Functionalization of Calcium Sulfate/Bioglass Scaffolds with Zinc Oxide Whisker
Abstract
:1. Introduction
2. Results and Discussion
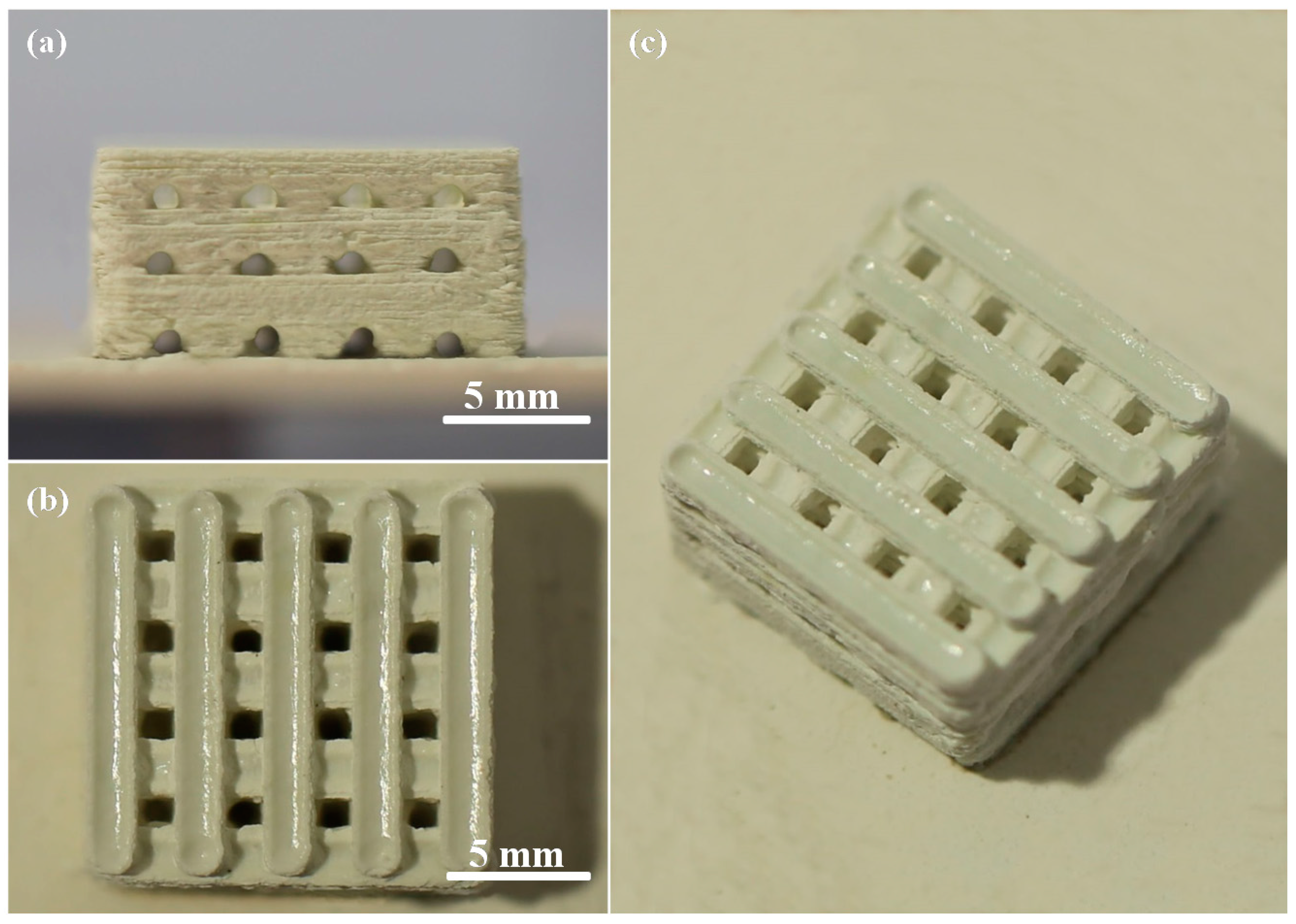
2.1. Scaffolds Fabrication
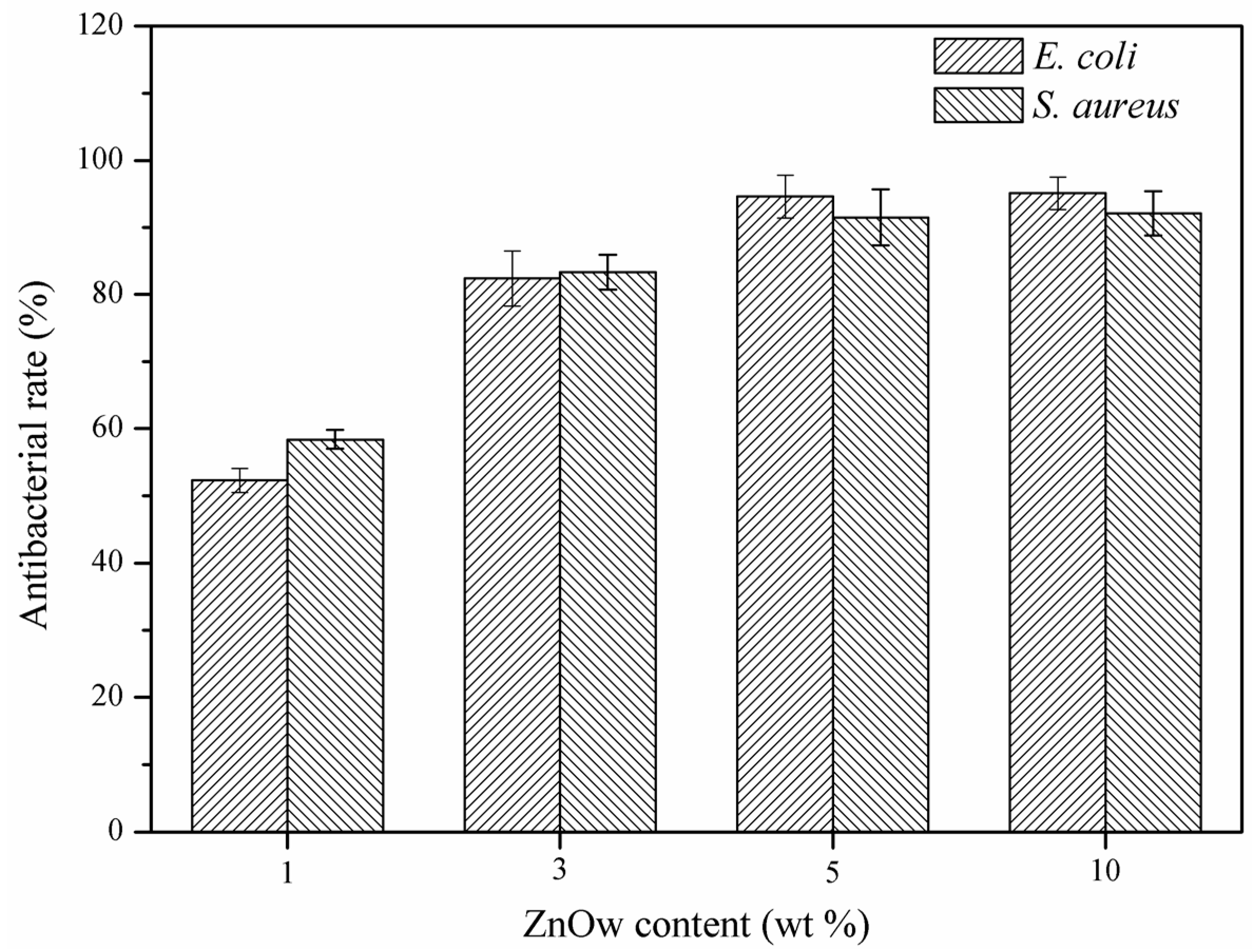
2.2. Antibacterial Activity
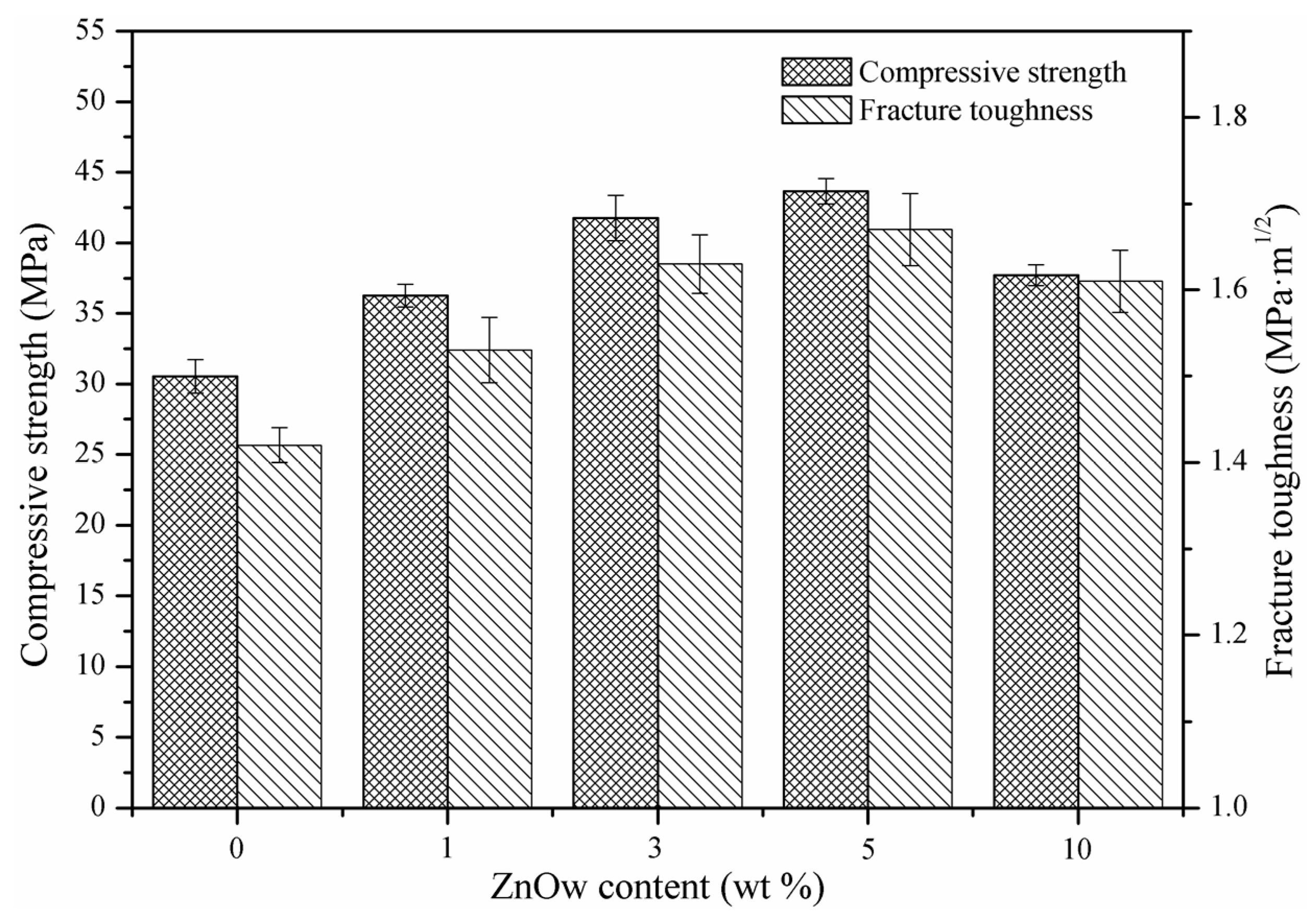
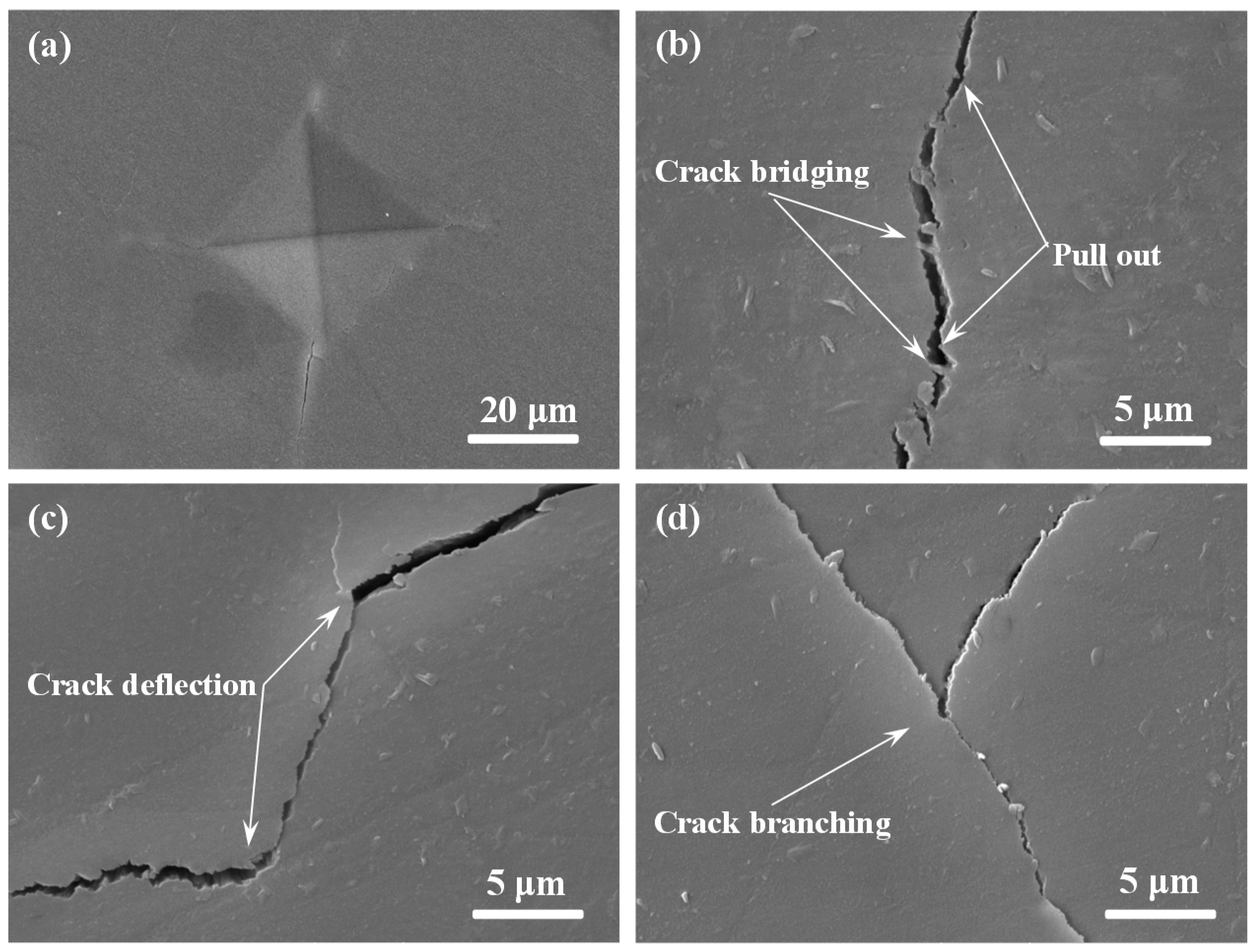
2.3. Mechanical Properties
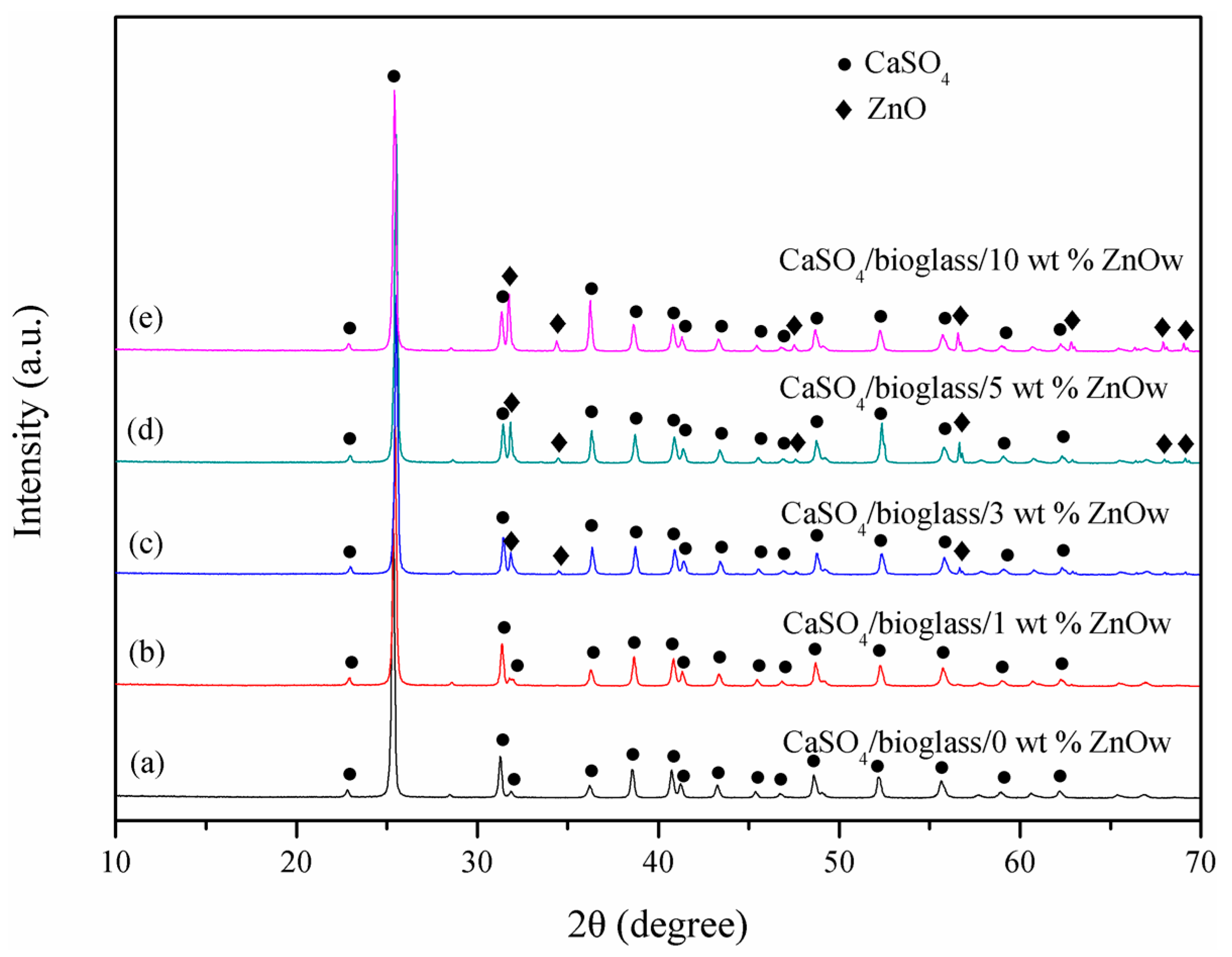
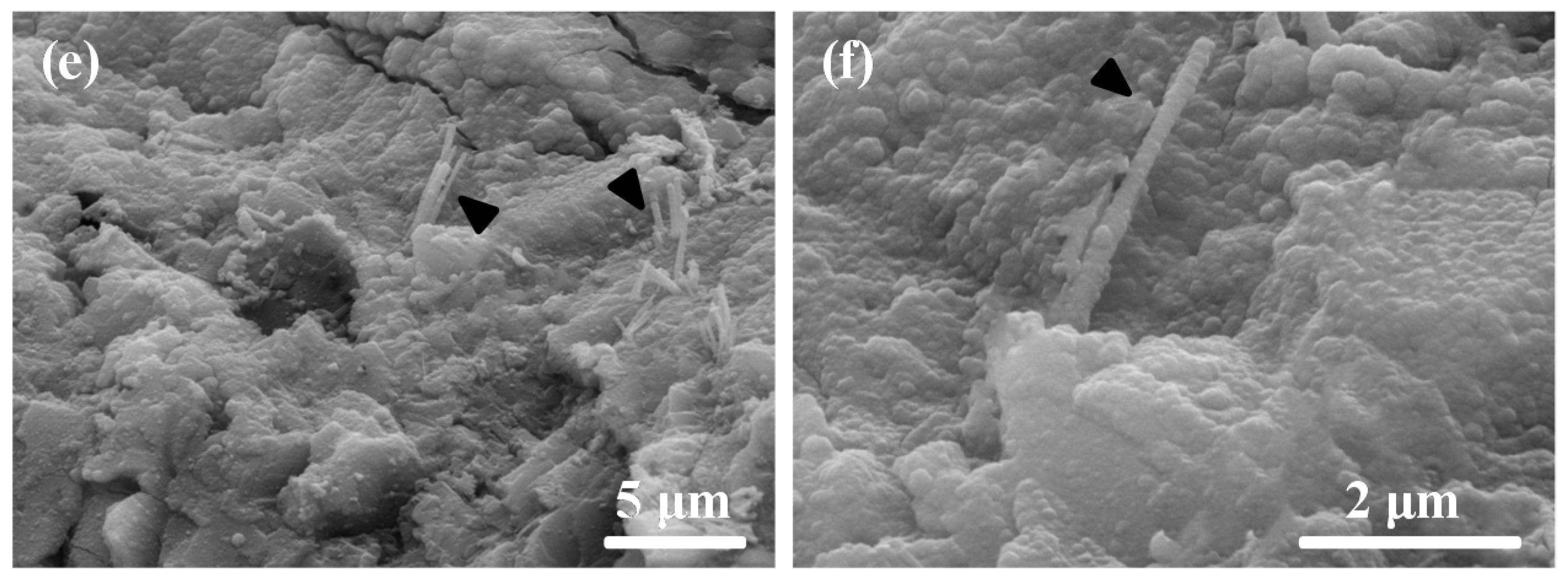
2.4. Composition and Microstructure
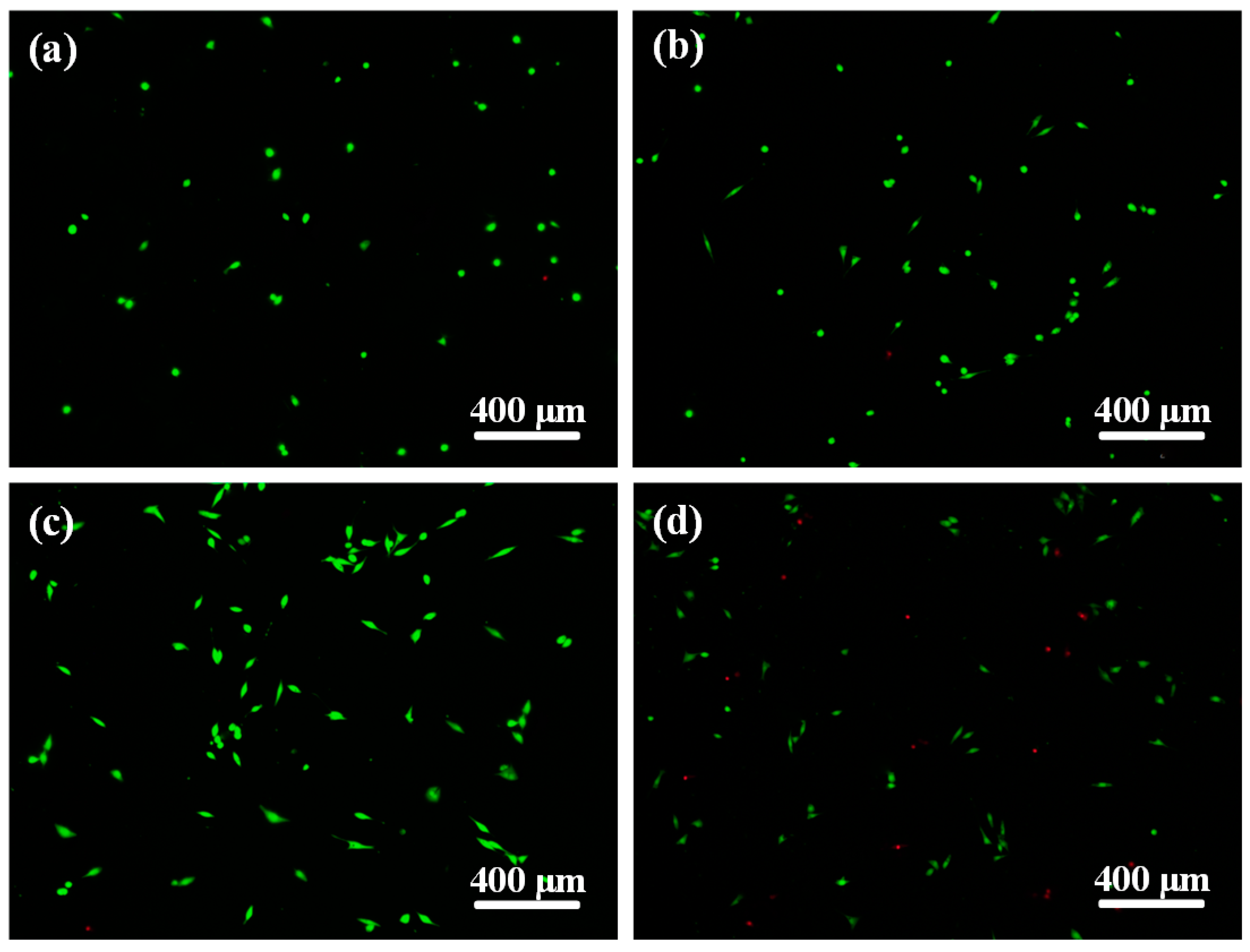
2.5. Biocompatibility
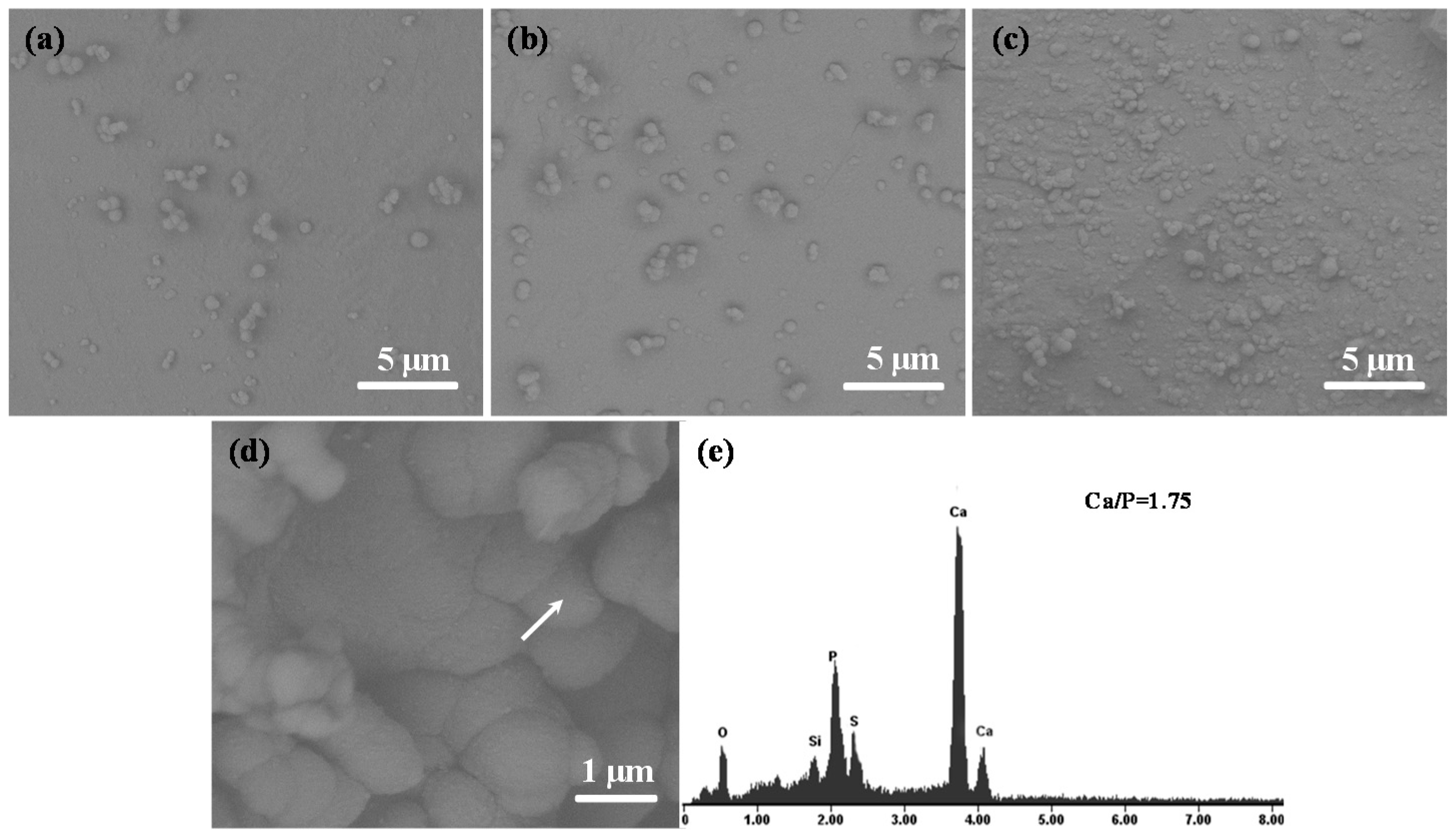
2.6. Bioactivity Evaluation
3. Experimental Procedures
3.1. Materials and Preparation
3.2. Antibacterial Behavior
3.3. Characterization
3.4. Cell Culture
3.5. Bioactivity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, S.; Weng, Z.; Liu, X.; Yeung, K.W.K.; Chu, P. Functionalized TiO2 based nanomaterials for biomedical applications. Adv. Funct. Mater. 2014, 24, 5464–5481. [Google Scholar] [CrossRef]
- Zhou, K.; Dong, C.; Zhang, X.; Shi, L.; Chen, Z.; Xu, Y.; Cai, H. Preparation and characterization of nanosilver-doped porous hydroxyapatite scaffolds. Ceram. Int. 2015, 41, 1671–1676. [Google Scholar] [CrossRef]
- Erakovic, S.; Jankovic, A.; Tsui, G.C.P.; Tang, C.Y.; Miskovic-Stankovic, V.; Stevanovic, T. Novel bioactive antimicrobial lignin containing coatings on titanium obtained by electrophoretic deposition. Int. J. Mol. Sci. 2014, 15, 12294–12322. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Kant, K.; Findlay, D.; Losic, D. Periodically tailored titania nanotubes for enhanced drug loading and releasing performances. J. Mater. Chem. B 2015, 3, 2553–2559. [Google Scholar] [CrossRef]
- Memarzadeh, K.; Sharili, A.S.; Huang, J.; Rawlinson, S.C.; Allaker, R.P. Nanoparticulate zinc oxide as a coating material for orthopedic and dental implants. J. Biomed. Mater. Res. Part A 2015, 103, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Meng, G.; He, J.; Wu, Y.; Wu, F.; Gu, Z. Antibiotic-loaded chitosan hydrogel with superior dual functions: Antibacterial efficacy and osteoblastic cell responses. ACS Appl. Mater. Interfaces 2014, 6, 10005–10013. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H.; Kim, D.; Seo, J.; Seo, K.; Han, H. Effect of tetrapod ZnO whiskers on the physical and moisture barrier properties of transparent polyimide/TZnO-W composite films. Macromol. Res. 2014, 22, 1243–1252. [Google Scholar] [CrossRef]
- Chang, B.P.; Akil, H.M.; Nasir, R.B.M.; Bandara, I.M.C.C.D.; Rajapakse, S. The effect of ZnO nanoparticles on the mechanical, tribological and antibacterial properties of ultra-high molecular weight polyethylene. J. Reinf. Plast. Compos. 2014, 33, 674–686. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Zhang, G.; Zhang, Z.; Liu, S.; Li, P.; Liao, Q.L.; Zhao, Y.G.; Zhang, Y. Investigation on the optimization, design and microwave absorption properties of reduced graphene oxide/tetrapod-like ZnO composites. RSC Adv. 2015, 5, 10197–10203. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Stöcker, H.; Bazhenov, V.V.; Langer, E.; Dobrowolska, A.; Czaczyk, K.; Galli, R.; Stelling, A.L.; Behm, T.; et al. An extreme biomimetic approach: Hydrothermal synthesis of β-chitin/ZnO nanostructured composites. J. Mater. Chem. B 2013, 1, 6469–6476. [Google Scholar] [CrossRef]
- Lakshmi Prasanna, V.; Vijayaraghavan, R. Insight into the mechanism of antibacterial activity of ZnO: Surface defects mediated reactive oxygen species even in the dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.B.P.; Aguilar, C.A.H.; Ramos, J.M.V.; Thangarasu, P. Synergistic antibacterial activity of nanohybrid materials ZnO–Ag and ZnO–Au: Synthesis, characterization, and comparative analysis of undoped and doped ZnO nanoparticles. Aust. J. Chem. 2015, 68, 288–297. [Google Scholar]
- Subhan, M.A.; Awal, M.R.; Ahmed, T.; Younus, M. Photocatalytic and antibacterial activities of Ag/ZnO nanocomposities fabricated by co-precipitation method. Acta Metall. Sin. (Engl. Lett.) 2014, 27, 223–232. [Google Scholar] [CrossRef]
- Cuozzo, R.C.; da Rocha Leão, M.H.M.; de Andrade Gobbo, L.; da Rocha, D.N.; Ayad, N.M.E.; Trindade, W.; Costa, A.M.; da Silva, M.H.P. Zinc alginate-hydroxyapatite composite microspheres for bone repair. Ceram. Int. 2014, 40, 11369–11375. [Google Scholar] [CrossRef]
- Duan, S.; Yang, X.; Tao, Y. Experimental study on strain-rate-dependent behavior and failure modes of long glass fiber-reinforced polypropylene composite. J. Reinf. Plast. Compos. 2015, 34, 1261–1270. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, C.; Feng, P.; Xiao, T.; Shuai, C.; Peng, S. Calcium sulfate bone scaffolds with controllable porous structure by selective laser sintering. J. Porous Mater. 2015, 22, 1171–1178. [Google Scholar] [CrossRef]
- D’Ayala, G.G.; Malinconico, M.; Laurienzo, P. Marine derived polysaccharides for biomedical applications: Chemical modification approaches. Molecules 2008, 13, 2069–2106. [Google Scholar] [CrossRef] [PubMed]
- García-García, J.M.; Garrido, L.; Quijada-Garrido, I.; Kaschta, J.; Schubert, D.W.; Boccaccini, A.R. Novel poly (hydroxyalkanoates)-based composites containing Bioglass® and calcium sulfate for bone tissue engineering. Biomed. Mater. 2012, 7, 054105. [Google Scholar] [CrossRef] [PubMed]
- Merolli, A.; Santin, M. Role of phosphatidyl-serine in bone repair and its technological exploitation. Molecules 2009, 14, 5367–5381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, M.P.; Tsung, Y.C.; Hsu, H.C.; Tuan, W.H.; Lai, P.L. Addition of a small amount of glass to improve the degradation behavior of calcium sulfate bioceramic. Ceram. Int. 2015, 41, 1155–1162. [Google Scholar] [CrossRef]
- Fang, M.; Chai, F.; Chen, J.H.; Neut, C.; Jia, M.; Liu, Y.; Zhao, S.J.; Hartmut, F.; Hildebrand, H.F. Antibacterial functionalization of an experimental self-etching primer by inorganic agents: Microbiological and biocompatibility evaluations. Biomol. Eng. 2007, 24, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Bini, M.; Grandi, S.; Capsoni, D.P.; Mustarelli, E.; Saino, E.; Visai, L. SiO2−P2O5−CaO glasses and glass-ceramics with and without ZnO: Relationships among composition, microstructure, and bioactivity. J. Phys. Chem. C 2009, 113, 8821–8828. [Google Scholar] [CrossRef]
- Jin, H.B.; Oktar, F.N.; Dorozhkin, S.; Agathopoulos, S. Sintering behavior and properties of reinforced hydroxyapatite/TCP biphasic bioceramics with ZnO-whiskers. J. Compos. Mater. 2011, 45, 1435–1445. [Google Scholar] [CrossRef]
- Fedorovich, N.E.; Alblas, J.; Hennink, W.E.; Öner, F.C.; Dhert, W.J. Organ printing: The future of bone regeneration? Trends Biotechnol. 2011, 29, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.S.; Nisha, M.M.; Joice, M.; Shilpa, P.N. Antimicrobial activity of zinc oxide (ZnO) nanoparticle against Klebsiella pneumoniae. Pharm. Boil. 2014, 52, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.H.; Djurišić, A.B.; Chan, C.M.N.; Xi, Y.Y.; Tse, C.W.; Leung, Y.H.; Chan, W.K.; Leung, F.C.C.; Au, D.W.T. Antibacterial activity of ZnO nanorods prepared by a hydrothermal method. Thin Solid Films 2008, 516, 6167–6174. [Google Scholar] [CrossRef]
- Ding, S.J.; Shie, M.Y.; Wei, C.K. In vitro physicochemical properties, osteogenic activity, and immunocompatibility of calcium silicate-gelatin bone grafts for load-bearing applications. ACS Appl. Mater. Interfaces 2011, 3, 4142–4153. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide—From synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef]
- Shuai, C.; Zhou, J.; Wu, P.; Gao, C.; Feng, P.; Xiao, T.; Deng, Y.W.; Peng, S. Enhanced stability of calcium sulfate scaffolds with 45S5 bioglass for bone repair. Materials 2015, 8, 7498–7510. [Google Scholar] [CrossRef]
- Veljović, D.; Zalite, I.; Palcevskis, E.; Smiciklas, I.; Petrović, R.; Janaćković, D. Microwave sintering of fine grained HAP and HAP/TCP bioceramics. Ceram. Int. 2010, 36, 595–603. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.


| Spot Diameter (mm) | Laser Power (W) | Scan Speed (mm·min−1) | Scan Spacing (mm) | Spot Diameter (mm) |
|---|---|---|---|---|
| 100 | 7.0 | 0.1 | 3.0 | 0.8 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuai, C.; Zhou, J.; Gao, D.; Gao, C.; Feng, P.; Peng, S. Functionalization of Calcium Sulfate/Bioglass Scaffolds with Zinc Oxide Whisker. Molecules 2016, 21, 378. https://doi.org/10.3390/molecules21030378
Shuai C, Zhou J, Gao D, Gao C, Feng P, Peng S. Functionalization of Calcium Sulfate/Bioglass Scaffolds with Zinc Oxide Whisker. Molecules. 2016; 21(3):378. https://doi.org/10.3390/molecules21030378
Chicago/Turabian StyleShuai, Cijun, Jianhua Zhou, Dan Gao, Chengde Gao, Pei Feng, and Shuping Peng. 2016. "Functionalization of Calcium Sulfate/Bioglass Scaffolds with Zinc Oxide Whisker" Molecules 21, no. 3: 378. https://doi.org/10.3390/molecules21030378






