Extraction, Preconcentration and Isolation of Flavonoids from Apocynum venetum L. Leaves Using Ionic Liquid-Based Ultrasonic-Assisted Extraction Coupled with an Aqueous Biphasic System
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of Ionic Liquid
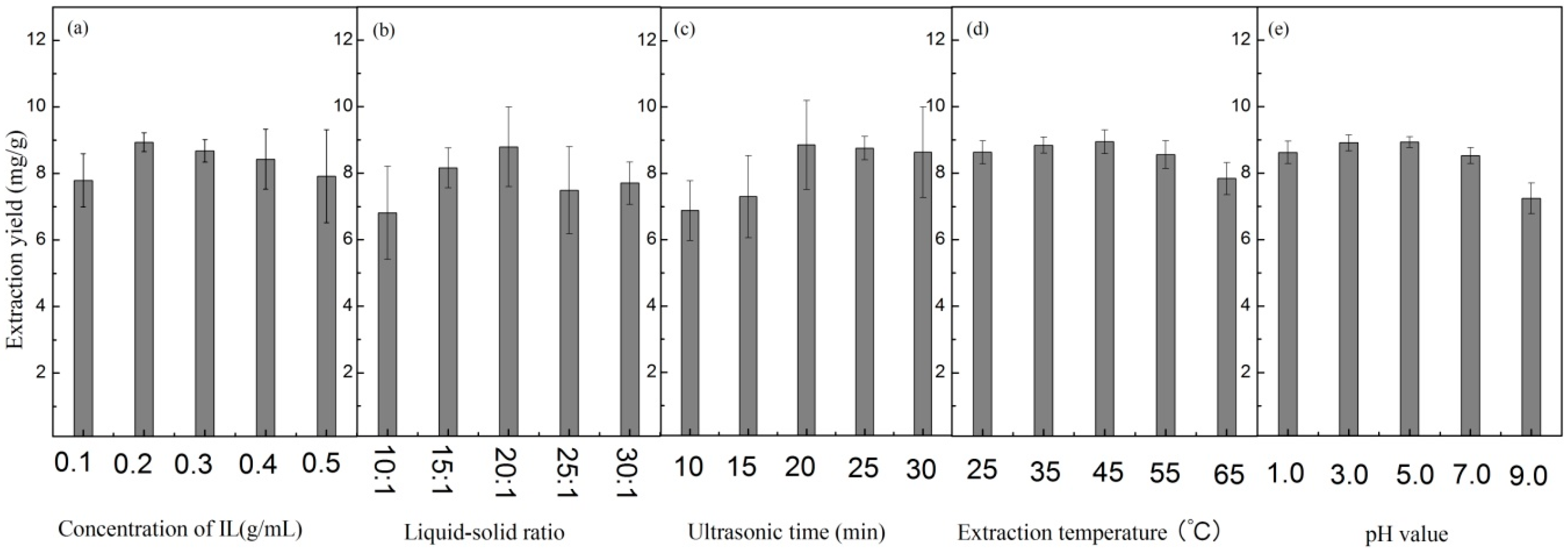
2.2. Optimization of UAE
2.2.1. Single Factor Analysis
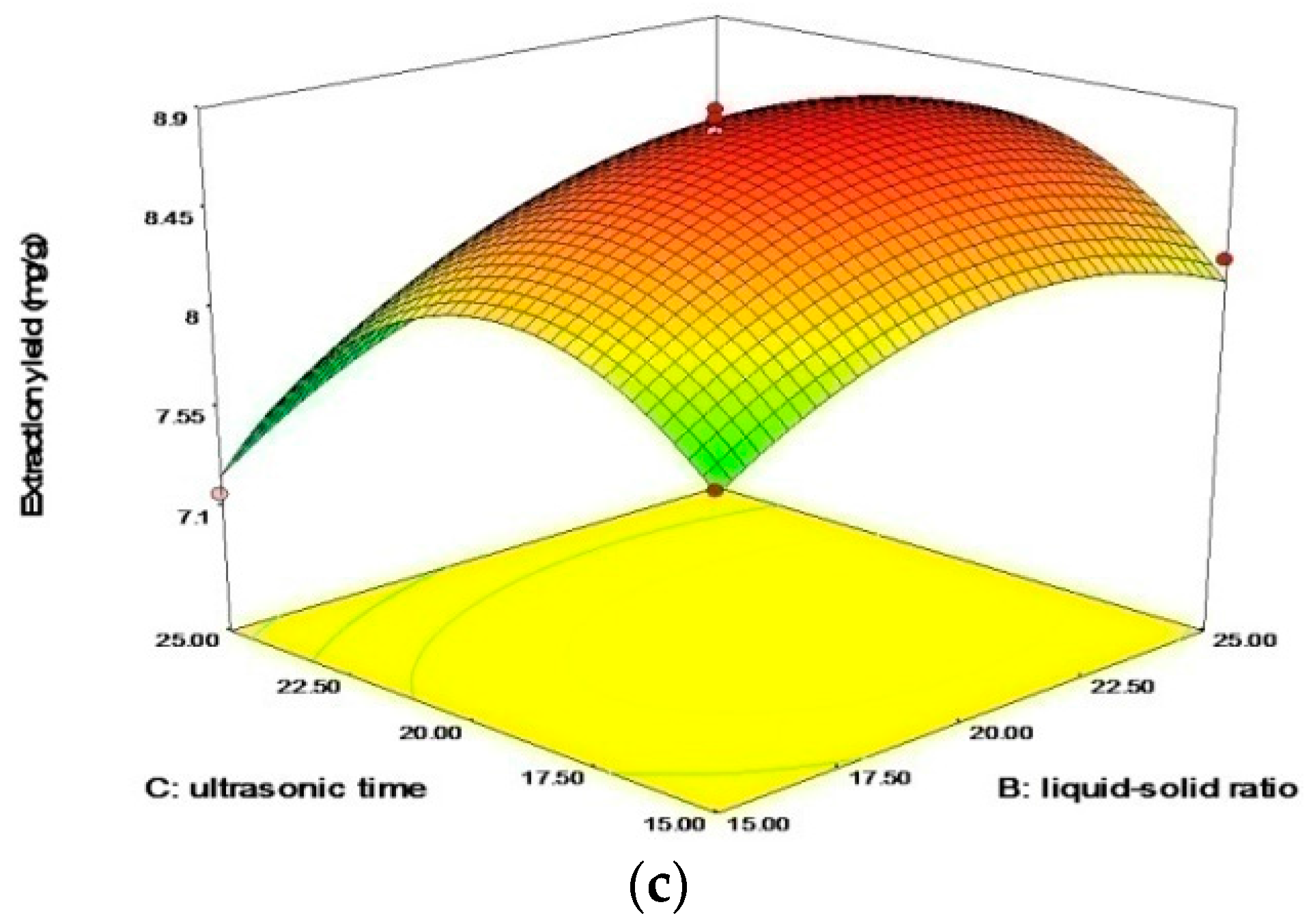
2.2.2. Response Surface Methodology
2.3. Comparison of Different Extraction Methods
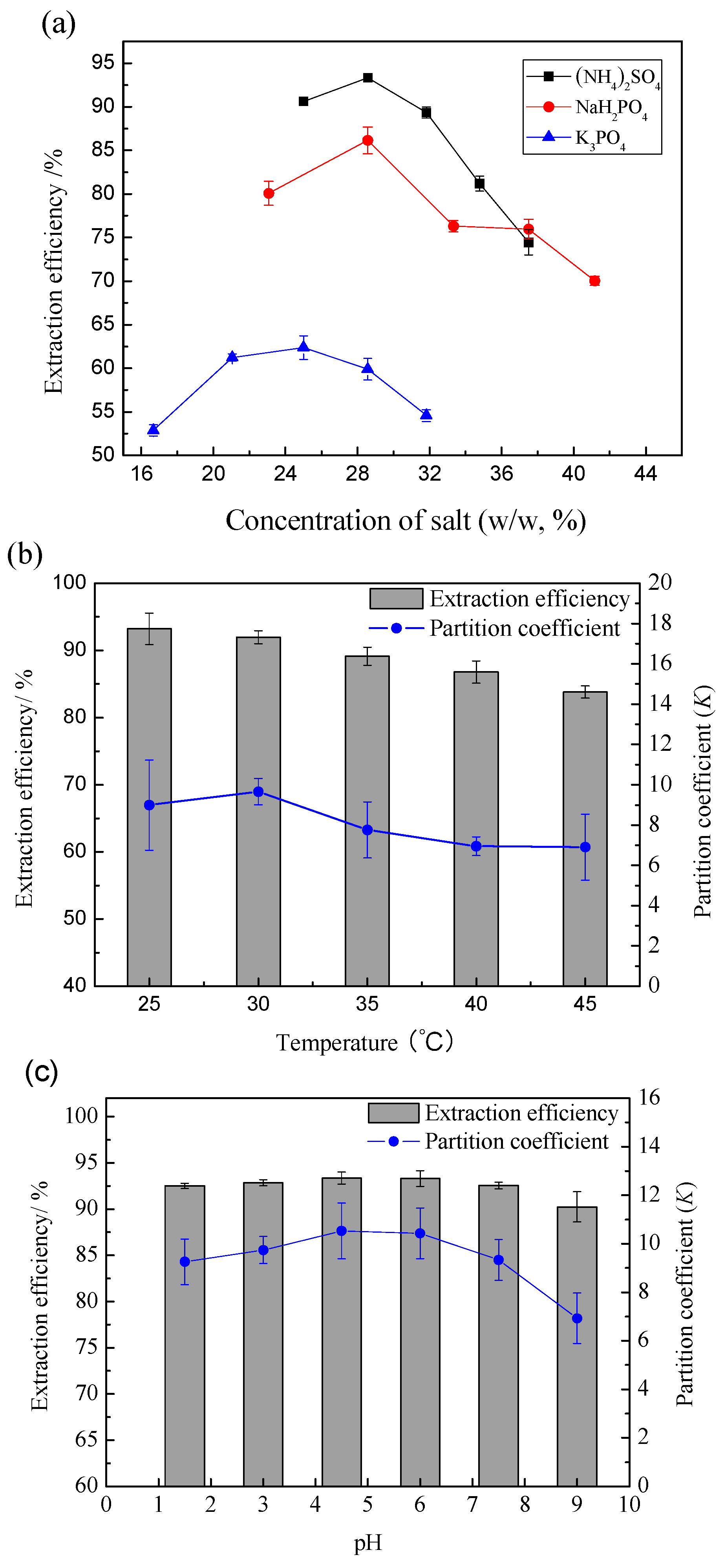
2.4. Extraction and Purification of Flavonoids by IL-ABS
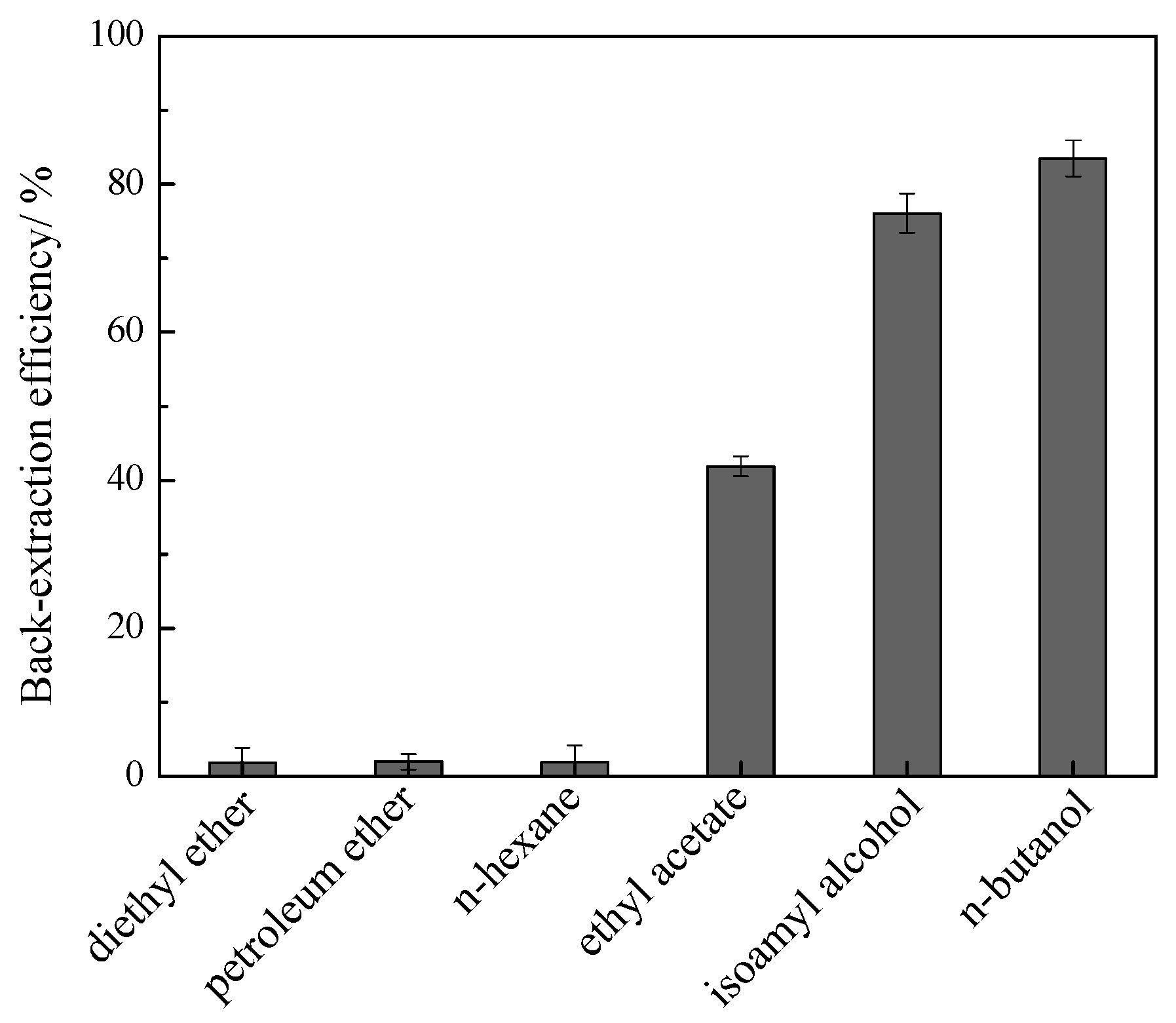
2.5. Isolation of Flavonoids and Recovery of IL
3. Materials and Methods
3.1. Materials and Reagents
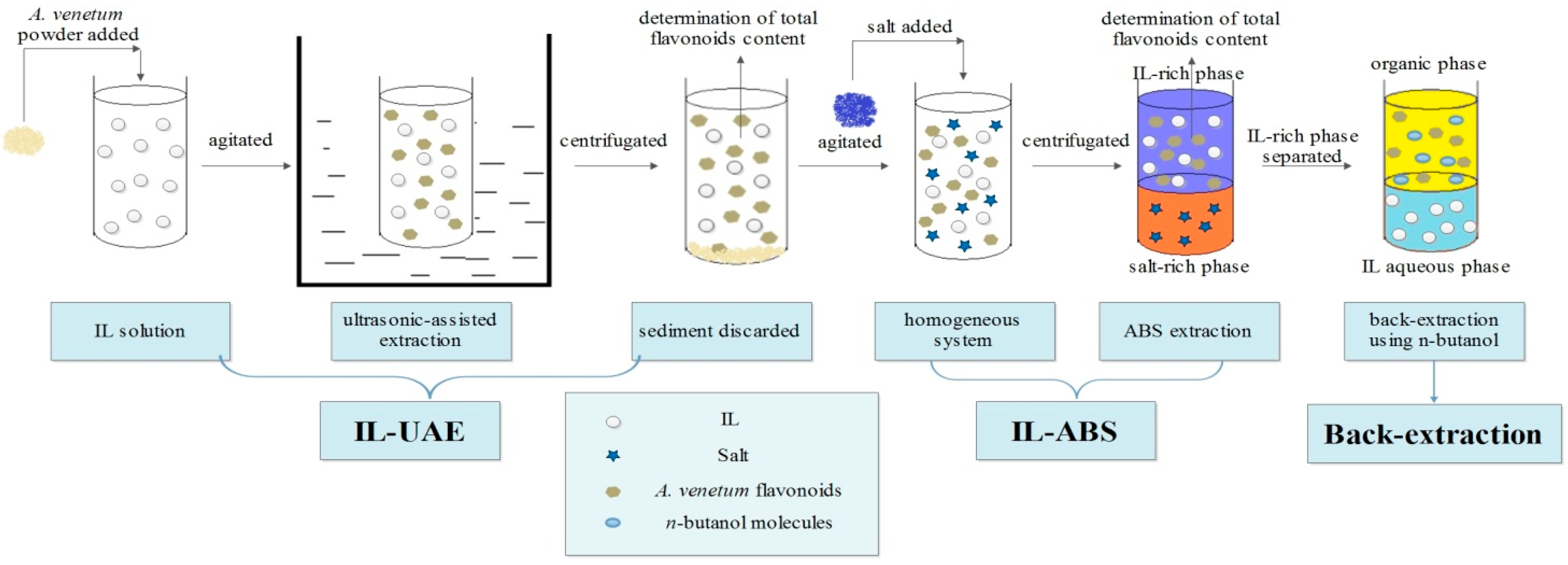
3.2. Ultrasonic-Assisted Extraction
3.3. Aqueous Biphasic System
3.4 Back-Extraction of Flavonoids and Recycle of IL
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Irie, K.; Sato, T.; Tanaka, I.; Nakajima, J.-I.; Kawaguchi, M.; Himi, T. Cardiotonic effect of Apocynum venetum L. Extracts on isolated guinea pig atrium. J. Nat. Med. 2009, 63, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Yue, W.; Li, Q. Comparison of the phenolic content and antioxidant activities of Apocynum venetum L. (luo-bu-ma) and two of its alternative species. Int. J. Mol. Sci. 2010, 11, 4452–4464. [Google Scholar] [CrossRef] [PubMed]
- Kamata, K.; Seo, S.; Nakajima, J.-I. Constituents from leaves of Apocynum venetum L. J. Nat. Med. 2008, 62, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhang, X.; Wang, T.; Hu, J. Botany, traditional uses, phytochemistry and pharmacology of Apocynum venetum L. (luobuma): A review. J. Ethnopharmacol. 2012, 141, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, O.; Nakajima, J.-I.; Seo, S.; Butterweck, V. Anti-anxiety effects of Apocynum venetum L. In the elevated plus maze test. J. Ethnopharmacol. 2007, 110, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, Y.; Liu, X.; Huang, S.; Zeng, Q. Ils-based microwave-assisted extraction coupled with aqueous two-phase for the extraction of useful compounds from Chinese medicine. Analyst 2012, 137, 4076–4085. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.H.; Zhang, X.; Fang, Y.H.; Ye, J.N. Determination of active ingredients of Apocynum venetum by capillary electrophoresis with electrochemical detection. Mikrochim. Acta 2001, 137, 57–62. [Google Scholar] [CrossRef]
- Ma, M.; Hong, C.L.; An, S.Q.; Li, B. Seasonal, spatial, and interspecific variation in quercetin in Apocynum venetum and Poacynum hendersonii, Chinese traditional herbal teas. J. Agric. Food Chem. 2003, 51, 2390–2393. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xia, S.; Ma, P. Use of ionic liquids as “Green” Solvents for extractions. J. Chem. Technol. Biotechnol. 2005, 80, 1089–1096. [Google Scholar] [CrossRef]
- Rogers, R.D. Materials science—Reflections on ionic liquids. Nature 2007, 447, 917–918. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, J.A.P.; Neves, C.M.S.S.; Ventura, S.P.M.; Freire, M.G.; Marrucho, I.M. Evaluation of cation influence on the formation and extraction capability of ionic-liquid-based aqueous biphasic systems. J. Phys. Chem. B 2009, 113, 5194–5199. [Google Scholar]
- Tang, S.; Baker, G.A.; Zhao, H. Ether- and alcohol-functionalized task-specific ionic liquids: Attractive properties and applications. Chem. Soc. Rev. 2012, 41, 4030–4066. [Google Scholar] [CrossRef] [PubMed]
- Albishri, H.M.; Abd El-Hady, D. Eco-friendly ionic liquid based ultrasonic assisted selective extraction coupled with a simple liquid chromatography for the reliable determination of acrylamide in food samples. Talanta 2014, 118, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Z.; Li, Y.; Chi, R. Optimization of ionic liquid-based microwave-assisted extraction of isoflavones from Radix puerariae by response surface methodology. Sep. Purif. Technol. 2014, 135, 285–285. [Google Scholar] [CrossRef]
- Duan, M.-H.; Luo, M.; Zhao, C.-J.; Wang, W.; Zu, Y.-G.; Zhang, D.-Y.; Yao, X.-H.; Fu, Y.-J. Ionic liquid-based negative pressure cavitation-assisted extraction of three main flavonoids from the pigeonpea roots and its pilot-scale application. Sep. Purif. Technol. 2013, 107, 26–36. [Google Scholar] [CrossRef]
- Chatel, G.; Macfarlane, D.R. Ionic liquids and ultrasound in combination: Synergies and challenges. Chem. Soc. Rev. 2014, 43, 8132–8149. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Y.; Zu, Y.-G.; Zhao, C.-J.; Zhang, L.; Chen, X.-Q.; Zhang, Z.-H. Optimize the process of ionic liquid-based ultrasonic-assisted extraction of aesculin and aesculetin from Cortex fraxini by response surface methodology. Chem. Eng. J. 2011, 175, 539–547. [Google Scholar] [CrossRef]
- Cao, X.; Ye, X.; Lu, Y.; Yu, Y.; Mo, W. Ionic liquid-based ultrasonic-assisted extraction of piperine from white pepper. Anal. Chim. Acta 2009, 640, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, Y.; Han, M.; Yang, L. Aqueous ionic liquid based ultrasonic assisted extraction of eight ginsenosides from ginseng root. Ultrason. Sonochem. 2013, 20, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, H.; Zu, Y.-G.; Zhao, C.; Zhang, L.; Chen, X.; Zhang, Z. Ultrasound-assisted extraction of the three terpenoid indole alkaloids vindoline, catharanthine and vinblastine from Catharanthus roseus using ionic liquid aqueous solutions. Chem. Eng. J. 2011, 172, 705–712. [Google Scholar] [CrossRef]
- Ma, C.-H.; Liu, T.-T.; Yang, L.; Zu, Y.-G.; Wang, S.-Y.; Zhang, R.-R. Study on ionic liquid-based ultrasonic-assisted extraction of biphenyl cyclooctene lignans from the fruit of Schisandra chinensis baill. Anal. Chim. Acta 2011, 689, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X. Hydrophobic ionic liquid-based ultrasound-assisted extraction of magnolol and honokiol from cortex Magnoliae officinalis. J. Sep. Sci. 2010, 33, 2035–2038. [Google Scholar] [CrossRef] [PubMed]
- Gutowski, K.E.; Broker, G.A.; Willauer, H.D.; Huddleston, J.G.; Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Controlling the aqueous miscibility of ionic liquids: Aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations. J. Am. Chem. Soc. 2003, 125, 6632–6633. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Li, F.; Xu, X. Isolation and purification of aloe anthraquinones based on an ionic liquid/salt aqueous two-phase system. Sep. Purif. Technol. 2012, 98, 150–157. [Google Scholar] [CrossRef]
- Claudio, A.F.M.; Ferreira, A.M.; Freire, C.S.R.; Silvestre, A.J.D.; Freire, M.G.; Coutinho, J.A.P. Optimization of the gallic acid extraction using ionic-liquid-based aqueous two-phase systems. Sep. Purif. Technol. 2012, 97, 142–149. [Google Scholar] [CrossRef]
- Fan, J.-P.; Cao, J.; Zhang, X.-H.; Huang, J.-Z.; Kong, T.; Tong, S.; Tian, Z.-Y.; Zhu, J.-H.; Ouyang, X.-K. Extraction of puerarin using ionic liquid based aqueous two-phase systems. Sep. Sci. Technol. 2012, 47, 1740–1747. [Google Scholar] [CrossRef]
- Freire, M.G.; Neves, C.M.S.S.; Marrucho, I.M.; Lopes, J.N.C.; Rebelo, L.P.N.; Coutinho, J.A.P. High-performance extraction of alkaloids using aqueous two-phase systems with ionic liquids. Green Chem. 2010, 12, 1715–1718. [Google Scholar] [CrossRef]
- Shi, J.; Li, G.; Zhang, R.; Zheng, J.; Suo, Y.; You, J.; Liu, Y.-J. A validated HPLC-DAD-MS method for identifying and determining the bioactive components of two kinds of luobuma. J. Liquid Chromatogr. Relat. Technol. 2011, 34, 537–547. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Zhang, Z.; Wang, J.; Wu, G.; Li, S. Comprehensive separation and identification of chemical constituents from Apocynum venetum leaves by high-performance counter-current chromatography and high performance liquid chromatography coupled with mass spectrometry. J. Chromatogr. B 2010, 878, 3149–3155. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Wang, H.; Lan, Y.; Hashi, Y.; Chen, S. Simultaneous qualitative and quantitative analysis of phenolic acids and flavonoids for the quality control of Apocynum venetum L. Leaves by HPLC-DAD-ESI-IT-TOF-MS and HPLC-DAD. J. Pharm. Biomed. 2013, 85, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Claudio, A.F.M.; Swift, L.; Hallett, J.P.; Welton, T.; Coutinho, J.A.P.; Freire, M.G. Extended scale for the hydrogen-bond basicity of ionic liquids. Phys. Chem. Chem. Phys. 2014, 16, 6593–6601. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, M.G. Ionic liquids as alternative solvents for extraction of natural products. In Alternative Solvents for Natural Products Extraction; Chemat, F., Abert-Vian, M., Eds.; Springer: Berlin, Germany; Heidelberg, Germany, 2014; pp. 127–166. [Google Scholar]
- Saleh, A.; Yamini, Y.; Faraji, M.; Rezaee, M.; Ghambarian, M. Ultrasound-assisted emulsification microextraction method based on applying low density organic solvents followed by gas chromatography analysis for the determination of polycyclic aromatic hydrocarbons in water samples. J. Chromatogr. A 2009, 1216, 6673–6679. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Zhu, T.; Row, K.H. Ultrasonic extraction of phenolic compounds from Laminaria japonica aresch using ionic liquid as extraction solvent. Bull. Korean Chem. Soc. 2011, 32, 2212–2216. [Google Scholar] [CrossRef]
- Cao, X.; Qiao, J.; Wang, L.; Ye, X.; Zheng, L.; Jiang, N.; Mo, W. Screening of glycoside isomers in P. Scrophulariiflora using ionic liquid-based ultrasonic-assisted extraction and ultra-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.G.; Neves, C.; Marrucho, I.M.; Coutinho, J.A.P.; Fernandes, A.M. Hydrolysis of tetrafluoroborate and hexafluorophosphate counter ions in imidazolium-based ionic liquids. J. Phys. Chem. A 2010, 114, 3744–3749. [Google Scholar] [CrossRef] [PubMed]
- Archer, D.G.; Widegren, J.A.; Kirklin, D.R.; Magee, J.W. Enthalpy of solution of 1-octyl-3-methylimidazolium tetrafluoroborate in water and in aqueous sodium fluoride. J. Chem. Eng. Data 2005, 50, 1484–1491. [Google Scholar] [CrossRef]
- Yoshida, Y.; Baba, O.; Larriba, C.; Saito, G. Imidazolium-based ionic liquids formed with dicyanamide anion: Influence of cationic structure on ionic conductivity. J. Phys. Chem. B 2007, 111, 12204–12210. [Google Scholar] [CrossRef] [PubMed]
- Fredlake, C.P.; Crosthwaite, J.M.; Hert, D.G.; Aki, S.N.V.K.; Brennecke, J.F. Thermophysical properties of imidazolium-based ionic liquids. J. Chem. Eng. Data 2004, 49, 954–964. [Google Scholar] [CrossRef]
- Stoppa, A.; Hunger, J.; Buchner, R. Conductivities of binary mixtures of ionic liquids with polar solvents. J. Chem. Eng. Data 2009, 54, 472–479. [Google Scholar] [CrossRef]
- Ozsoy, N.; Can, A.; Yanardag, R.; Akev, N. Antioxidant activity of Smilax excelsa L. Leaf extracts. Food Chem. 2008, 110, 571–583. [Google Scholar] [CrossRef]
- Bogdanov, M.G.; Keremedchieva, R.; Svinyarov, I. Ionic liquid-supported solid-liquid extraction of bioactive alkaloids. III. Ionic liquid regeneration and glaucine recovery from ionic liquid-aqueous crude extract of Glaucium flavum cr.(papaveraceae). Sep. Purif. Technol. 2015, 155, 13–19. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds flavonoids from A. venetum L. leaves are available from the authors.


| Run | Factor A: Concentration of IL (g/mL) | Factor B: Liquid/Solid Ratio | Factor C: Ultrasonic Time (min) | Response Average Extraction Yield (mg/g) |
|---|---|---|---|---|
| 1 | 0.20 | 15:1 | 20 | 7.84 |
| 2 | 0.40 | 15:1 | 20 | 6.59 |
| 3 | 0.20 | 25:1 | 20 | 8.50 |
| 4 | 0.40 | 25:1 | 20 | 6.61 |
| 5 | 0.20 | 20:1 | 15 | 8.10 |
| 6 | 0.40 | 20:1 | 15 | 6.24 |
| 7 | 0.20 | 20:1 | 25 | 7.21 |
| 8 | 0.40 | 20:1 | 25 | 6.39 |
| 9 | 0.30 | 15:1 | 15 | 7.86 |
| 10 | 0.30 | 25:1 | 15 | 8.23 |
| 11 | 0.30 | 15:1 | 25 | 7.15 |
| 12 | 0.30 | 25:1 | 25 | 7.79 |
| 13 | 0.30 | 20:1 | 20 | 8.77 |
| 14 | 0.30 | 20:1 | 20 | 8.79 |
| 15 | 0.30 | 20:1 | 20 | 8.89 |
| 16 | 0.30 | 20:1 | 20 | 8.76 |
| 17 | 0.30 | 20:1 | 20 | 8.85 |
| Source | Degrees of Freedom | Sum of Squares | Mean Square | F-Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 9 | 13.90 | 1.54 | 147.00 | <0.0001 |
| A | 1 | 4.23 | 4.23 | 402.94 | <0.0001 |
| B | 1 | 0.36 | 0.36 | 53.98 | 0.0006 |
| C | 1 | 0.45 | 0.45 | 42.49 | 0.0003 |
| AB | 1 | 0.1 | 0.1 | 9.75 | 0.0168 |
| AC | 1 | 0.27 | 0.27 | 25.73 | 0.0014 |
| BC | 1 | 0.018 | 0.018 | 1.73 | 0.2293 |
| A2 | 1 | 5.09 | 5.09 | 484.63 | <0.0001 |
| B2 | 1 | 0.45 | 0.45 | 42.91 | 0.0003 |
| C2 | 1 | 2.23 | 2.23 | 211.93 | <0.0001 |
| Residual | 7 | 0.074 | 0.011 | ||
| Lack of fit | 3 | 0.061 | 0.02 | 6.53 | 0.0508 |
| Pure Error | 4 | 0.012 | 3.12 × 10−3 | ||
| Cor total | 16 | 13.98 |
| Methods | Temperature (°C) | Time (h) | Yield (mg/g) | RSD (%) |
|---|---|---|---|---|
| IL-HRE(40%(w/w) IL) | 100 | 6 | 7.31 | 3.5 |
| Ethanol-SE(100%(w/w) ethanol) | 80 | 6 | 6.45 | 2.8 |
| Ethanol-UAE(40%(w/w) ethanol) | 30 | 0.5 | 7.65 | 2.4 |
| Ethanol-UAE(70%(w/w) ethanol) | 30 | 0.5 | 7.88 | 1.8 |
| Ethanol-UAE(100%(w/w) ethanol) | 30 | 0.5 | 7.14 | 2.6 |
| IL-UAE(40%(w/w) IL) | 30 | 0.5 | 8.67 | 1.7 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Z.; Yi, Y.; Wang, H.; Zhou, W.; Wang, C. Extraction, Preconcentration and Isolation of Flavonoids from Apocynum venetum L. Leaves Using Ionic Liquid-Based Ultrasonic-Assisted Extraction Coupled with an Aqueous Biphasic System. Molecules 2016, 21, 262. https://doi.org/10.3390/molecules21030262
Tan Z, Yi Y, Wang H, Zhou W, Wang C. Extraction, Preconcentration and Isolation of Flavonoids from Apocynum venetum L. Leaves Using Ionic Liquid-Based Ultrasonic-Assisted Extraction Coupled with an Aqueous Biphasic System. Molecules. 2016; 21(3):262. https://doi.org/10.3390/molecules21030262
Chicago/Turabian StyleTan, Zhijian, Yongjian Yi, Hongying Wang, Wanlai Zhou, and Chaoyun Wang. 2016. "Extraction, Preconcentration and Isolation of Flavonoids from Apocynum venetum L. Leaves Using Ionic Liquid-Based Ultrasonic-Assisted Extraction Coupled with an Aqueous Biphasic System" Molecules 21, no. 3: 262. https://doi.org/10.3390/molecules21030262






