Quercetin-Rich Guava (Psidium guajava) Juice in Combination with Trehalose Reduces Autophagy, Apoptosis and Pyroptosis Formation in the Kidney and Pancreas of Type II Diabetic Rats
Abstract
:1. Introduction
2. Results
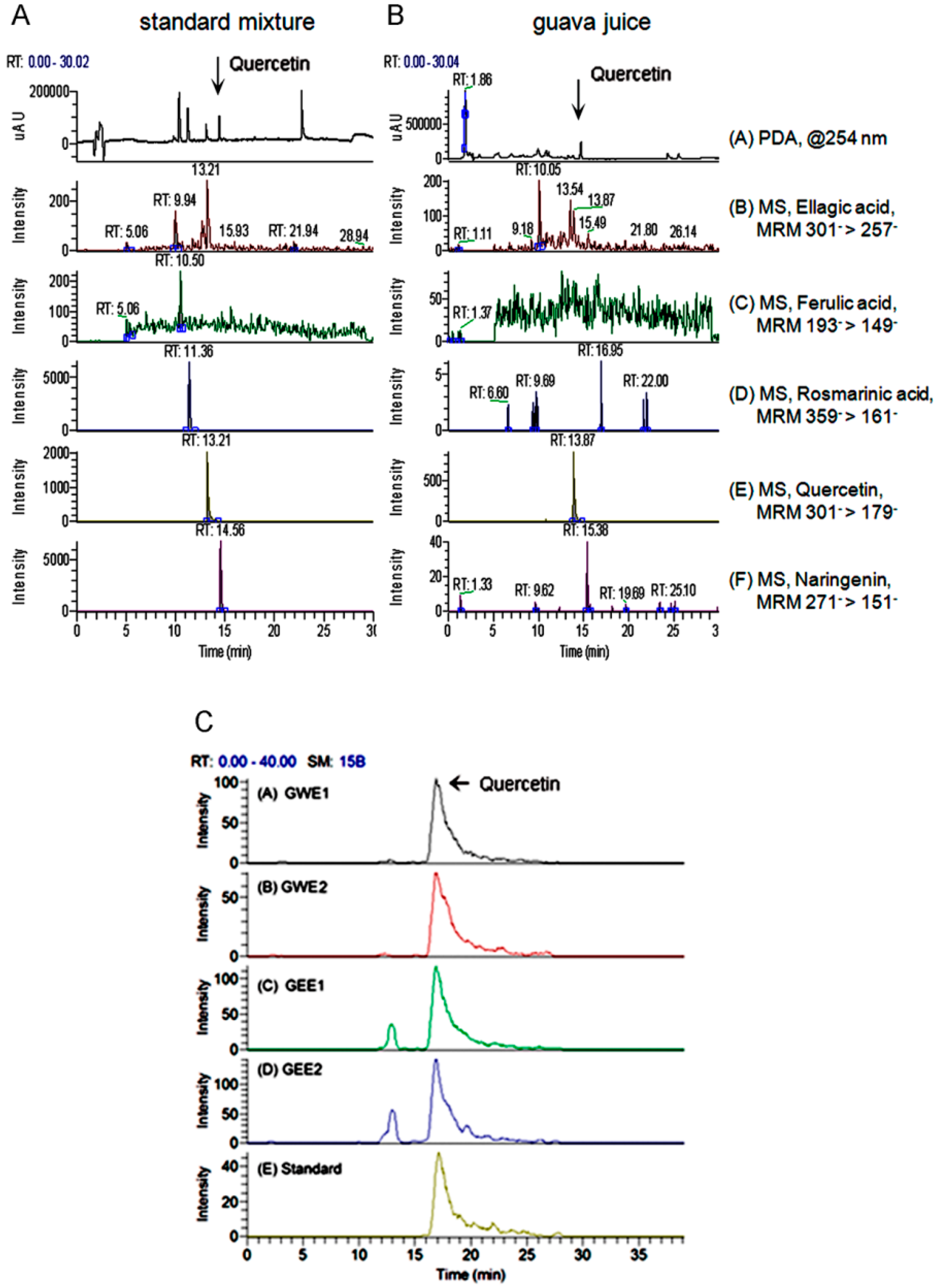
2.1. Quercetin Content in Guava Extraction
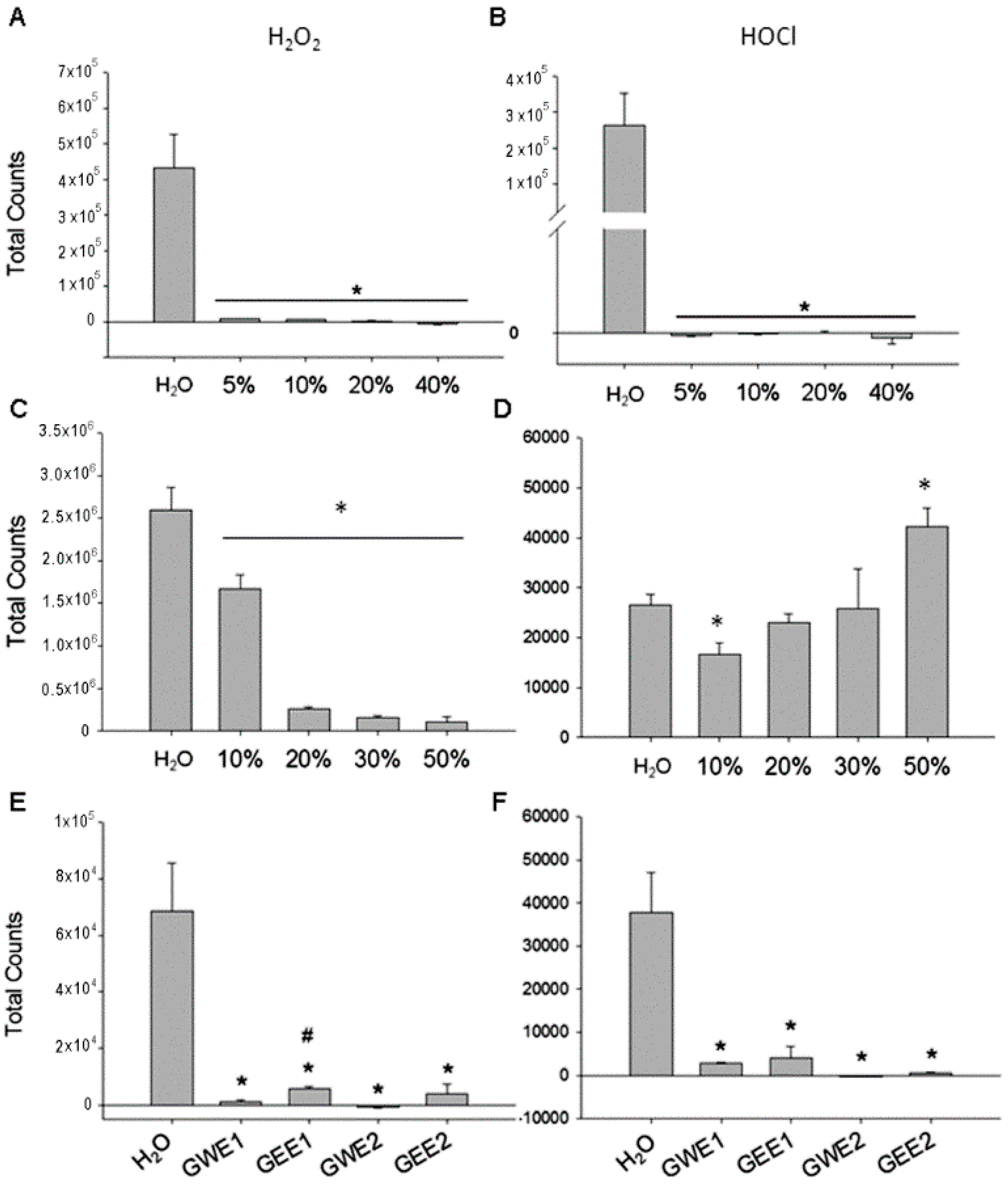
2.2. Guava Juice in Vitro ROS Level
2.3. Trehalose in Vitro ROS Levels
2.4. Guava Extraction in Vitro ROS Levels
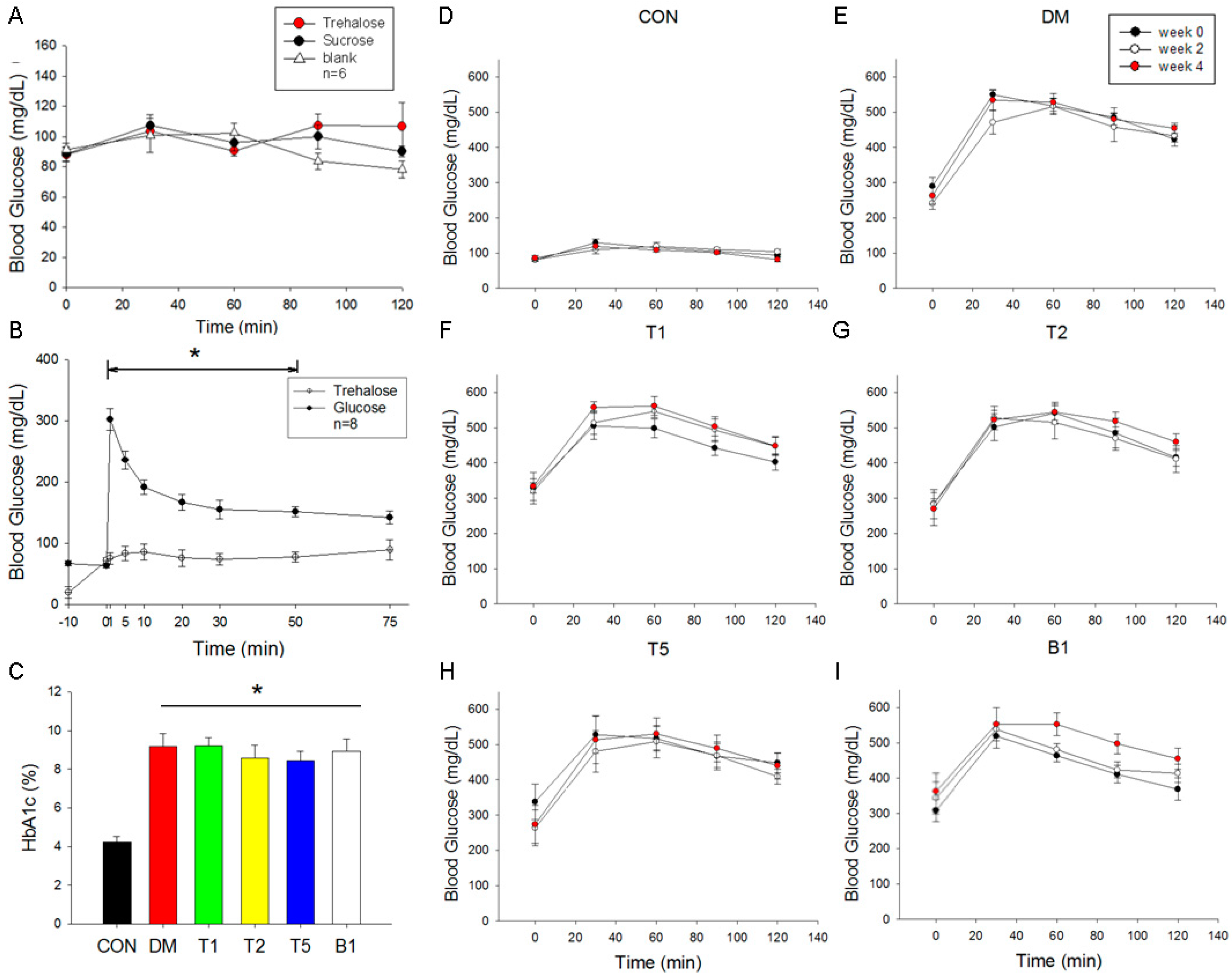
2.5. Oral Guava Juice Tolerance Test
2.6. Intravenous Glucose and Trehalose Tolerance Test
2.7. HbA1c Levels
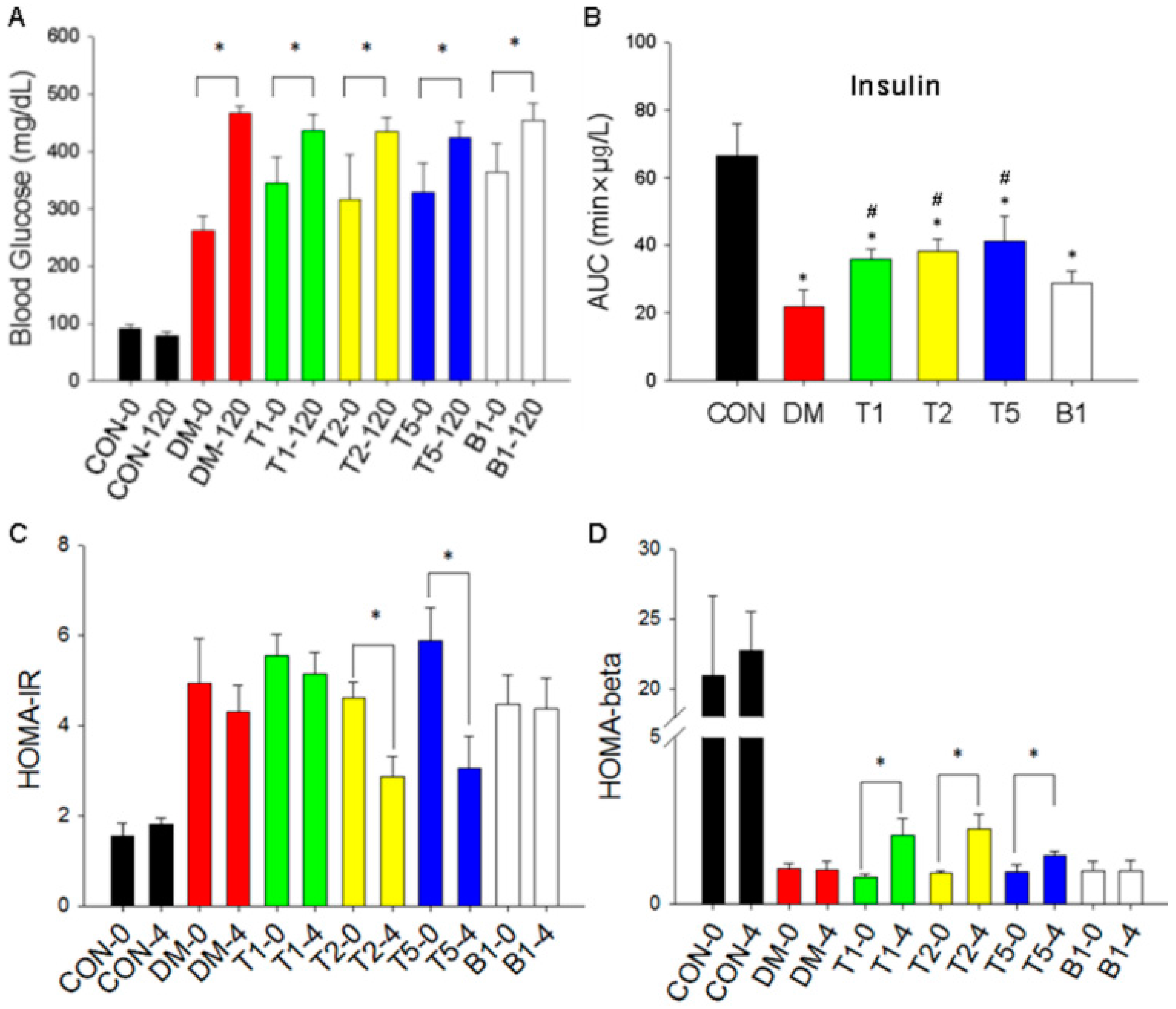
2.8. Oral Glucose Tolerance Test (OGTT)
2.9. Blood Glucose Changes
2.10. Insulin Levels
2.11. HOMA-IR
2.12. HOMA-β
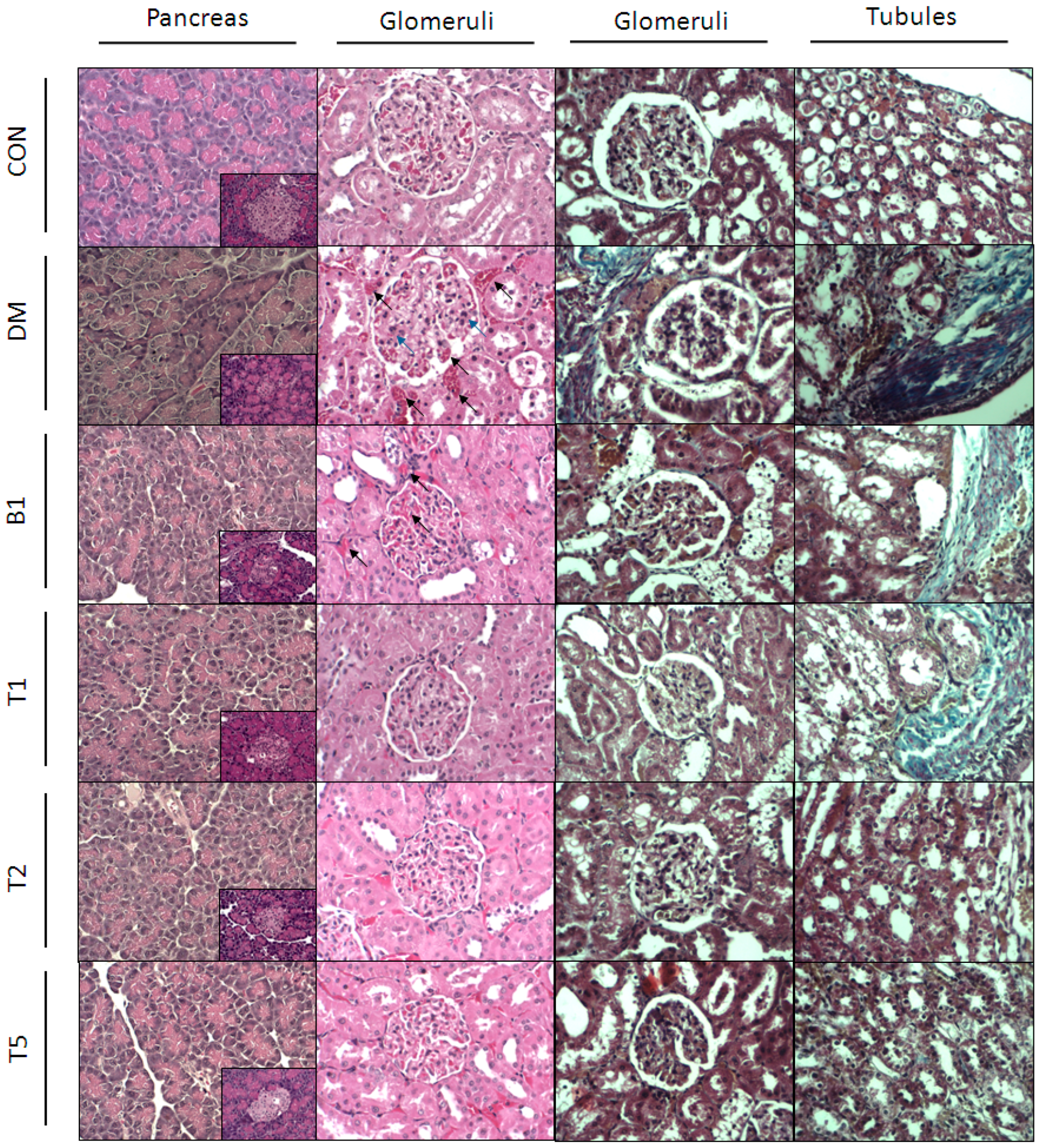
2.13. HE (Hematoxylin and Eosin) Stain
2.14. Masson’s Stain
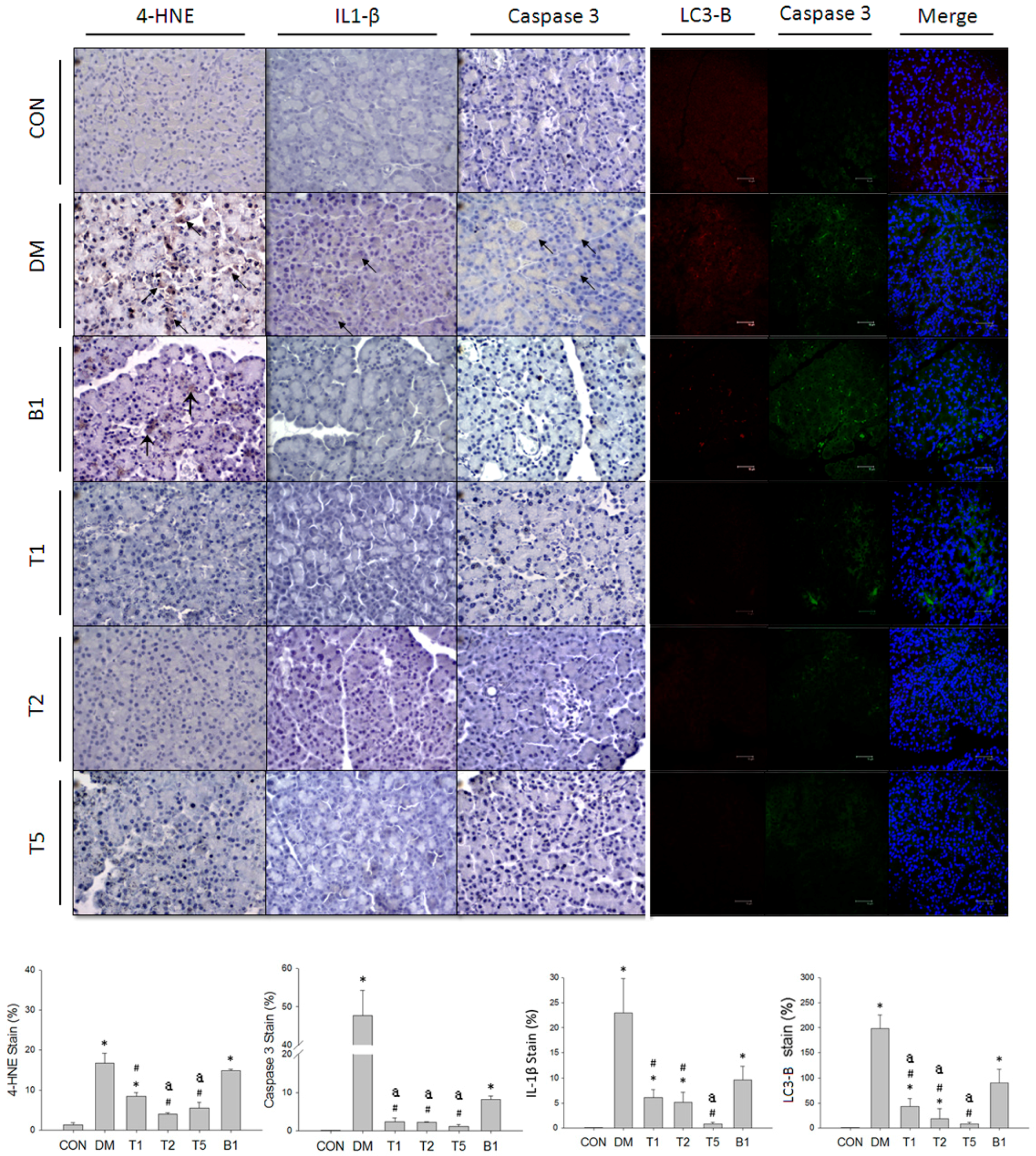
2.15. IHC and Fluorescent Stain in Kidney
2.16. Renal in Vivo ROS Levels
2.17. Serum in Vitro ROS Level
2.18. IHC and Fluorescent Stain in Pancreas
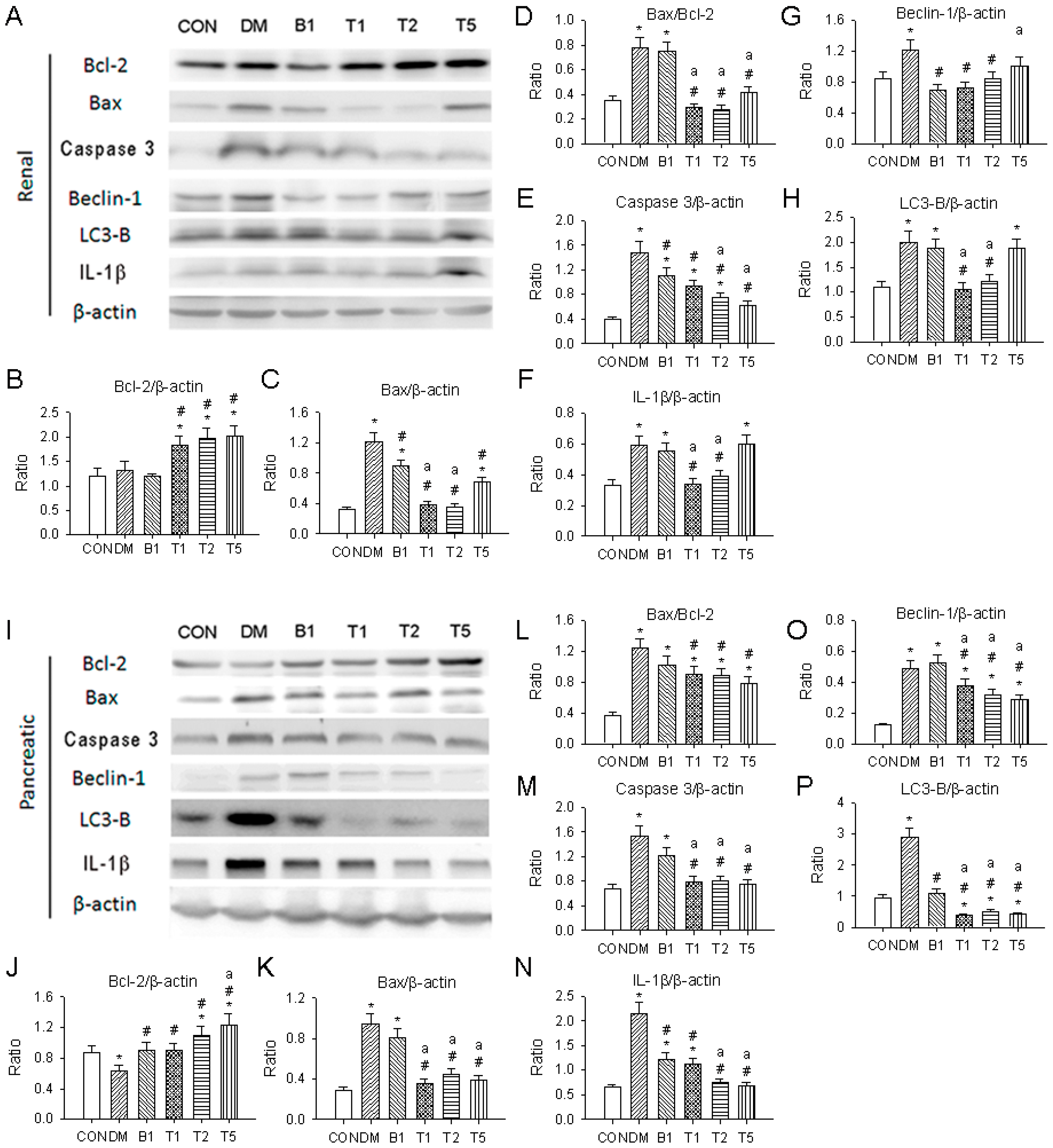
2.19. Western Blotting
3. Discussion
4. Experimental Section
4.1. Guava Extraction
4.2. LC/MS
4.3. Animals
4.4. Induction of T2DM
4.5. Animal Treatment
4.6. Determine Blood Glucose
4.7. Determine Insulin Levels
4.8. HOMA-IR and HOMA-β
4.9. Glucose Tolerance Test
4.9.1. Intra Venous Glucose Tolerance Test (IVGTT)
4.9.2. Oral Glucose Tolerance Test (OGTT)
4.10. Determine HbA1c
4.11. In Vitro ROS Level
4.12. In Vivo ROS Level
4.13. Immunohistochemistry (IHC)
4.14. Masson’s Stain
4.15. Fluorescent Stain
4.16. Western Blotting
4.17. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 4-HNE | 4-Hydroxynonenal |
| AUC | Area under curve |
| AGE | Advanced glycation end products |
| B1 | T2DM with 4 mL/kg BW of guava juice without trehalose |
| CON | Control group |
| FBG | Fasted blood glucose |
| G1 | Thailand guava |
| G2 | Pearl guava |
| GWE1 | Guava water extraction of G1 |
| GWE2 | Guava water extraction of G2 |
| GEE1 | Guava ethanol extraction of G1 |
| GEE2 | Guava ethanol extraction of G2 |
| HbA1c | Glycated hemoglobin A1c |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| HOMA-β | Homeostasis model assessment of the function of β cell in pancreas and insulin secretion index |
| IHC | Immunohistochemisty |
| IVGTT | Intra venous glucose tolerance test |
| IR | Insulin resistance |
| MCLA | 2-Methyl-6-(4-methoxyphenyl)-3, 7-dihydroimidazo-(1,2-a)-pyrazin-3-one hydrochloride |
| NA | Nicotinamide |
| OGTT | Oral glucose tolerance test |
| ROS | Reactive oxygen species |
| STZ | Streptozotocin |
| T1 | T2DM with 4 mL/kg BW of guava juice with 2 mL/kg BW of trehalose |
| T2 | T2DM with 8 mL/kg BW of guava juice with 4 mL/kg BW of trehalose |
| T5 | T2DM with 20 mL/kg BW of guava juice with 1 mL/kg BW of trehalose |
| T2DM | Type II diabetes |
References
- Hunyadi, A.; Martins, A.; Hsieh, T.J.; Seres, A.; Zupkó, I. Chlorogenic acid and rutin play a major role in the in vivo anti-diabetic activity of Morus alba leaf extract on type II diabetic rats. PLoS ONE 2012, e50619. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Terajima, T.; Ogata, T.; Ueno, K.; Hashimoto, N.; Ono, K.; Yano, S. Establishment and pathophysiological characterization of type 2 diabetic mouse model produced by streptozotocin and nicotinamide. Biol. Pharm. Bull. 2006, 29, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Sarah, W.; Gojka, R.; Anders, G.; Richard, S.; Hilary, K. Global prevalence of diabetes. Diabetes Care 2004, 27, 1047–1053. [Google Scholar]
- Yoon, K.H.; Lee, J.H.; Kim, J.W.; Cho, J.H.; Choi, Y.H.; Ko, S.H.; Son, H.Y. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006, 368, 1681–1688. [Google Scholar] [CrossRef]
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- Kakehi, T.; Yabe-Nishimura, C. NOX enzymes and diabetic complications. In Seminars in Immunopathology; Springer-Verlag: Berlin, Germany, 2008; Volume 30, pp. 301–314. [Google Scholar]
- Jha, J.C.; Gray, S.P.; Barit, D.; Okabe, J.; El-Osta, A.; Namikoshi, T.; Thallas-Bonke, V.; Wingler, K.; Szyndralewiez, C.; Heitz, F.; et al. Genetic targeting or pharmacologic inhibition of NADPH oxidase Nox4 provides renoprotection in long-term diabetic nephropathy. J. Am. Soc. Nephrol. 2014, 25, 1237–1254. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E.; Defronzo, R.A.; Franz, M.J.; Keane, W.F.; Mogensen, C.E.; Parving, H.H.; Steffes, M.W. Nephropathy in diabetes. Diabetes Care 2004, 27, S79–S83. [Google Scholar] [PubMed]
- Calcutt, N.A.; Cooper, M.E.; Kern, T.S.; Schmidt, A.M. Therapies for hyperglycaemia-induced diabetic complications: From animal models to clinical trials. Nat. Rev. Drug Discov. 2009, 8, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Yin, M.C. Renal protective effects of extracts from guava fruit (Psidium guajava L.) in diabetic mice. Plant Foods Hum. Nutr. 2012, 67, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Li, P.Y.; Hsu, C.C.; Yin, M.C.; Kuo, Y.H.; Tang, F.Y.; Chao, C.Y. Protective effects of red guava on inflammation and oxidative stress in streptozotocin-induced diabetic mice. Molecules 2015, 20, 22341–22350. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.; Dastmalchi, K.; Wu, S.B.; Whalen, K.; Dabo, A.J.; Reynertson, K.A.; Foronjy, R.F.; D’Armiento, J.M.; Kennelly, E.J. Phenolic-rich extract from the Costa Rican guava (Psidium friedrichsthalianum) pulp with antioxidant and anti-inflammatory activity. Potential for COPD therapy. Food Chem. 2013, 141, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Eidenberger, T.; Selg, M.; Krennhuber, K. Inhibition of dipeptidyl peptidase activity by flavonol glycosides of guava (Psidium guajava L.): A key to the beneficial effects of guava in type II diabetes mellitus. Fitoterapia 2013, 89, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Haddad, G.G. Role of trehalose phosphate synthase and trehalose during hypoxia: From flies to mammals. J. Exp. Biol. 2004, 207, 3125–3129. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, X.; Wang, F.; Weng, H.; Yang, P. Trehalose prevents neural tube defects by correcting maternal diabetes-suppressed autophagy and neurogenesis. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E667–E678. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Davies, J.E.; Huang, Z.; Tunnacliffe, A.; Rubinsztein, D.C. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and α-synuclein. J. Biol. Chem. 2007, 282, 5641–5652. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Barkhordarian, H.; Emadi, S.; Park, C.B.; Sierks, M.R. Trehalose differentially inhibits aggregation and neurotoxicity of beta-amyloid 40 and 42. Neurobiol. Dis. 2005, 20, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, A.; Russo, M.J.; Bieganski, R.; Fowler, A.; Cheley, S.; Bayley, H.; Toner, M. Intracellular trehalose improves the survival of cryopreserved mammalian cells. Nat. Biotechnol. 2000, 18, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, A.; Sumida, Y.; Gabazza, E.C.; Murashima, S.; Furuta, M.; Araki-Sasaki, R.; Hori, Y.; Yano, Y.; Adachi, Y. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care 2001, 24, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.T.; Lee, P.H.; Chen, C.F.; Ma, M.C.; Lai, M.K.; Hsu, S.M. De novo demonstration and co-localization of free-radical production and apoptosis formation in rat kidney subjected to ischemia reperfusion. J. Am. Soc. Nephrol. 2001, 12, 973–982. [Google Scholar] [PubMed]
- Uchida, T.; Rossignol, F.; Matthay, M.A.; Mounier, R.; Couette, S.; Clottes, E.; Clerici, C. Prolonged hypoxia differentially regulates hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression in lung epithelial cells: Implication of natural antisense HIF-1alpha. J. Biol. Chem. 2004, 279, 14871–14878. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Schmid, T.; Frank, R.; Brüne, B. PI3K/Akt is required for heat shock proteins to protect hypoxia-inducible factor 1alpha from pVHL-independent degradation. J. Biol. Chem. 2004, 279, 13506–13513. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.L.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Jain, S.; Sinha, P.R. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition 2007, 23, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Bieger, W.P.; Michel, G.; Barwich, D.; Biehl, K.; Wirth, A. Diminished insulin receptors on monocytes and erythrocytes in hypertriglyceridemia. Metabolism 1984, 33, 982–987. [Google Scholar] [CrossRef]
- Masiello, P.; Broca, C.; Gross, R.; Roye, M.; Manteghetti, M.; Hillaire-Buys, D.; Novelli, M.; Ribes, G. Experimental NIDDM: Development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes 1998, 47, 224–229. [Google Scholar] [CrossRef]
- Chen, H.Y.; Yen, G.C. Antioxidant activity and free radical-scavenging capacity of extracts from guava (Psidium guajava L.) leaves. Food Chem. 2007, 101, 686–694. [Google Scholar] [CrossRef]
- Huang, C.S.; Yin, M.C.; Chiu, L.C. Antihyperglycemic and antioxidative potential of Psidium guajava fruit in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2011, 49, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Tas, S.; Sarandol, E.; Ayvalik, S.Z.; Serdar, Z.; Dirican, M. Vanadyl sulfate, taurine, and combined vanadyl sulfate and taurine treatments in diabetic rats: Effects on the oxidative and antioxidative systems. Arch. Med. Res. 2007, 38, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Masini, M.; Bugliani, M.; Lupi, R.; Del Guerra, S.; Boggi, U.; Filipponi, F.; Marselli, L.; Masiellom, P.; Marchetti, P. Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia 2009, 52, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, L.A.; Li, T.Y.; Joshipura, K.J.; Hu, F.B. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care 2008, 31, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-F.; Kuo, Y.-T.; Chen, T.-Y.; Chien, C.-T. Quercetin-Rich Guava (Psidium guajava) Juice in Combination with Trehalose Reduces Autophagy, Apoptosis and Pyroptosis Formation in the Kidney and Pancreas of Type II Diabetic Rats. Molecules 2016, 21, 334. https://doi.org/10.3390/molecules21030334
Lin C-F, Kuo Y-T, Chen T-Y, Chien C-T. Quercetin-Rich Guava (Psidium guajava) Juice in Combination with Trehalose Reduces Autophagy, Apoptosis and Pyroptosis Formation in the Kidney and Pancreas of Type II Diabetic Rats. Molecules. 2016; 21(3):334. https://doi.org/10.3390/molecules21030334
Chicago/Turabian StyleLin, Chia-Fa, Yen-Ting Kuo, Tsung-Ying Chen, and Chiang-Ting Chien. 2016. "Quercetin-Rich Guava (Psidium guajava) Juice in Combination with Trehalose Reduces Autophagy, Apoptosis and Pyroptosis Formation in the Kidney and Pancreas of Type II Diabetic Rats" Molecules 21, no. 3: 334. https://doi.org/10.3390/molecules21030334





