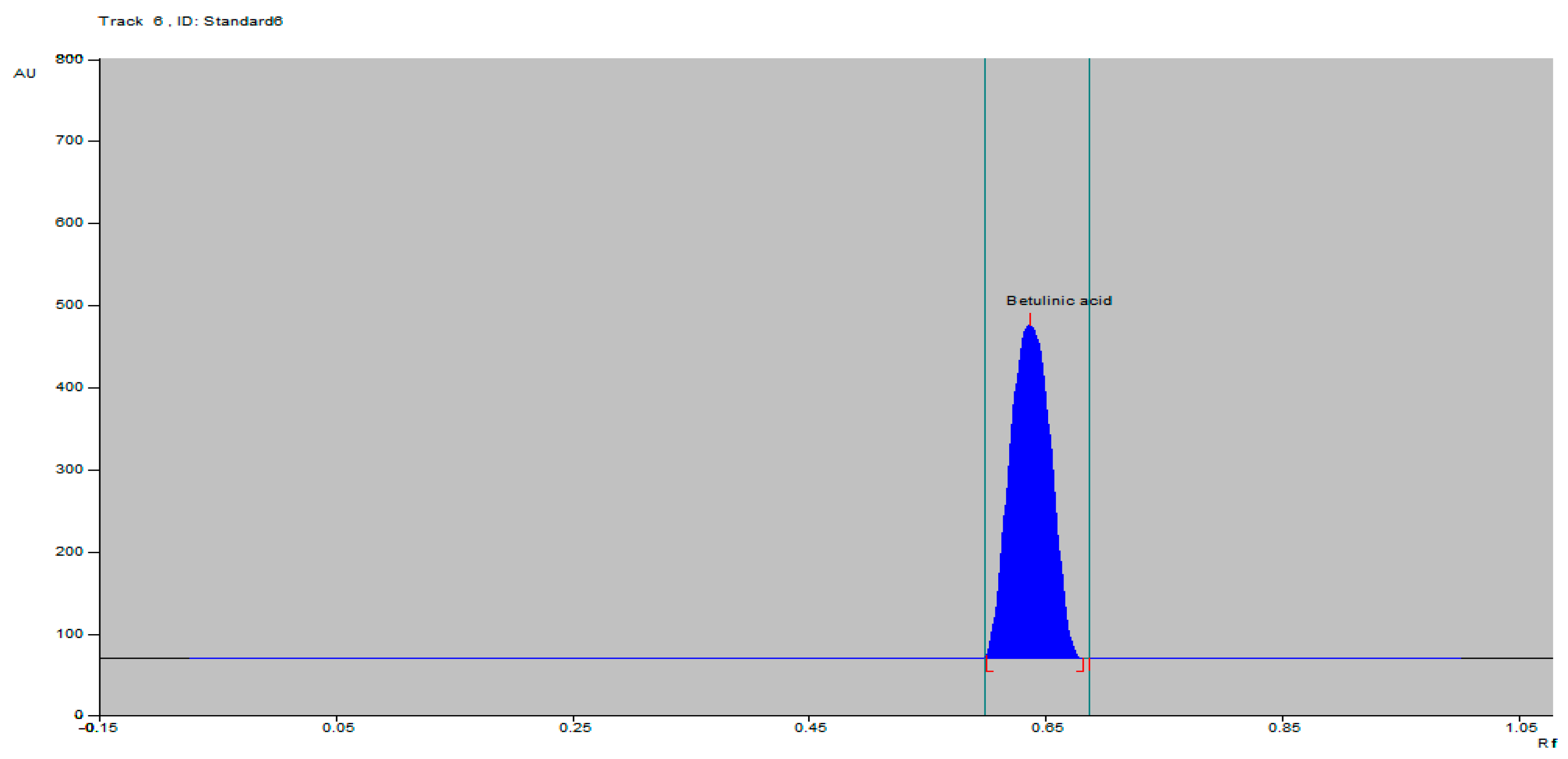
2.1. HPTLC-VIS Analysis of BA
2.2. Model Fitting
2.3. Effect of Extraction Parameters on BA Yield of Tecomella Undulata and RSM Analysis
2.4. RSM Validation
2.5. Optimization and Verification of the Model for Extraction Parameters
3. Experimental Section
3.1. Plant Material and Chemicals
3.2. Soxhlet Extraction of BA
3.3. Experimental Design
3.4. HPTLC-VIS Analysis of BA
3.5. Calibration Curve Preparation
3.6. Validation
3.6.1. Accuracy
3.6.2. Precision
3.6.3. Robustness
3.6.4. Limit of Detection (LOD) and Limit of Quantification (LOQ)
3.6.5. Specificity
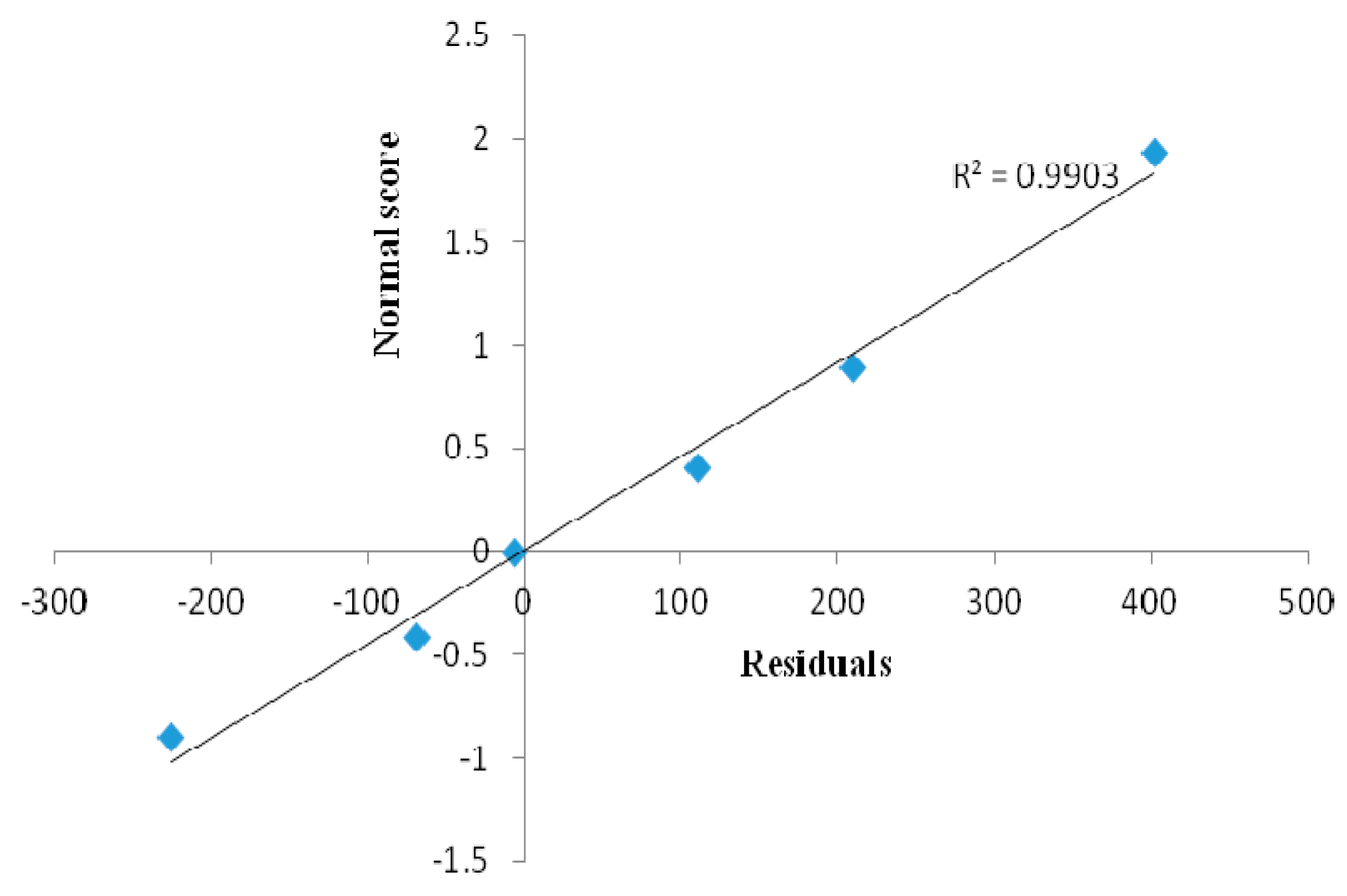
3.7. RSM Model and Validity Testing
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yun, Y.; Han, S.; Park, E.; Yim, D.; Lee, S.; Lee, C.K.; Cho, K.; Kim, K. Immunomodulatory activity of betulinic acid by producing pro-inflammatory cytokines and activation of macrophages. Arch. Pharm. Res. 2003, 26, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Perumal, Y.; Dharmarajan, S. Betulinic acid and its derivatives: A review on their biological properties. Curr. Med. Chem. 2005, 12, 657–666. [Google Scholar]
- The Ayurvedic Pharmacopoeia of India, 1st ed.Ministry of Health and Family Welfare, Government of India: New Delhi, India, 2008; pp. 58–59.
- Jain, M.; Kapadia, R.; Jadeja, R.N.; Thounaojam, M.C.; Devkar, R.V.; Mishra, S.H. Hepatoprotective potential of Tecomell aundulata stem bark is partially due to the presence of betulinic acid. J. Ethnopharmacol. 2012, 143, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, A.S.; Szerszen, M.K. Protective effects of betulin and betulinic acid against ethanol-induced cytotoxicity in HepG2 cells. Pharmacol. Rep. 2005, 57, 588–595. [Google Scholar]
- Ciesielska, A.S.; Plewka, K.; Daniluk, J.; Szerszen, M.K. Betulin and betulinic acid attenuate ethanol-induced liver stellate cell activation by inhibiting reactive oxygen species (ROS), cytokine (TNF-α, TGF-β) production and by influencing intracellular signalling. Toxicology 2011, 280, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, A.M. Hepatoprotective triterpenes from hairy root cultures of Ocimum basilicum L. J. Biosci. 2009, 64, 201–209. [Google Scholar] [CrossRef]
- Quan, H.Y.; Kim, D.Y.; Kim, S.J.; Jo, H.K.; Kim, G.W.; Chung, S.H. Betulinic acid alleviates non-alcoholic fatty liver by inhibiting SREBP1 activity via the AMPK–mTOR–SREBP signaling pathway. Biochem. Pharmacol. 2013, 85, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Yi, j.; Xia, W.; Wu, J.; Yuan, L.; Wu, J.; Tu, D.; Fang, J.; Tan, Z. Betulinic acid prevents alcohol-induced liver damage by improving the antioxidant system in mice. J. Vet. Sci. 2014, 15, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Genet, C.; Strehle, A.; Schmidt, C.; Boudjelal, G.; Lobstein, A.; Schoonjans, K.; Souchet, M.; Auwerx, J.; Saladin, R.; Wagner, A. Structure-activity relationship study of betulinic acid, a novel and selective TGR5 agonist, and its synthetic derivatives: potential impact in diabetes. J. Med. Chem. 2010, 53, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.S.; Kim, C.S.; Kim, J.S. Betulinic acid has an inhibitory effect on pancreatic lipase and induces adipocyte lipolysis. Phytother. Res. 2012, 26, 1103–1106. [Google Scholar] [CrossRef] [PubMed]
- Viji, V.; Shobha, B.; Kavitha, S.K.; Ratheesh, M.; Kripa, K.; Helen, A. Betulinic acid isolated from Bacopa monniera (L.) Wettst suppresses lipopolysaccharide stimulated interleukin-6 production through modulation of nuclear factor-κB in peripheral blood mononuclear cells. Int. Immunopharmacol. 2010, 10, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Chintharlapalli, S.; Papineni, S.; Ramaiah, S.K.; Safe, S. Betulinic acid inhibits prostate cancer growth through inhibition of specificity protein transcription factors. Cancer Res. 2007, 67, 2816–2823. [Google Scholar] [CrossRef] [PubMed]
- Dorina, G.; Oana, D.; Cristina, D.; Codruta, S.; Danina, M. Betulinic acid as a potent and complex antitumor phytochemical: A mini review. Anticancer Agents. Med. Chem. 2014, 14, 936–945. [Google Scholar]
- Chandramu, C.; Manohar, R.D.; Krupadanam, D.G.L.; Dashavantha, R.V. Isolation, characterization and biological activity of betulinic acid and ursolic acid from Vitex negundo L. Phytother. Res. 2003, 17, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends. Food. Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Yin, G.H.; Dang, Y.L. Optimization of extraction technology of the Lycium barbarum polysaccharides by Box–Behnken statistical design. Carbohydr. Polym. 2008, 74, 603–610. [Google Scholar] [CrossRef]
- Pandey, D.K.; Banik, R.M. Optimization of extraction conditions for colchicines from Gloriosa superb tubers using response surface methodology. J. Agric. Technol. 2012, 8, 1301–1315. [Google Scholar]
- Zhao, L.; Liang, J.; Li, W.; Cheng, K.; Xia, X.; Deng, X.; Yang, G. The use of response surface methodology to optimize the ultrasound-assisted extraction of five anthraquinones from Rheum palmatum L. Molecules 2011, 16, 5928–5937. [Google Scholar] [CrossRef] [PubMed]
- Cacace, J.E.; Mazza, G. Optimization of extraction of anthocyanins from black currants with aqueous ethanol. J. Food. Sci. 2003, 68, 240–248. [Google Scholar] [CrossRef]
- Jeganathan, P.M.; Venkatachalam, S.; Karichappan, T.; Ramasamy, S. Model development and process optimization for solvent extraction of polyphenols from red grapes using Box–Behnken design. Prep. Biochem. Biotechnol. 2014, 44, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandao, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lightfoot, D.A.; Kinsel, M.; Watson, D.G. Employing Response Surface Methodology for the Optimization of Ultrasound Assisted Extraction of Lutein and β-Carotene from Spinach. Molecules 2015, 20, 6611–6625. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.D. Skewness and Kurtosis. Shiken JALT Test. Eval. SIG Newsl. 1997, 1, 20–23. [Google Scholar]
- Mukherjee, D.; Kumar, N.S.; Khatua, T.; Mukherjee, P.K. Rapid validated HPTLC method for estimation of betulinic acid in Nelumbo nucifera (Nymphaeaceae) rhizome extract. Phytochem. Anal. 2010, 21, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Motwani, S.K.; Iqbal, Z.; Talengaonkar, S.; Ahmad, F.J.; Khar, R.K. Optimisation of polyherbal gels for vaginal drug delivery by Box-Behnken statistical design. Eur. J. Pharm. Biopharm. 2007, 67, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.; Taylor, L.T. Extraction of polyphenolic compounds from grape seeds with near criticalcarbon dioxide. J. Chromatogr. A 1999, 849, 117–124. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite Guideline, Validation of Analytical Procedures: Text and Methodology, Q2 (R1). International Conference on Harmonization, Geneva, Switzerland. November 2005. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf (accessed on 21 September 2015).
- Ibrahim, A.A.; Kalam, M.A.; Sultana, Y.; Imran, A.; Alshamsan, A. Development and validation of stability-indicating high performance liquid chromatography method to analyze gatifloxacin in bulk drug and pharmaceutical preparations. Saudi Pharm. J. 2015, 23, 85–94. [Google Scholar]
- Sample Availability: Sample of the compound is available from the author.



| Parameters | |
|---|---|
| Linearity range | 100–600 ng |
| Correlation coefficient | 0.9902 |
| Regression equation (peak area) | Y= −174.788 + 3.090X |
| LOD (ng) | 6.37 |
| LOQ (ng) | 19.32 |
| Rf value | 0.65 |
| BA yield | 2.4% |
| Amount of Betulinic Acid/Spot (ng) | Mean | SD | %RSD | SEM |
|---|---|---|---|---|
| Intra-day | ||||
| 200 | 871.73 | 0.86 | 0.098 | 0.35 |
| 400 | 1317.12 | 1.06 | 0.080 | 0.43 |
| Inter-day | ||||
| 200 | 860.71 | 1.41 | 0.16 | 0.58 |
| 400 | 1328.073 | 1.74 | 0.13 | 0.71 |
| Amount of Betulinic Acid in Sample (mg) | % Age of Standard Betulinic Acid Added in a Sample | % Recovery of Betulinic Acid (Peak Area) | %RSD | SEM |
|---|---|---|---|---|
| 0.06 | 0 | 98.69 | 0.5 | 0.29 |
| 0.06 | 50 | 97.44 | 1.05 | 0.59 |
| 0.06 | 100 | 97.58 | 1.27 | 0.72 |
| 0.06 | 150 | 99.26 | 1.03 | 0.59 |
| Betulinic Acid (ng/Spot) | Ratio of Solvent System | |
|---|---|---|
| Toluene–Ethyl Acetate–Glacial Acetic Acid (8.2:1.8:0.02 v/v/v) %RSD via Area | Toluene–Ethyl acetate–Glacial Acetic Acid (8.5:1.5:0.02 v/v/v) %RSD via Area | |
| 200 | 0.16 | 0.57 |
| 400 | 0.075 | 0.49 |
| Run | Temperature (A) (°C) | Time (B) (h) | Solvent to Drug Ratio (C) (mL/100 g) | Yield (Y) BA (% w/w) |
|---|---|---|---|---|
| 01 | 60 (+1) | 6 (0) | 500 (+1) | 2.305 ± 0.011 |
| 02 | 45 (0) | 6 (0) | 400 (0) | 2.413 ± 0.003 |
| 03 | 30 (−1) | 6 (0) | 300 (−1) | 1.771 ± 021 |
| 04 | 60 (+1) | 6 (0) | 300 (−1) | 2.186 ± 0.008 |
| 05 | 45 (0) | 8 (+1) | 500 (+1) | 2.241 ± 0.019 |
| 06 | 45 (0) | 6 (0) | 400 (0) | 2.421 ± 0.045 |
| 07 | 30 (−1) | 4 (−1) | 400 (0) | 1.696 ± 0.038 |
| 08 | 45 (0) | 6 (0) | 400 (0) | 2.397 ± 0.098 |
| 09 | 60 (+1) | 8 (+1) | 400 (0) | 2.358 ± 0.083 |
| 10 | 60 (+1) | 4 (−1) | 400 (0) | 2.247 ± 0.051 |
| 11 | 45 (0) | 6 (0) | 400 (0) | 2.451 ± 0.091 |
| 12 | 45 (0) | 4 (−1) | 500 (+1) | 2.154 ± 0.004 |
| 13 | 30 (−1) | 6 (0) | 500 (+1) | 1.789 ± 0.013 |
| 14 | 45 (0) | 8 (+1) | 300 (−1) | 2.253 ± 0.021 |
| 15 | 45 (0) | 6 (0) | 400 (0) | 2.388 ± 0.063 |
| 16 | 45 (0) | 4 (−1) | 300 (−1) | 2.011 ± 0.051 |
| 17 | 30 (−1) | 8 (+1) | 400 (0) | 1.961 ± 0.078 |
| Model F Value | R2 | Adjusted R2 | Predicted R2 | SD | CV% | |
|---|---|---|---|---|---|---|
| Linear | 0.5287 | 0.4199 | 0.2828 | 0.20 | - | |
| Yield of BA | 2F1 | 0.5464 | 0.2742 | −0.2106 | 0.22 | - |
| Cubic | 0.9978 | 0.9911 | - | 0.024 | - | |
| Quadratic | 0.9962 | 0.9914 | 0.9717 | 0.024 | 1.11 |
| Source | Sum of Square | Degree of Freedom | Mean Square | F-Value | Prob > F |
|---|---|---|---|---|---|
| Model | 1.07 | 9 | 0.12 | 205.75 | <0.0001 Significant |
| Residual | 4.05 | 7 | 5.78 | ||
| Lack of fit | 1.67 | 3 | 5.56 | 0.93 | 0.5030 Non significant |
| Pure error | 2.38 | 4 | 5.96 |
| Variable | Degree of Freedom | Sum of Square | F-Value | p Value |
|---|---|---|---|---|
| AB | 1 | 9.22 | 15.92 | 0.0053 |
| AC | 1 | 3.84 | 6.64 | 0.0366 |
| BC | 1 | 6.01 | 10.38 | 0.0146 |
| A2 | 1 | 0.31 | 528.89 | <0.0001 |
| B2 | 1 | 0.04 | 70.41 | <0.0001 |
| C2 | 1 | 0.09 | 165.61 | <0.0001 |
| Independent Variable | Ranges of Independent Variable | Dependent Variable | Goal | |
|---|---|---|---|---|
| Low (−1) | High (+1) | |||
| Temperature (°C) | 30 | 60 | Yield of BA (% w/w) | Maximized |
| Time (h) | 4 | 6 | ||
| Solvent to drug ratio (mL/100 g) | 300 | 500 | ||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, N.; Aeri, V. Optimization of Betulinic Acid Extraction from Tecomella undulata Bark Using a Box-Behnken Design and Its Densitometric Validation. Molecules 2016, 21, 393. https://doi.org/10.3390/molecules21040393
Siddiqui N, Aeri V. Optimization of Betulinic Acid Extraction from Tecomella undulata Bark Using a Box-Behnken Design and Its Densitometric Validation. Molecules. 2016; 21(4):393. https://doi.org/10.3390/molecules21040393
Chicago/Turabian StyleSiddiqui, Nahida, and Vidhu Aeri. 2016. "Optimization of Betulinic Acid Extraction from Tecomella undulata Bark Using a Box-Behnken Design and Its Densitometric Validation" Molecules 21, no. 4: 393. https://doi.org/10.3390/molecules21040393





