Self-Assembled Modified Soy Protein/Dextran Nanogel Induced by Ultrasonication as a Delivery Vehicle for Riboflavin
Abstract
:1. Introduction
2. Results and Discussion
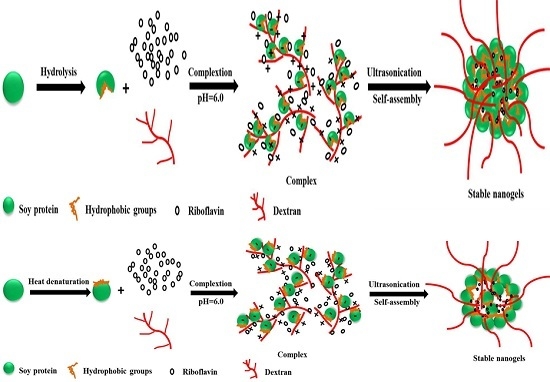
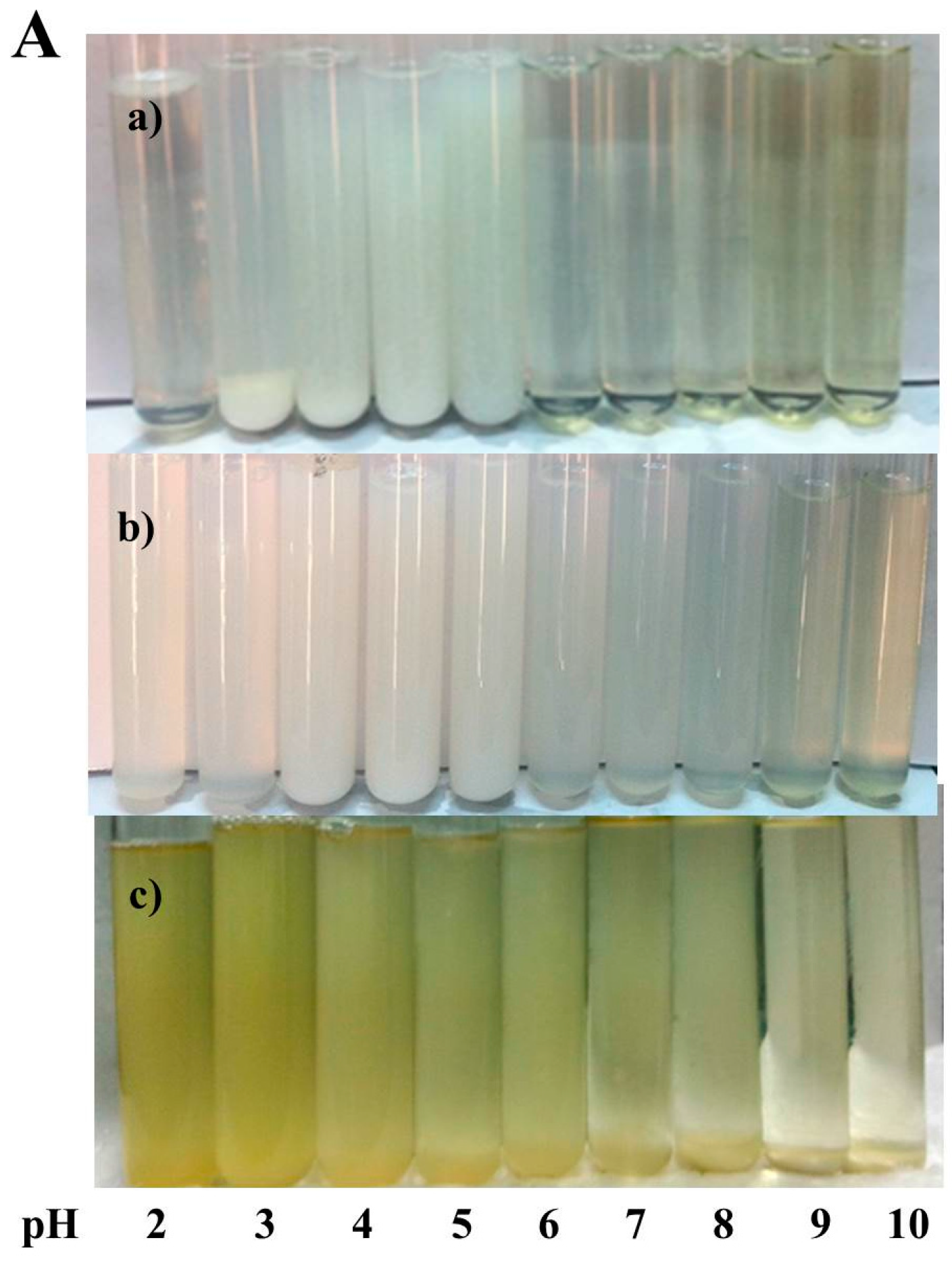
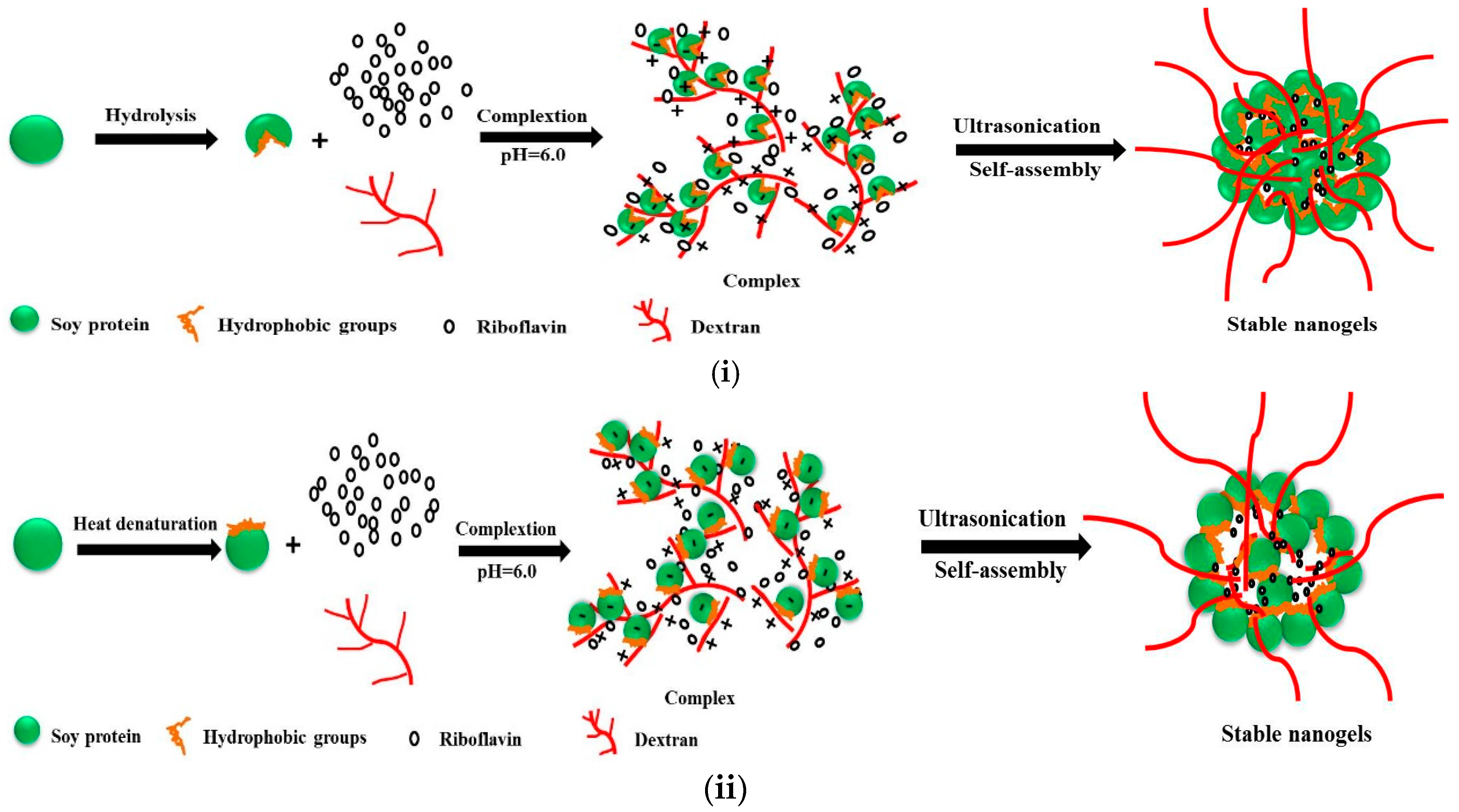
2.1. Optimization of the Fabrication of Modified Soy Protein/Dextran Nanogels
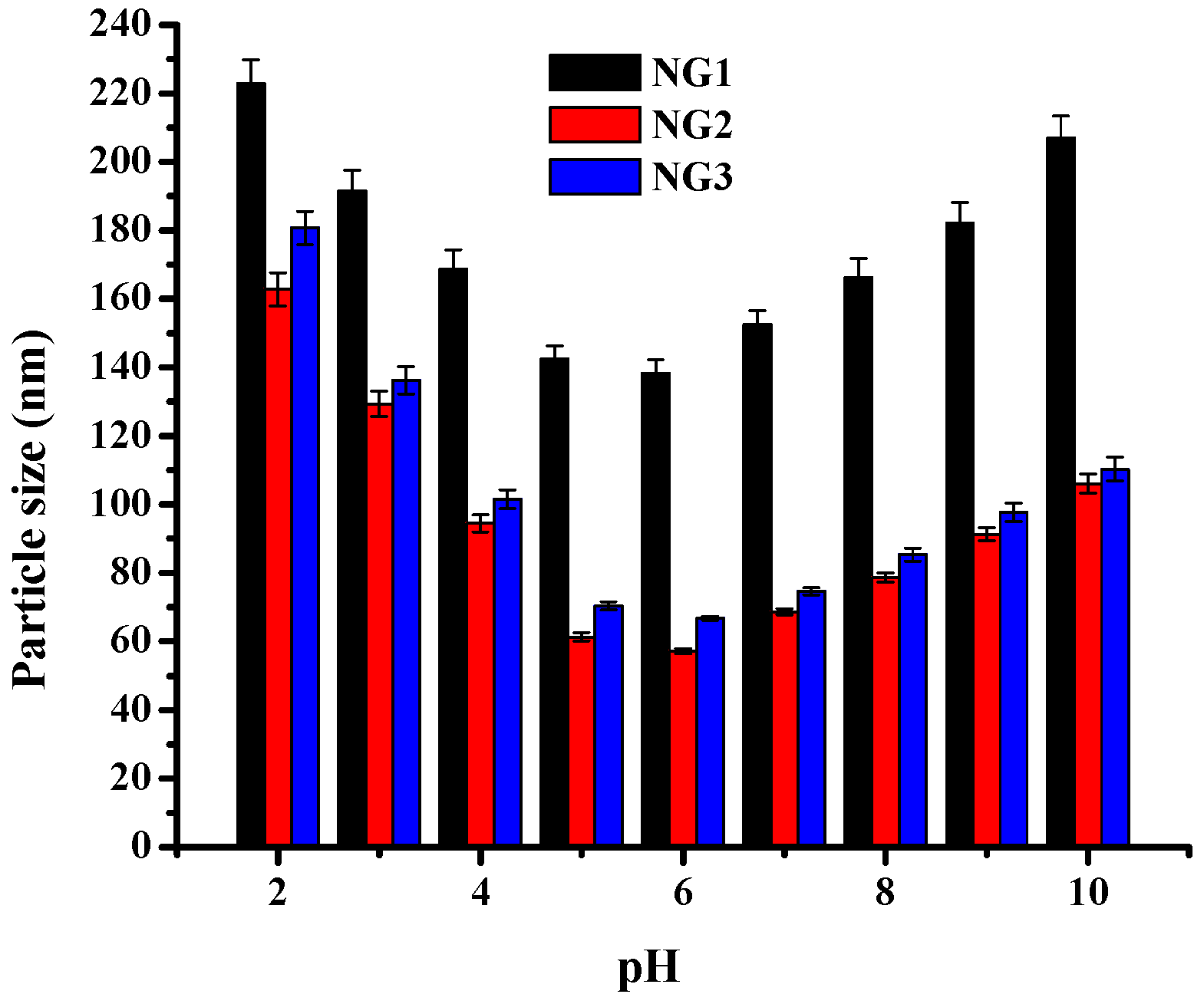
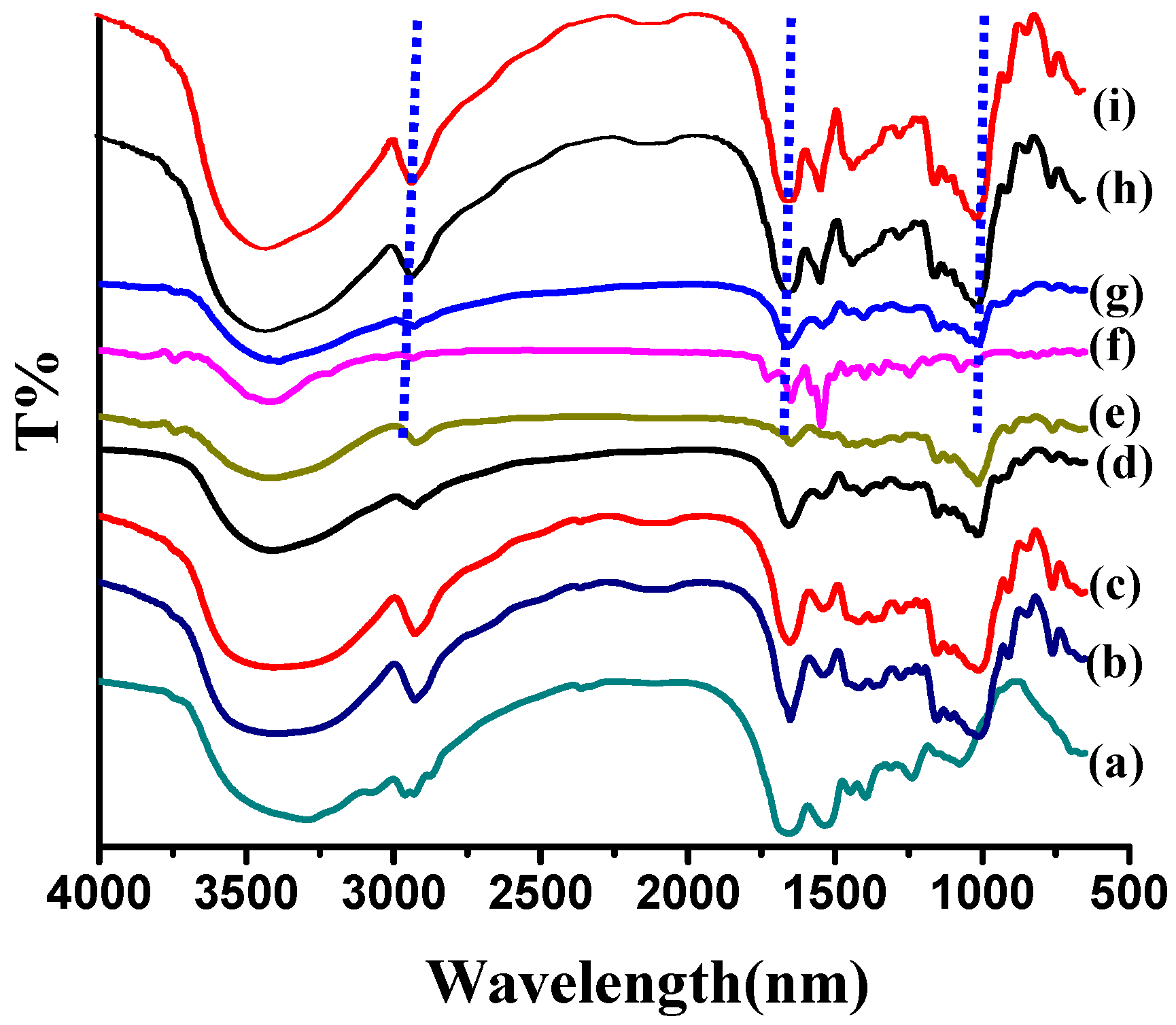
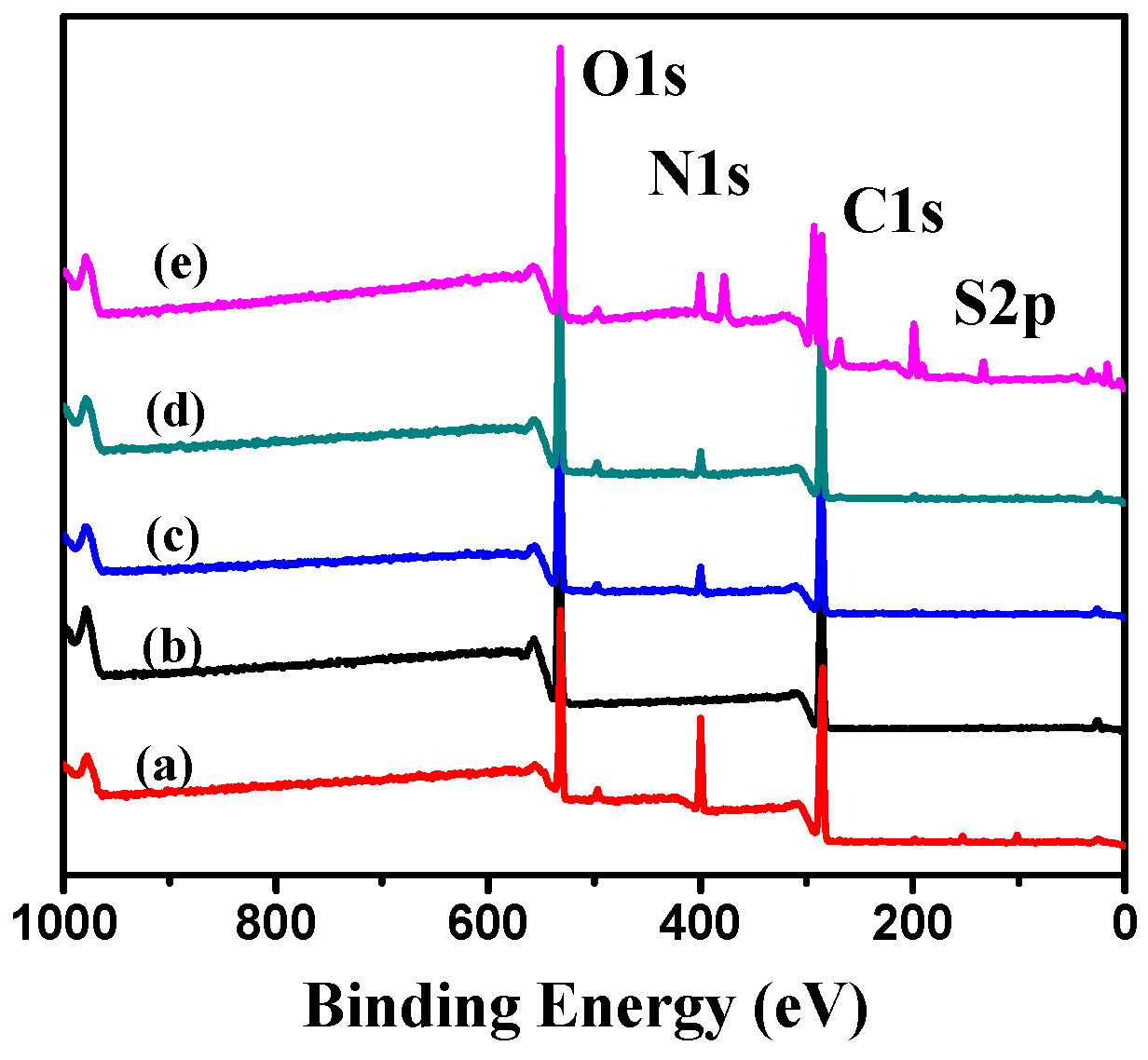
2.2. Characterization of Modified Soy Protein/Dextran Nanogels
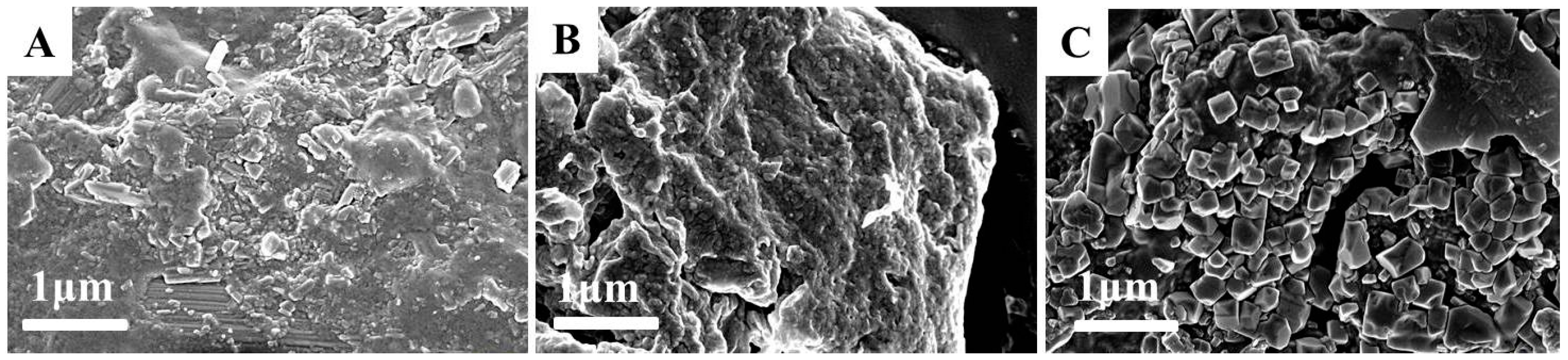
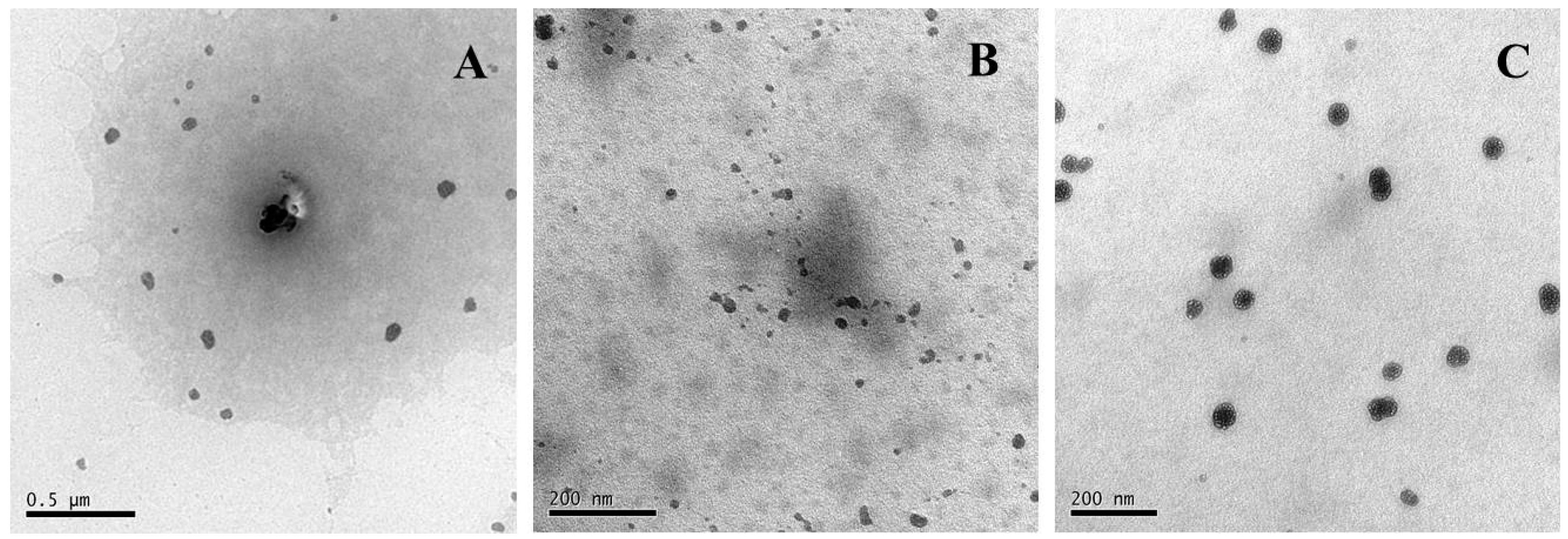
2.3. Morphology Characterization
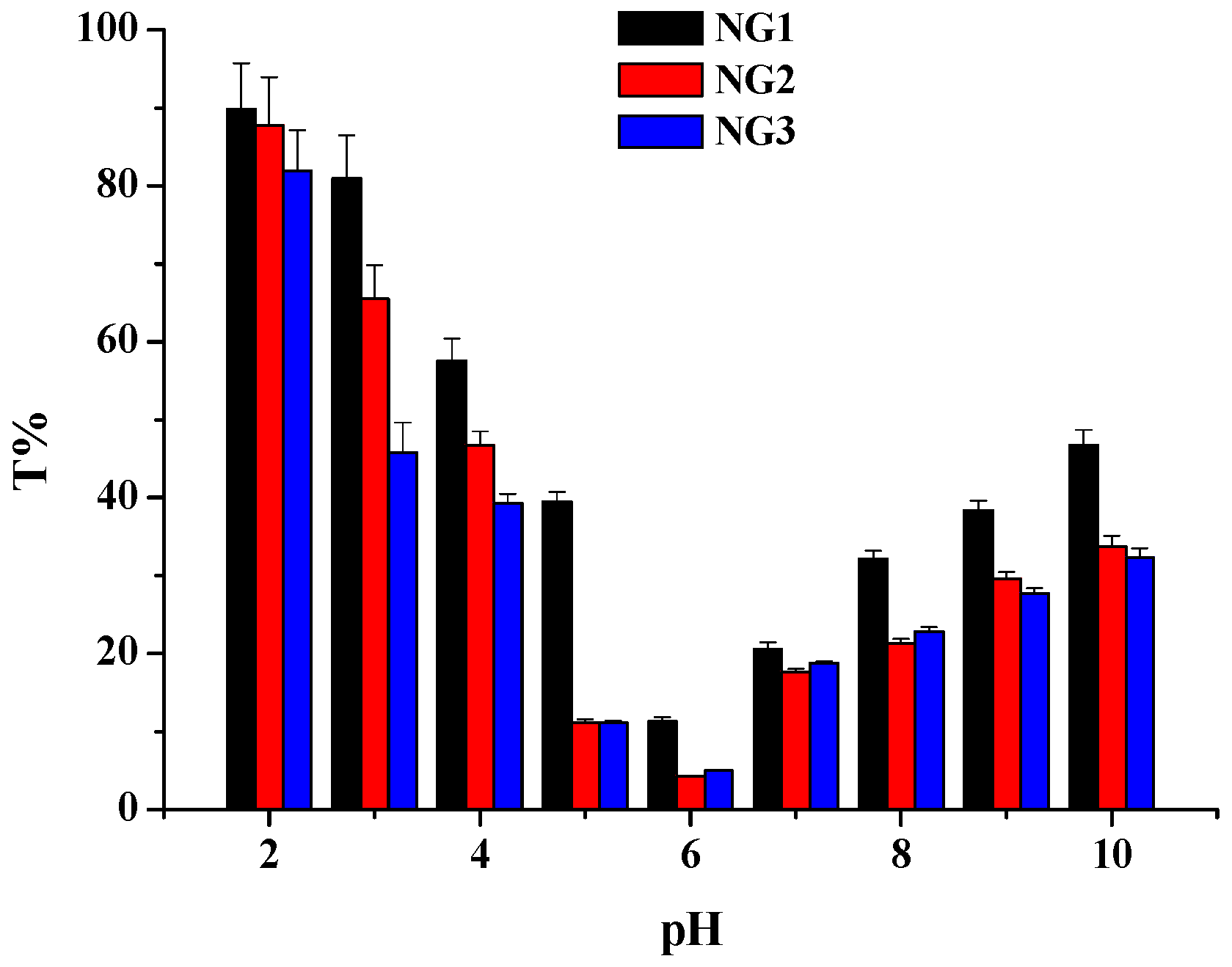
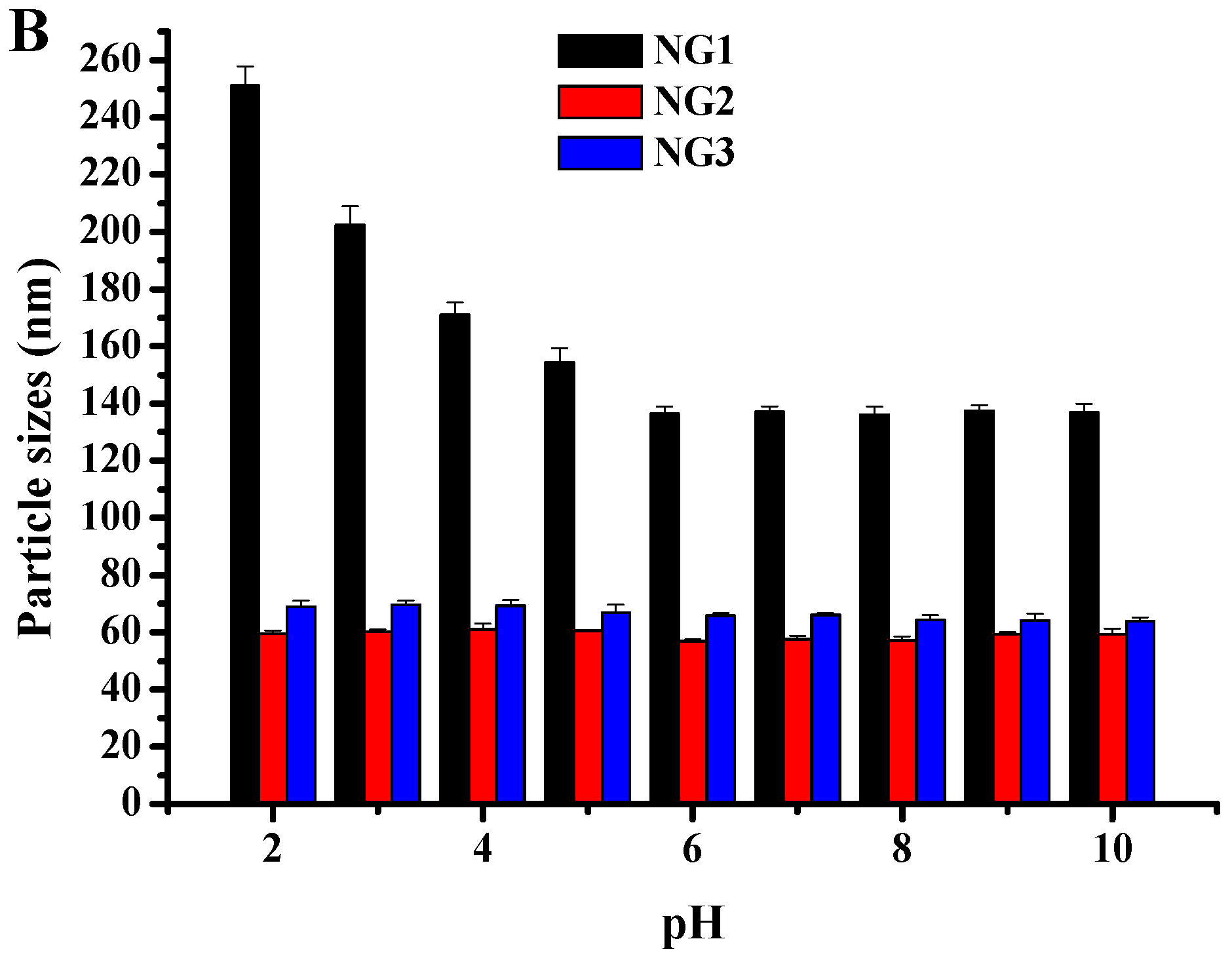
2.4. Physical Stability of Nanogels
2.5. Encapsulation Riboflavin in Modified Soy Protein/Dextran Nanogels
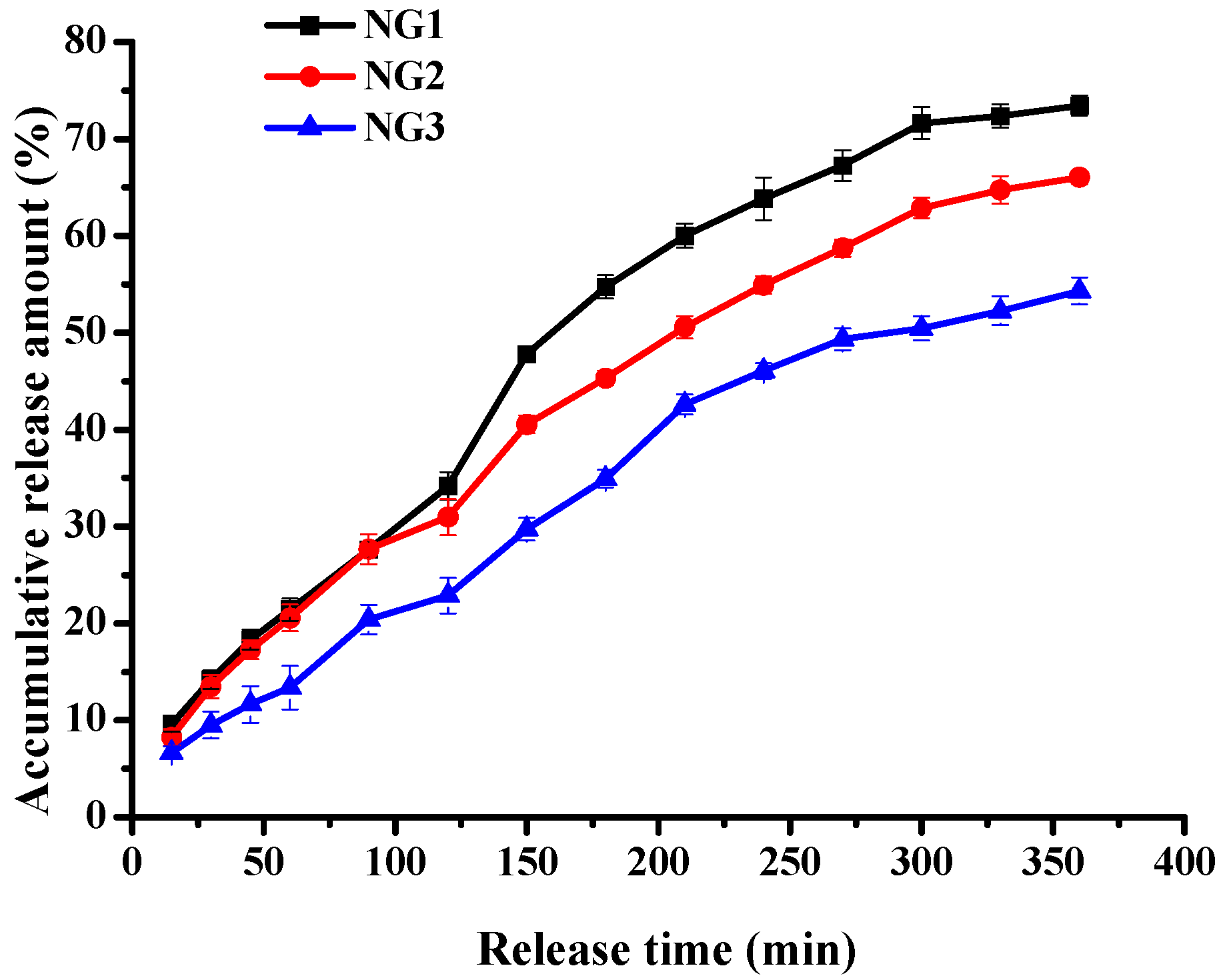
2.6. Riboflavin Release from Modified Soy Protein/Dextran Nanogels
3. Experimental Section
3.1. Materials
3.2. Preparation of Complex Nanogels
3.3. Riboflavin Encapsulation
3.4. Characterization of Blank Nanogels and Riboflavin-Loaded Nanogels
3.5. In vitro Release Studies
3.6. Statistical Analysis
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Sasaki, Y.; Akiyoshi, K. Nanogel engineering for new nano biomaterials: From chaperoning engineering to biomedical applications. Chem. Rec. 2010, 10, 366–376. [Google Scholar] [PubMed]
- Tsuchido, Y.; Sasaki, Y.; Sawada, S.I.; Akiyoshi, K. Protein nanogelation using vitamin B6-bearingpullulan: Effect of zinc ions. Polym. J. 2014, 47, 201–205. [Google Scholar] [CrossRef]
- Mano, J.F. Stimuli-responsive polymeric systems forbiomedical applications. Adv. Eng. Mater. 2008, 10, 515–527. [Google Scholar] [CrossRef]
- Yu, S.; Hu, J.; Pan, X.; Yao, P.; Jiang, M. Stable and pH-sensitive nanogels prepared by self-assembly of chitosan and ovalbumin. Langmuir 2006, 22, 2754–2759. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Ye, T.; Liu, J.; Peng, Z.; Xu, S.; Lei, J.; Deng, H.; Li, B. Nanogels fabricated by lysozyme and sodium carboxymethyl cellulose for 5-fluorouracil controlled release. Int. J. Pharm. 2013, 441, 721–727. [Google Scholar] [CrossRef]
- Ding, X.; Yao, P. Soy protein/soy polysaccharide complex nanogels: Folic acid loading, protection, and controlled delivery. Langmuir 2013, 29, 8636–8644. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.G.; McClements, D.J. Recent progress in biopolymer nanoparticle and microparticle formation by heat-treating electrostatic protein-polysaccharide complexes. Adv. Colloid Interface Sci. 2011, 167, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Bengoechea, C.; Peinado, I.; McClements, D.J. Formation of protein nanoparticlesby controlled heat treatment of lactoferrin: Factors affecting particle characteristics. Food Hydrocoll. 2011, 25, 1354–1360. [Google Scholar] [CrossRef]
- Li, X.; Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Yao, X.; Zhang, Y.; Zhao, M.; Zhang, K.; Jiang, F. Complexation of bovine serum albumin and sugar beet pectin: Structural transitions and phase diagram. Langmuir 2012, 28, 10164–10176. [Google Scholar] [CrossRef] [PubMed]
- Maltais, A.; Remondetto, G.E.; Subirade, M. Soy protein cold-set hydrogels as controlled delivery devicesfor nutraceutical compounds. Food Hydrocoll. 2009, 23, 1647–1653. [Google Scholar] [CrossRef]
- Casadei, M.A.; Cesa, S.; Pacelli, S.; Paolicelli, P.; Tita, B.; Vitali, F. Dextran-based hydrogel microspheres obtained in W/O emulsion: Preparation, characterisation and in vivo studies. J. Microencapsul. 2014, 31, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Zhou, X.S.; Chen, C.Y.; Zhang, X.S.; Chen, S.Q. Preparation, characterization and In vitro evaluation of theophylline nanoparticles prepared with dextran-conjugated soy protein. Trop. J. Pharm. Res. 2015, 14, 1323–1332. [Google Scholar] [CrossRef]
- Feng, J.L.; Qi, J.R.; Yin, S.W.; Wang, J.M.; Guo, J.; Weng, J.Y.; Liu, Q.R.; Yang, X.Q. Fabrication and characterization of stable soy β-conglycinin-dextran core-shell nanogels prepared via a self-assembly approach at the isoelectric point. J. Agric. Food Chem. 2015, 63, 6075–6083. [Google Scholar] [CrossRef] [PubMed]
- Maltais, A.; Remondetto, G.E.; Subirade, M. Tabletted soy protein cold-set hydrogels as carriers of nutraceutical substances. Food Hydrocoll. 2010, 24, 518–524. [Google Scholar] [CrossRef]
- Teng, Z.; Luo, Y.C.; Wang, Q. Nanoparticles synthesized from soy protein: Preparation, characterization, and application for nutraceutical encapsulation. J. Agric. Food Chem. 2012, 60, 2712–2720. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.H.; Dou, H.J.; Sun, K. One-step synthesis of dextran-based stable nanoparticles assistedby self-assembly. Polymer 2006, 47, 728–734. [Google Scholar] [CrossRef]
- Yu, S.Y.; Yao, P.; Jiang, M.; Zhang, G.Z. Nanogels Prepared by Self-Assembly of Oppositely Charged Globular Proteins. Biopolymers 2006, 83, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.A.; Jones, O.G.; McClements, D.J. Comparison of protein-polysaccharide nanoparticle fabrication methods: Impact of biopolymer complexation before or after particle formation. J. Colloid Interface Sci. 2010, 344, 21–29. [Google Scholar]
- Abd EI-Ghaffar, M.A.; Hashem, M.S.; EI-Awady, M.K.; Rabie, A.M. pH-sensitive sodium alginate hydrogels for riboflavin controlled release. Carbohydr. Polym. 2012, 89, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Yang, L.Q.; Zhang, L.M.; Wu, Q. Evaluation of amylose used as a drug delivery carrier. Carbohydr. Res. 2010, 345, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.H.; Kuo, M.I. Ultra-high pressure homogenization effect on the proteins in soy flour. Food Hydrocoll. 2016, 52, 741–748. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Tan, C.; Abbas, S.; Eric, K.; Xia, S.Q.; Zhang, X.M. Modified SPI improves the emulsion properties and oxidative stability of fish oil microcapsules. Food Hydrocoll. 2015, 51, 108–117. [Google Scholar] [CrossRef]
- Chen, N.N.; Lin, L.Z.; Sun, W.Z.; Zhao, M.M. Stable and pH-Sensitive Protein Nanogels Made by Self-Assembly of Heat Denatured Soy Protein. J. Agric. Food Chem. 2014, 62, 9553–9561. [Google Scholar] [PubMed]
- Pujana, M.A.; Pérez-Álvarez, L.; Iturbe, L.C.C.; He, L.; Katime, I. Water soluble folate-chitosan nanogels crosslinked by genipin. Carbohydr. Polym. 2014, 101, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.


| Storage Time (Days) | Particle Size (nm) | ||
|---|---|---|---|
| NG1 | NG2 | NG3 | |
| 0 | 138.3 ± 3.9 | 57.2 ± 0.7 | 66.8 ± 0.6 |
| 30 | 141.3 ± 5.2 | 60.7 ± 1.8 | 69.4 ± 1.2 |
| Riboflavin Concentration (μg/mL) | NG1 | NG2 | NG3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LC (%) | EE (%) | Particle Size (nm) | LC (%) | EE (%) | Particle Size (nm) | LC (%) | EE (%) | Particle Size (nm) | |
| 50 | 1.9 ± 0.1 | 29.8 ± 0.6 | 152.7 ± 4.2 | 3.9 ± 0.2 | 38.9 ± 1.6 | 42.8 ± 2.6 | 4.2 ± 0.4 | 43 ± 1.8 | 49.1 ± 3.4 |
| 100 | 2.6 ± 0.2 | 35.1 ± 0.5 | 147.3 ± 5.1 | 4.7 ± 0.2 | 45.5 ± 2.2 | 47 ± 3.7 | 5.9 ± 0.2 | 47.7 ± 2.7 | 52.2 ± 2.7 |
| 150 | 3.8 ± 0.4 | 41.7 ± 1.6 | 141 ± 4.5 | 6.2 ± 0.8 | 49.3 ± 3.2 | 45.9 ± 3.5 | 7.5 ± 0.7 | 54.9 ± 3.4 | 57.1 ± 2.3 |
| 200 | 4.7 ± 0.3 | 46.6 ± 2.3 | 138.6 ± 5.6 | 8.8 ± 0.9 | 55.4 ± 2.6 | 50.8 ± 2.2 | 9.9 ± 0.5 | 58.7 ± 3.8 | 61.8 ± 4.1 |
| 250 | 7 ± 0.4 | 55.6 ± 0.9 | 143.4 ± 3.3 | 10.4 ± 0.5 | 60.1 ± 2.1 | 53.6 ± 1.8 | 12 ± 0.9 | 65.9 ± 1.2 | 64.9 ± 2.5 |
| 300 | 9.6 ± 0.8 | 56.9 ± 1.0 | 144.7 ± 3.6 | 12.3 ± 0.6 | 57.4 ± 1.4 | 56.6 ± 2.4 | 13.8 ± 1.1 | 60.1 ± 0.9 | 65.2 ± 1.9 |
| 350 | 11 ± 0.7 | 58 ± 0.8 | 148.2 ± 2.5 | 14.6 ± 0.8 | 54.6 ± 1.8 | 55.2 ± 2.8 | 15.9 ± 0.4 | 58.8 ± 1.1 | 65.8 ± 2.6 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, B.; Zhou, X.; Li, X.; Lin, W.; Chen, G.; Qiu, R. Self-Assembled Modified Soy Protein/Dextran Nanogel Induced by Ultrasonication as a Delivery Vehicle for Riboflavin. Molecules 2016, 21, 282. https://doi.org/10.3390/molecules21030282
Jin B, Zhou X, Li X, Lin W, Chen G, Qiu R. Self-Assembled Modified Soy Protein/Dextran Nanogel Induced by Ultrasonication as a Delivery Vehicle for Riboflavin. Molecules. 2016; 21(3):282. https://doi.org/10.3390/molecules21030282
Chicago/Turabian StyleJin, Bei, Xiaosong Zhou, Xiangzhong Li, Weiqin Lin, Guangbin Chen, and Riji Qiu. 2016. "Self-Assembled Modified Soy Protein/Dextran Nanogel Induced by Ultrasonication as a Delivery Vehicle for Riboflavin" Molecules 21, no. 3: 282. https://doi.org/10.3390/molecules21030282