Achillolide A Protects Astrocytes against Oxidative Stress by Reducing Intracellular Reactive Oxygen Species and Interfering with Cell Signaling
Abstract
:1. Introduction
2. Results
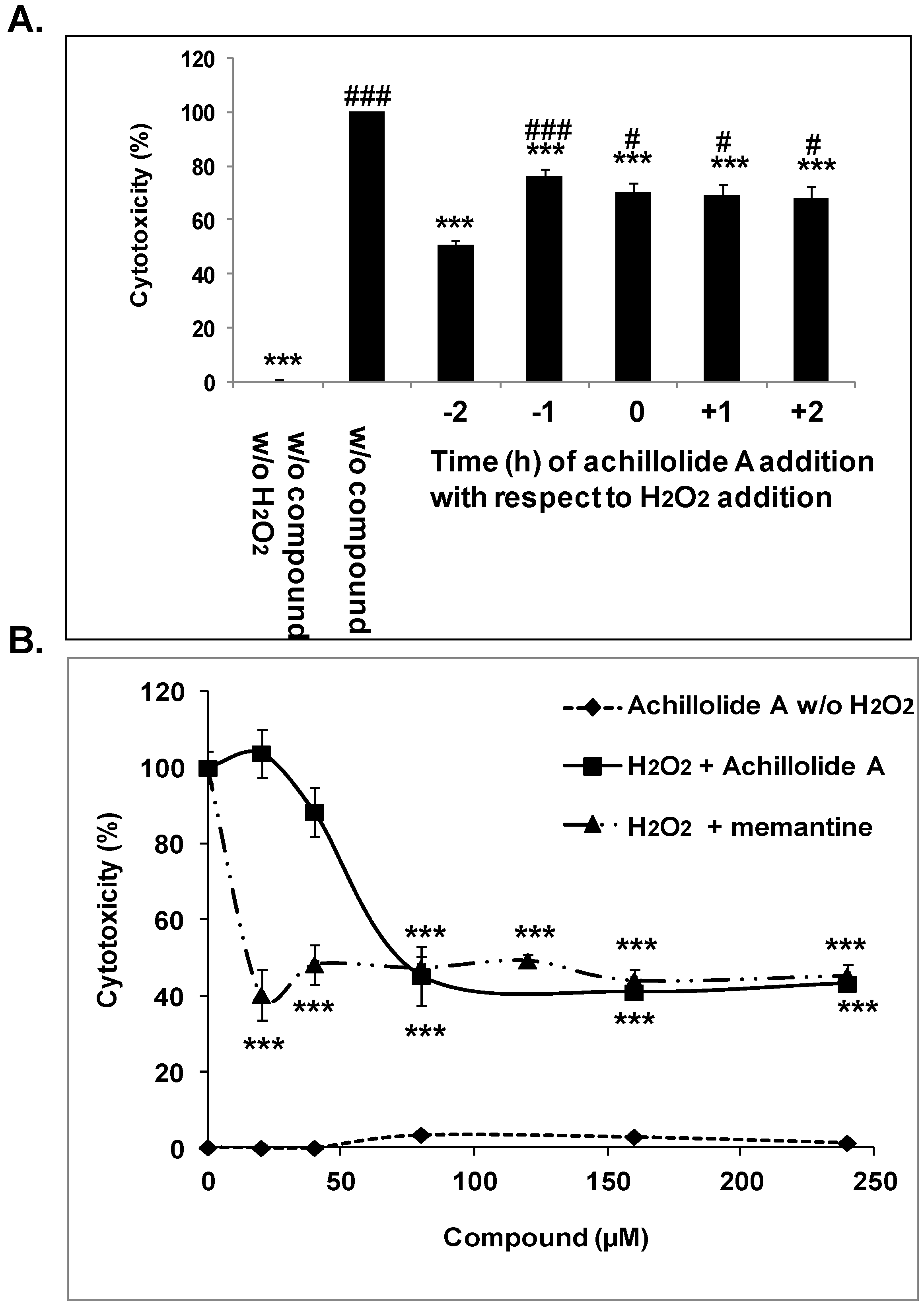
2.1. Achillolide A Protects Astrocytes against H2O2-Induced Cell Death
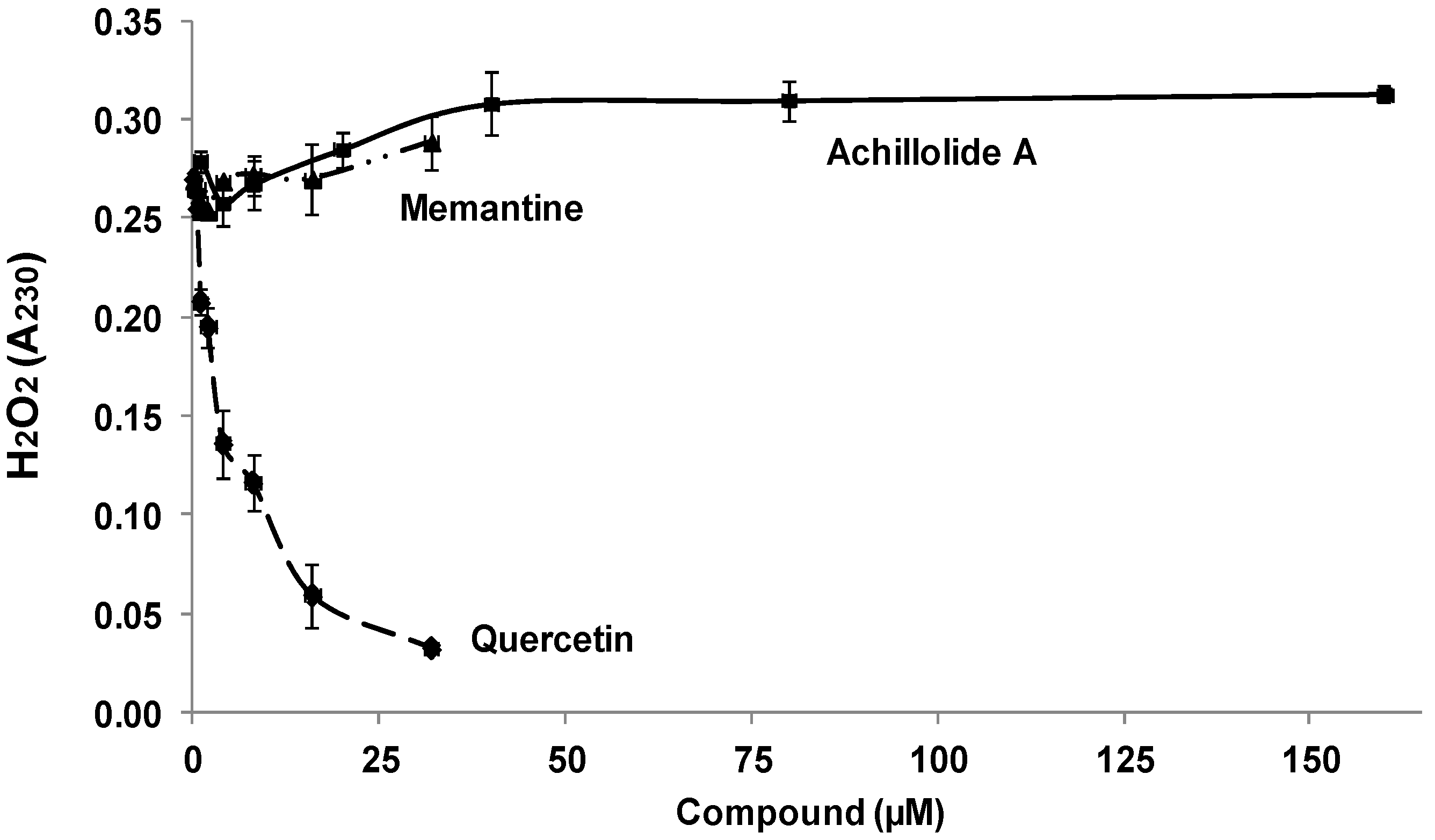
2.2. Achillolide A Does Not Have a Hydrogen-Peroxide Scavenging Activity
2.3. Achillolide A Inhibits H2O2-Induced Phosphorylation of MEK1 and p44/42 MAPK in Astrocytes
2.4. Achillolide A Inhibited the H2O2-Induced Generation of ROS
2.5. Differential Pulse Voltammetry (DPV) Analysis of the Antioxidant Capacity of Achilloide A
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plant Material
4.3. Purification of Achillolide A
4.4. Preparation of Primary Cultures of Astrocytes
4.5. Treatment of Astrocytes with H2O2
4.6. Determination of Cell Viability
4.7. Enzyme-Linked Immunosorbent Assays (ELISA) for Total and Phosphorylated-MEK1 and p44/42 MAPK
4.8. Evaluation of Intracellular ROS Levels
4.9. Determination of H2O2 Scavenging Activity
4.10. Diffrential Pulse Voltammetry (DPV)
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DPV | Differential pulse voltammetry; |
| MAPK | Mitogen-activated protein kinases; |
| MEK | MAP/ERK kinases; |
| ROS | Reactive oxygen species. |
References
- Chan, P.H. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cereb. Blood Flow Metab. 2001, 21, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003, 53, S26–S36; discussion S36–S38. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Fernandez, S.; Almeida, A.; Bolanos, J.P. Antioxidant and bioenergetic coupling between neurons and astrocytes. Biochem. J. 2012, 443, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Thanan, R.; Oikawa, S.; Hiraku, Y.; Ohnishi, S.; Ma, N.; Pinlaor, S.; Yongvanit, P.; Kawanishi, S.; Murata, M. Oxidative Stress and Its Significant Roles in Neurodegenerative Diseases and Cancer. Int. J. Mol. Sci. 2015, 16, 193–217. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar] [PubMed]
- Melo, A.; Monteiro, L.; Lima, R.M.; Oliveira, D.M.; Cerqueira, M.D.; El-Bacha, R.S. Oxidative stress in neurodegenerative diseases: Mechanisms and therapeutic perspectives. Oxid. Med. Cell. Longev. 2011, 2011, 467180. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, E.; Factor-Litvak, P.; Santella, R.M.; Mitsumoto, H. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2013, 65, 509–527. [Google Scholar] [CrossRef] [PubMed]
- Casetta, I.; Govoni, V.; Granieri, E. Oxidative stress, antioxidants and neurodegenerative diseases. Curr. Pharm. Des. 2005, 11, 2033–2052. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Mendes Arent, A.; de Souza, L.F.; Walz, R.; Dafre, A.L. Perspectives on molecular biomarkers of oxidative stress and antioxidant strategies in traumatic brain injury. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Ferrero-Gutierrez, A.; Perez-Gomez, A.; Novelli, A.; Fernandez-Sanchez, M.T. Inhibition of protein phosphatases impairs the ability of astrocytes to detoxify hydrogen peroxide. Free Radic. Biol. Med. 2008, 44, 1806–1816. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, D.; Pamantung, T.F.; Basille, M.; Rousselle, C.; Fournier, A.; Vaudry, H.; Beauvillain, J.C.; Gonzalez, B.J. PACAP protects cerebellar granule neurons against oxidative stress-induced apoptosis. Eur. J. Neurosci. 2002, 15, 1451–1460. [Google Scholar] [CrossRef]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta 2006, 1758, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. The Biological Chemistry of Hydrogen Peroxide. Method Enzymol. 2013, 528, 3–25. [Google Scholar]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. BBA Gen. Subj. 2014, 1840, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Volterra, A.; Meldolesi, J. Astrocytes, from brain glue to communication elements: The revolution continues. Nat. Rev. Neurosci. 2005, 6, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Parpura, V.; Heneka, M.T.; Montana, V.; Oliet, S.H.; Schousboe, A.; Haydon, P.G.; Stout, R.F., Jr.; Spray, D.C.; Reichenbach, A.; Pannicke, T.; et al. Glial cells in (patho)physiology. J. Neurochem. 2012, 121, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Sidoryk-Wegrzynowicz, M.; Wegrzynowicz, M.; Lee, E.; Bowman, A.B.; Aschner, M. Role of astrocytes in brain function and disease. Toxicol. Pathol. 2011, 39, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, R.B.; Munoz, J.P.; Barbeito, L. The molecular bases of Alzheimer’s disease and other neurodegenerative disorders. Arch. Med. Res. 2001, 32, 367–381. [Google Scholar] [CrossRef]
- Vila, M.; Jackson-Lewis, V.; Guegan, C.; Wu, D.C.; Teismann, P.; Choi, D.K.; Tieu, K.; Przedborski, S. The role of glial cells in Parkinson’s disease. Curr. Opin. Neurol. 2001, 14, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Fang, Z.H.; Yu, Z.X.; Wang, C.E.; Li, S.H.; Li, X.J. Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J. Cell Biol. 2005, 171, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Feeney, C.J.; Frantseva, M.V.; Carlen, P.L.; Pennefather, P.S.; Shulyakova, N.; Shniffer, C.; Mills, L.R. Vulnerability of glial cells to hydrogen peroxide in cultured hippocampal slices. Brain Res. 2008, 1198. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Jiang, B.; Liu, J.H.; Lei, C.; Zhang, X.L.; An, L.J. Protective effects of catalpol against H2O2-induced oxidative stress in astrocytes primary cultures. Neurosci. Lett. 2008, 442, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Chan, P.H.; Swanson, R.A. Astrocytes overexpressing Cu,Zn superoxide dismutase have increased resistance to oxidative injury. Glia 2001, 33, 343–347. [Google Scholar] [CrossRef]
- Takuma, K.; Baba, A.; Matsuda, T. Astrocyte apoptosis: Implications for neuroprotection. Prog. Neurobiol. 2004, 72, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Gabryel, B.; Adamczyk, J.; Huzarska, M.; Pudelko, A.; Trzeciak, H.I. Aniracetam attenuates apoptosis of astrocytes subjected to simulated ischemia in vitro. Neurotoxicology 2002, 23, 385–395. [Google Scholar] [CrossRef]
- Giffard, R.G.; Swanson, R.A. Ischemia-induced programmed cell death in astrocytes. Glia 2005, 50, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, Z.; Dafni, A.; Friedman, J.; Palevitch, D. Plants used for the treatment of diabetes in Israel. J. Ethnopharmacol. 1987, 19, 145–151. [Google Scholar] [CrossRef]
- Segal, R.A.; Dor, A.; Duddeck, D.H.; Snatzke, G.; Rosenbaum, D.; Kajtar, M. The sesquiterpene lactones from Achillea fragrantissima, I. Achillolide A and B, two novel germacranolides. Tetrahedron 1987, 43, 4125–4132. [Google Scholar] [CrossRef]
- Shabana, M.M.; Mirhom, Y.W.; Genenah, A.A.; Aboutabl, E.A.; Amer, H.A. Study into wild Egyptian plants of potential medicinal activity. Ninth communication: Hypoglycaemic activity of some selected plants in normal fasting and alloxanised rats. Arch. Exp. Vet. 1990, 44, 389–394. [Google Scholar]
- Mustafa, E.H.; Abu Zarga, M.; Abdalla, S. Effects of cirsiliol, a flavone isolated from Achillea fragrantissima, on rat isolated ileum. Gen. Pharmacol. 1992, 23, 555–560. [Google Scholar] [CrossRef]
- Hamdan, I.; Afifi, F.U. Studies on the in vitro and in vivo hypoglycemic activities of some medicinal plants used in treatment of diabetes in Jordanian traditional medicine. J. Ethnopharmacol. 2004, 93, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Al-Mustafa, A.H.; Al-Thunibat, O.Y. Antioxidant activity of some Jordanian medicinal plants used traditionally for treatment of diabetes. Pak. J. Biol. Sci. 2008, 11, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Mandour, M.A.; Al-Shami, S.A.; Al-Eknah, M.M.; Hussein, Y.A.; El-Ashmawy, I.M. The Acute And Long-Term Safety Evaluation Of Aqueous, Methanolic And Ethanolic Extracts Of Achillea Fragrantissima. Afr. J. Pharm. Pharmacol. 2013, 7, 2282–2290. [Google Scholar]
- Elmann, A.; Telerman, A.; Mordechay, S.; Erlank, H.; Rindner, M.; Kashman, Y.; Ofir, R. Downregulation of microglial activation by achillolide A. Planta Med. 2015, 81, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Merfort, I. Perspectives on sesquiterpene lactones in inflammation and cancer. Curr. Drug Targets 2011, 12, 1560–1573. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Cho, S.B.; Moon, H.I. Neuroprotective effects of a sesquiterpene lactone and flavanones from Paulownia tomentosa Steud. against glutamate-induced neurotoxicity in primary cultured rat cortical cells. Phytother. Res. 2010, 24, 1898–1900. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.M.; Kim, G.H.; Lee, Y.S. Protective effects of dehydrocostus lactone against hydrogen peroxide-induced dysfunction and oxidative stress in osteoblastic MC3T3-E1 cells. Toxicol. Vitr. 2009, 23, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Gach, K.; Dlugosz, A.; Janecka, A. The role of oxidative stress in anticancer activity of sesquiterpene lactones. Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Hyslop, P.A.; Zhang, Z.; Pearson, D.V.; Phebus, L.A. Measurement of striatal H2O2 by microdialysis following global forebrain ischemia and reperfusion in the rat: Correlation with the cytotoxic potential of H2O2 in vitro. Brain Res. 1995, 671, 181–186. [Google Scholar] [CrossRef]
- Barnes, J.S.; Schug, K.A. Oxidative Degradation of Quercetin with Hydrogen Peroxide Using Continuous-Flow Kinetic Electrospray-Ion Trap-Time-of-Flight Mass Spectrometry. J. Agric. Food Chem. 2014, 62, 4322–4331. [Google Scholar] [CrossRef] [PubMed]
- Bin, L.; Yang, X.; Zhang, B.; Yong, H.; Yun, Y.; Ming-Chang, H. MEK inhibition reduces glial scar formation and promotes the recovery of sensorimotor function in rats following spinal cord injury. Exp. Ther. Med. 2014, 7, 66–72. [Google Scholar]
- Mori, T.; Wang, X.; Jung, J.C.; Sumii, T.; Singhal, A.B.; Fini, M.E.; Dixon, C.E.; Alessandrini, A.; Lo, E.H. Mitogen-activated protein kinase inhibition in traumatic brain injury: In vitro and in vivo effects. J. Cereb. Blood Flow Metab. 2002, 22, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Cho, C.L.; Liang, C.L.; Chen, S.D.; Liliang, P.C.; Wang, S.Y.; Chen, H.J. Inhibition of the MEK/ERK pathway reduces microglial activation and interleukin-1-beta expression in spinal cord ischemia/reperfusion injury in rats. J. Thorac. Cardiovasc. Surg. 2007, 133, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Lander, H.M.; Milbank, A.J.; Tauras, J.M.; Hajjar, D.P.; Hempstead, B.L.; Schwartz, G.D.; Kraemer, R.T.; Mirza, U.A.; Chait, B.T.; Burk, S.C.; et al. Redox regulation of cell signalling. Nature 1996, 381, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Bar-Sagi, D.; Hall, A. Ras and Rho GTPases: A family reunion. Cell 2000, 103, 227–238. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef] [PubMed]
- Tournier, C.; Thomas, G.; Pierre, J.; Jacquemin, C.; Pierre, M.; Saunier, B. Mediation by arachidonic acid metabolites of the H2O2-induced stimulation of mitogen-activated protein kinases (extracellular-signal-regulated kinase and c-Jun NH2-terminal kinase). Eur. J. Biochem. 1997, 244, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Urzua, C.; Perez-Ortiz, M.; Bravo, M.; Olivieri, A.C.; Alvarez-Lueje, A. Simultaneous voltammetric determination of levodopa, carbidopa and benserazide in pharmaceuticals using multivariate calibration. Talanta 2010, 82, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Smyth, W.F.; Woolfson, A.D. Drug assays—The role of modern voltammetric techniques. J. Clin. Pharm. Ther. 1987, 12, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Kohen, R.; Beit-Yannai, E.; Berry, E.M.; Tirosh, O. Overall low molecular weight antioxidant activity of biological fluids and tissues by cyclic voltammetry. Methods Enzymol. 1999, 300, 285–296. [Google Scholar] [PubMed]
- Piljac, J.; Martinez, S.; Stipcevic, T.; Petrovic, Z.; Metikos-Hukovic, M. Cyclic voltammetry investigation of the phenolic content of Croatian wines. Am. J. Enol. Vitic. 2004, 55, 417–422. [Google Scholar]
- Yakovleva, K.E.; Kurzeev, S.A.; Stepanova, E.V.; Fedorova, T.V.; Kuznetsov, B.A.; Koroleva, O.V. Characterization of plant phenolic compounds by cyclic voltammetry. Appl. Biochem. Microbiol. 2007, 43, 661–668. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Jeong, G.S.; Pae, H.O.; Jeong, S.O.; Kim, Y.C.; Kwon, T.O.; Lee, H.S.; Kim, N.S.; Park, S.D.; Chung, H.T. The alpha-methylene-gamma-butyrolactone moiety in dehydrocostus lactone is responsible for cytoprotective heme oxygenase-1 expression through activation of the nuclear factor E2-related factor 2 in HepG2 cells. Eur. J. Pharmacol. 2007, 565, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Umemura, K.; Itoh, T.; Hamada, N.; Fujita, Y.; Akao, Y.; Nozawa, Y.; Matsuura, N.; Iinuma, M.; Ito, M. Preconditiopning by sesquiterpene lactone enhances H2O2-induced Nrf2/ARE activation. Biochem. Biophys. Res. Commun. 2008, 368, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Beauchet, O. Possibility of a new anti-alzheimer’s disease pharmaceutical composition combining memantine and vitamin D. Drugs Aging 2012, 29, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Ruch, R.J.; Cheng, S.-J.; Klaunig, J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods; Fundamentals and Applications; 1st ed.; Wiley-Interscience: New-York, NY, USA, 1980; p. 92. [Google Scholar]
- Sample Availability: Samples of the compound achillolide A are available from A. Elmann.



© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmann, A.; Telerman, A.; Erlank, H.; Ofir, R.; Kashman, Y.; Beit-Yannai, E. Achillolide A Protects Astrocytes against Oxidative Stress by Reducing Intracellular Reactive Oxygen Species and Interfering with Cell Signaling. Molecules 2016, 21, 301. https://doi.org/10.3390/molecules21030301
Elmann A, Telerman A, Erlank H, Ofir R, Kashman Y, Beit-Yannai E. Achillolide A Protects Astrocytes against Oxidative Stress by Reducing Intracellular Reactive Oxygen Species and Interfering with Cell Signaling. Molecules. 2016; 21(3):301. https://doi.org/10.3390/molecules21030301
Chicago/Turabian StyleElmann, Anat, Alona Telerman, Hilla Erlank, Rivka Ofir, Yoel Kashman, and Elie Beit-Yannai. 2016. "Achillolide A Protects Astrocytes against Oxidative Stress by Reducing Intracellular Reactive Oxygen Species and Interfering with Cell Signaling" Molecules 21, no. 3: 301. https://doi.org/10.3390/molecules21030301





