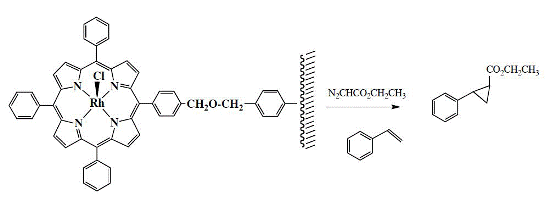
Rhodium Porphyrin Bound to a Merrifield Resin as Heterogeneous Catalyst for the Cyclopropanation Reaction of Olefins
Abstract
:1. Introduction
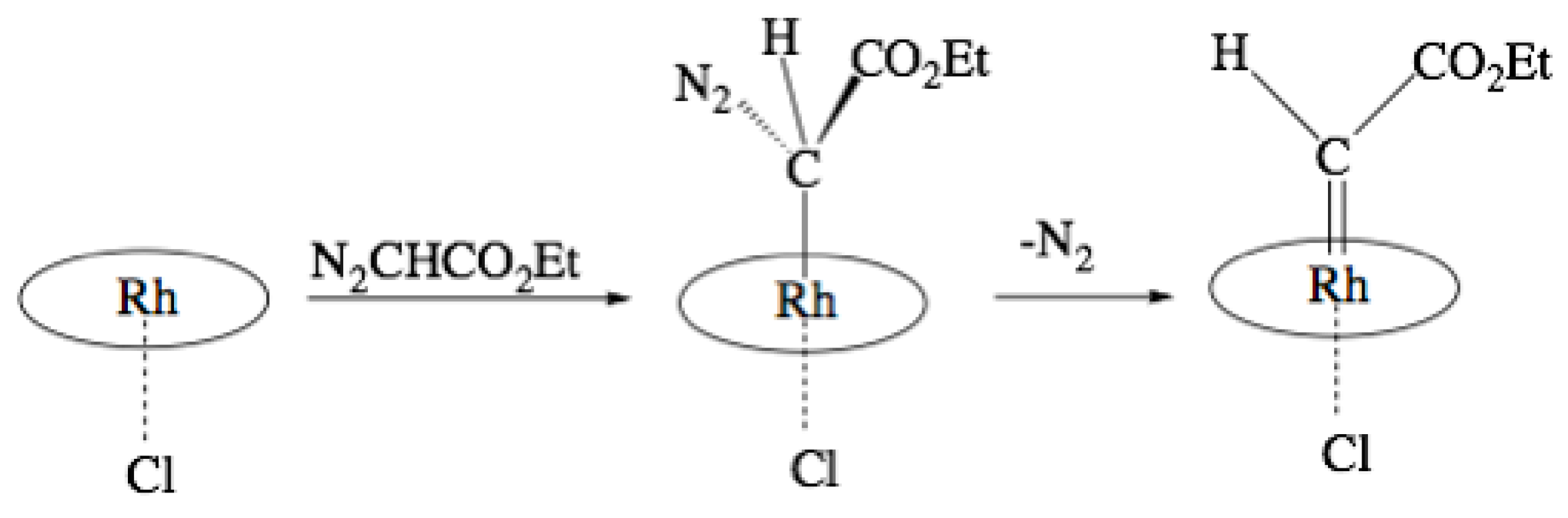
2. Results
3. Discussion
4. Materials and Methods
4.1. General
4.2. Chemicals
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Pogacic, V.; Bullock, A.N.; Federov, O.; Filippakopoulos, P.; Gasser, C.; Biondi, A.; Meyer-Monard, S.; Knapp, S.; Schwaller, J. Structural Analysis Identifies Imidazo[1,2-b]Pyridazines as PIM Kinase Inhibitors with in vitro Antileukemic Activity. Cancer Res. 2007, 67, 6916–6924. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.P. Catalytic methods for metal carbene transformations. Chem. Rev. 1986, 86, 919–939. [Google Scholar] [CrossRef]
- Robbins Wolf, J.; Hamaker, C.G.; Djukic, J.-P.; Kodadek, T.; Woo, L.K. Shape and stereoselective cyclopropanation of alkenes catalyzed by iron porphyrins. J. Am. Chem. Soc. 1995, 117, 9194–9199. [Google Scholar] [CrossRef]
- Maxwell, J.L.; Brown, K.C.; Bartley, D.W.; Kodadek, T. Mechanism of the Rhodium Porphyrin-Catalyzed Cyclopropanation of Alkenes. Science 1992, 256, 1544–1547. [Google Scholar] [CrossRef] [PubMed]
- Hamaker, C.G.; Djukic, J.-P.; Smith, D.A.; Woo, L.K. Mechanism of Cyclopropanation Reactions Mediated by (5,10,15,20-Tetra-p-tolylporphyrinato)osmium(II) Complexes. Organometallics 2001, 20, 5189–5199. [Google Scholar] [CrossRef]
- Intrieri, D.; Caselli, A.; Gallo, E. Cyclopropanation Reactions Mediated by Group 9 Metal Porphyrin Complexes. Eur. J. Inorg. Chem. 2011, 2011, 5071–5081. [Google Scholar] [CrossRef]
- Galardon, E.; le Maux, P.; Simonneaux, G. Cyclopropanation of Alkenes with Ethyldiazoacetate Catalysed by Ruthenium Porphyrin Complexes. Chem. Commun. 1997, 927–928. [Google Scholar] [CrossRef]
- Tollari, S.; Gallo, E.; Musella, D.; Ragaini, F.; Demartin, F.; Cenini, S. Cyclopropanation of Olefins with Diazoalkanes, Catalyzed by CoII(porphyrin) Complexes—A Synthetic and Mechanistic Investigation and the Molecular Structure of CoIII(TPP)(CH2CO2Et) (TPP = Dianion of meso-Tetraphenylporphyrin). Eur. J. Inorg. Chem. 2003, 2003, 1452–1460. [Google Scholar]
- Xu, X.; Lu, H.-J.; Ruppel, J.V.; Cui, X.; de Mesa, S.L.; Wojtas, L.; Zhang, X.P. Highly Asymmetric Intramolecular Cyclopropanation of Acceptor-Substituted Diazoacetates by Co(II)-Based Metalloradical Catalysis: Iterative Approach for Development of New Generation Catalysts. J. Am. Chem. Soc. 2011, 133, 15292–15295. [Google Scholar] [CrossRef] [PubMed]
- Callot, H.J.; Metz, E.; Piechocki, C. Sterically crowded cyclopropanation catalysts. Syn-selectivity using rhodium(III)porphyrins. Tetrahedron 1982, 38, 2365–2369. [Google Scholar] [CrossRef]
- Tagliatesta, P.; Floris, B.; Galloni, P.; Leoni, A.; D’Arcangelo, G. The First Solvent-free Cyclotrimerization Reaction of Arylethynes Catalyzed by Rhodium(III) Porphyrins. Inorg. Chem. 2003, 42, 7701–7703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elakkari, E.; Floris, B.; Galloni, P.; Tagliatesta, P. The Formation of 1-Arylsubstituted Naphthalenes by an Unusual Cyclization of Arylethynes Catalyzed by Ruthenium and Rhodium Porphyrins. Eur. J. Org. Chem. 2005, 2005, 889–894. [Google Scholar] [CrossRef]
- Conte, V.; Elakkari, E.; Floris, B.; Mirruzzo, V.; Tagliatesta, P. The Cyclooligomerization of Arylethynes in Ionic Liquids Catalysed by Ruthenium Porphyrins: A Case of Real Catalyst Recycling. Chem. Commun. 2005, 12, 1587–1588. [Google Scholar] [CrossRef] [PubMed]
- Tagliatesta, P.; Elakkari, E.; Leoni, A.; Lembo, A.; Cicero, D. High Selective Biaryls Formation by the Cyclooligomerization of Arylethynes Catalyzed by Ruthenium and Rhodium Porphyrins. New J. Chem. 2008, 32, 1847–1849. [Google Scholar] [CrossRef]
- Cicero, D.; Lembo, A.; Leoni, A.; Tagliatesta, P. The Highly Selective Formation of Biaryls by the Cyclization of Arylethynes Catalyzed by Vanadyl Phthalocyanine. New J. Chem. 2009, 33, 2162–2165. [Google Scholar] [CrossRef]
- Ciammaichella, A.; Leoni, A.; Tagliatesta, P. Ruthenium porphyrin bound to a Merrifield resin as heterogeneous catalyst for the cyclooligomerization of arylethynes. New J. Chem. 2010, 34, 2122–2124. [Google Scholar] [CrossRef]
- Merrifield, R.B. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Paolesse, R.; Macagnano, A.; Monti, D.; Tagliatesta, P.; Boschi, T.J. Synthesis and Characterization of meso-Tetraphenylporphyrin-Corrole Unsymmetrical Dimers. J. Porphyrins Phthalocyanines 1998, 2, 501–510. [Google Scholar] [CrossRef]
- Fleisher, E.B.; Lavallee, D. Phenylrhodium Tetraphenylporphine. Novel Synthesis of a rhodium-carbon sigma bond. J. Am. Chem. Soc. 1967, 89, 7132–7133. [Google Scholar] [CrossRef]
- Baker, R.H.; Martin, W.B. A Side Reaction in the Williamson Synthesis. J. Org. Chem. 1960, 25, 1496–1498. [Google Scholar] [CrossRef]
- Battioni, P.; Bartoli, J.F.; Mansuy, D.; Byun, Y.S.; Traylor, T.G. An Easy Access to Polyhalogenated Metalloporphyrins Covalently Bound to Polymeric Supports as Efficient Catalysts for Hydrocarbon Oxidation. J. Chem. Soc. Chem. Commun. 1992, 15, 1051–1053. [Google Scholar] [CrossRef]
- Hilal, H.S.; Kim, C.; Sito, M.L.; Schreiner, A.F. Preparation and Characterization of tetra(4-pyridyl)porphyrinatomanganese(III) Cation Supported Covalently on Poly(siloxane). J. Mol. Cat. 1991, 64, 133–142. [Google Scholar] [CrossRef]
- Razenberg, J.A.S.J.; van der Made, A.W.; Smeets, J.W.H.; Nolte, R.J.M. Cyclohexene Epoxidation by the Mono-oxygenase Model (tetraphenylporphyrinato)manganese(III) acetate-sodium Hypochlorite. J. Mol. Cat. 1985, 31, 271–285. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Chan, P.W.H.; Che, C.M. Ruthenium(II) Porphyrin Catalyzed Cyclopropanation of Alkenes with Tosylhydrazones. Tetrahedron Lett. 2003, 44, 8733–8737. [Google Scholar] [CrossRef]
- Galardon, E.; Le Maux, P.; Simonneaux, G. Cyclopropanation of Alkenes, N–H and S–H Insertion of Ethyl Diazoacetate Catalysed by Ruthenium Porphyrin Complexes. Tetrahedron 2000, 56, 615–621. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the all the reported compounds are available from the authors.

| Entry | Substrate | Yield (%) b | syn/anti Ratio c | syn/anti Ratio from Lit. d |
|---|---|---|---|---|
| 1 | Styrene a | 60(71 d) | 1.12 | 1.13 |
| 2 | 4-chlorostyrene | 55 | 1.10 | - |
| 3 | 4-methylstyrene | 53 | 1.30 | - |
| 4 | 4-methoxystyrene | 59 | 1.85 | - |
| 5 | Cyclohexene b | 48(62 d) | 1.03 | 0.84 |
| 6 | Norbonene b | 51(71 d) | 1.27 | 1.28 |
| 7 | 1-Methylcyclopentene b | 45 e | 0.9 e | - |
| 8 | 2,4,4-Trimethylpentene a | 70 e | 0.72 e | - |
| 9 | trans-β-Methylstyrene b | 52 | 0.15 | - |
| Entry | Catalyst | Yield(%) a,b | syn/anti Ratio |
|---|---|---|---|
| 1 | 2 | 59 | 1.04 |
| 2 | 3 | 58 | 0.92 |
| 3 | 4 c | 50 | 1.06 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciammaichella, A.; Cardoni, V.; Leoni, A.; Tagliatesta, P. Rhodium Porphyrin Bound to a Merrifield Resin as Heterogeneous Catalyst for the Cyclopropanation Reaction of Olefins. Molecules 2016, 21, 278. https://doi.org/10.3390/molecules21030278
Ciammaichella A, Cardoni V, Leoni A, Tagliatesta P. Rhodium Porphyrin Bound to a Merrifield Resin as Heterogeneous Catalyst for the Cyclopropanation Reaction of Olefins. Molecules. 2016; 21(3):278. https://doi.org/10.3390/molecules21030278
Chicago/Turabian StyleCiammaichella, Alina, Valeria Cardoni, Alessandro Leoni, and Pietro Tagliatesta. 2016. "Rhodium Porphyrin Bound to a Merrifield Resin as Heterogeneous Catalyst for the Cyclopropanation Reaction of Olefins" Molecules 21, no. 3: 278. https://doi.org/10.3390/molecules21030278