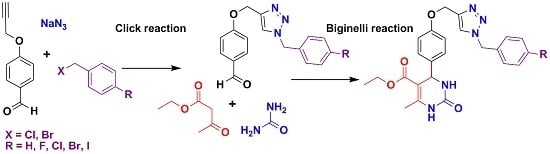
Multicomponent Synthesis and Evaluation of New 1,2,3-Triazole Derivatives of Dihydropyrimidinones as Acidic Corrosion Inhibitors for Steel
Abstract
:1. Introduction
2. Results and Discussion
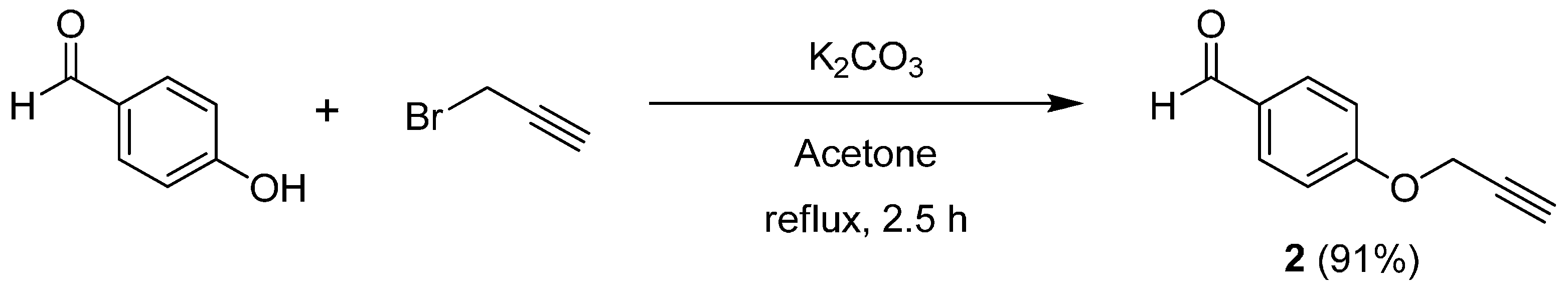
2.1. Synthesis
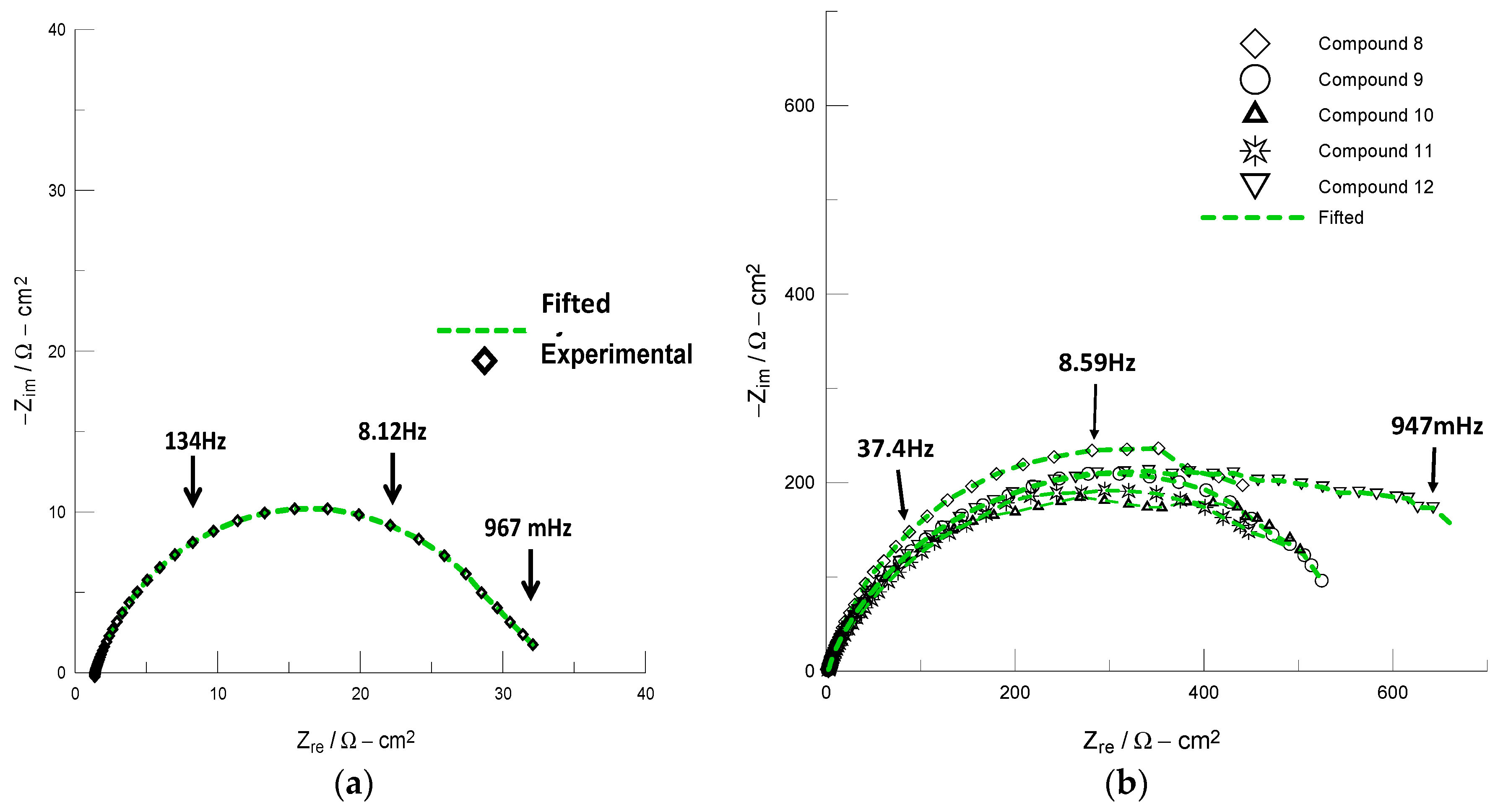
2.2. Corrosion Inhibition Efficiencies
3. Experimental Section
3.1. General Information
3.2. Product Synthesis and Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dömling, A.; Ugi, I. Multicomponent reactions with Isocianides. Angew. Chem. Int. Ed. 2000, 39, 3168–3210. [Google Scholar] [CrossRef]
- Hulme, C.; Gore, V. Multi-component reactions: Emerging chemistry in drug discovery from Xylocain to Crixivan. Curr. Med. Chem. 2003, 10, 51–80. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Bienymé, H. (Eds.) Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005.
- Ganem, B. Strategies for innovation in multicomponent reaction design. Acc. Chem. Res. 2009, 42, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Dömling, A.; Wang, W.; Wang, K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijden, G.; Ruijter, E.; Orru, R.V.A. Efficiency, diversity, and complexity with multicomponent reactions. Synlett 2013, 24, 666–685. [Google Scholar]
- Appkkuttan, P.; Dehaen, W.; Fokin, V.V.; van der Eycken, E. A microwave-assisted click chemistry synthesis of 1,4-disubstituted 1,2,3-triazoles via a copper(I)-catalyzed three-component reaction. Org. Lett. 2004, 6, 4223–4225. [Google Scholar] [CrossRef] [PubMed]
- Bock, V.D.; Hiemstra, H.; van Maarseveen, J.H. CuI-catalyzed alkyne-azide “click” cycloadditions from a mechanistic and synthetic perspective. Eur. J. Org. Chem. 2006. [Google Scholar] [CrossRef]
- Meldal, M.; Tornøe, C.W. Cu-catalyzed azide-alkyne cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed]
- Hein, J.E.; Fokin, V.V. Copper-catalyzed azide-alkyne cycloaddition (CuAAC) and beyond: New reactivity of Copper(I) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Ding, N.-N.; Wei, X.-L.; Cai, L.; He, X.-P.; Long, Y.-T.; Chen, G.-R.; Chen, K. Identification of diverse 1,2,3-triazole-connected benzyl glycoside-serine/threonine conjugates as potent corrosion inhibitors for mild steel in HCl. Corros. Sci. 2012, 64, 64–73. [Google Scholar] [CrossRef]
- Deng, Q.; Shi, H.-W.; Ding, N.-N.; Chen, B.-Q.; He, X.-P.; Liu, G.; Tang, Y.; Long, Y.-T.; Chen, G.-R. Novel triazolyl bis-amino acid derivatives readily synthesized via click chemistry as potential corrosion inhibitors for mild steel in HCl. Corros. Sci. 2012, 57, 220–227. [Google Scholar] [CrossRef]
- Zhang, H.-L.; He, X.-P.; Deng, Q.; Long, Y.-T.; Chen, G.-R.; Chen, K. Research on the structure-surface adsorptive activity relationships of triazolyl glycolipid derivatives for mild steel in HCl. Carbohydr. Res. 2012, 354, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; He, X.-P.; Shi, H.-W.; Chen, B.-Q.; Liu, G.; Tang, Y.; Long, Y.-T.; Chen, G.-R.; Chen, K. Concise Cu(I)-catalyzed azide-alkyne 1,3-dipolar cycloaddition reaction ligation remarkably enhances the corrosion inhibitive potency of natural amino acids for mild steel in HCl. Ind. Eng. Chem. Res. 2012, 51, 7160–7169. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, S.; Quan, H.; Huang, Z.; Xu, S. Synthesis and corrosion inhibition performance of alkyl triazole derivatives. Res. Chem. Intermed. 2015, 41, 2709–2724. [Google Scholar] [CrossRef]
- Ramaganthan, B.; Gopiraman, M.; Olasunkanmi, L.O.; Kabanda, M.M.; Yesudass, S.; Bahadur, I.; Adekunle, A.S.; Obot, I.B.; Ebenso, E.E. Synthesized photo-cross-linking chalcones as novel corrosion inhibitors for mild steel in acidic medium: experimental, quantum chemical and Monte Carlo simulation studies. RSC Adv. 2015, 5, 76675–76688. [Google Scholar] [CrossRef]
- Ganesh, A. Potential biological activity of 1,4-sustituted-1H-[1,2,3]triazoles. Int. J. Chem. Sci. 2013, 11, 573–578. [Google Scholar]
- He, X.-P.; Xie, J.; Tang, Y.; Li, J.; Chen, G.-R. CuAAC click chemistry accelerates the discovery of novel chemical scaffolds as promising protein tyrosine phospatases inhibitors. Curr. Med. Chem. 2012, 19, 2399–2405. [Google Scholar] [PubMed]
- Xu, S.; Zhuang, X.; Pan, X.; Zhang, Z.; Duan, L.; Liu, Y.; Zhang, L.; Ren, X.; Ding, K. 1-Phenyl-4-benzoyl-1H-1,2,3-traizoles as orally bioavailable transcriptional function suppressors of estrogen-related receptor α. J. Med. Chem. 2013, 56, 4631–4640. [Google Scholar] [CrossRef] [PubMed]
- Sirivolu, V.R.; Vernekar, S.K.V.; Ilina, T.; Myshakina, N.S.; Parniak, M.A.; Wang, Z. Clicking 3’-azidothymidine into novel potent inhibitors of human immunodeficiency virus. J. Med. Chem. 2013, 56, 8765–8780. [Google Scholar] [CrossRef] [PubMed]
- Pingaew, R.; Prachayasittikul, V.; Mandi, P.; Nantasenamat, C.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis and molecular docking of 1,2,3-triazole-based sulfonamides as aromatase inhibitors. Bioorg. Med. Chem. 2015, 23, 3472–3480. [Google Scholar] [CrossRef] [PubMed]
- Keck, T.M.; Banal, A.K.; Slack, R.D.; Burzynski, C.; Bonifazi, A.; Okunola-Bakare, O.M.; Moore, M.; Deschamps, J.R.; Rais, R.; Slusher, B.S.; et al. Using click chemistry toward novel 1,2,3-triazole-linked dopamine D3 receptors ligands. Bioorg. Med. Chem. 2015, 23, 4000–4012. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Patil, S.A.; Budagumpi, S.; Nagaraja, B.M. Triazole: A promising antitubercular agent. Chem. Biol. Drug Des. 2015, 86, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Negrón-Silva, G.E.; González-Olvera, R.; Angeles-Beltrán, D.; Maldonado-Carmona, N.; Espinoza-Vázquez, A.; Palomar-Pardavé, M.E.; Romero-Romo, M.A.; Santillan, R. Synthesis of new 1,2,3-triazole derivatives of uracil and thymine with potential inhibitory activity against acidic corrosion of steels. Molecules 2013, 18, 4613–4627. [Google Scholar] [CrossRef] [PubMed]
- González-Olvera, R.; Espinoza-Vázquez, A.; Negrón-Silva, G.E.; Palomar-Pardavé, M.E.; Romero-Romo, M.A.; Santillan, R. Multicomponent click synthesis of new 1,2,3-triazole derivatives of pyrimidine nucleobases: promising acidic corrosion inhibitors for steel. Molecules 2013, 18, 15064–15079. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Gonzalez, D.Y.; González-Olvera, R.; Negrón-Silva, G.E.; Lomas-Romero, L.; Gutiérrez-Carrillo, A.; Palomar-Pardavé, M.E.; Romero-Romo, M.A.; Santillan, R.; Uruchurtu, J. One-pot three-component synthesis of new mono- and bis-1,2,3-triazole derivatives of 2-benzimidazolethiol with a promising inhibitory activity against acidic corrosion of steel. Synthesis 2014, 46, 1217–1223. [Google Scholar] [CrossRef]
- Espinoza-Vázquez, A.; Negrón-Silva, G.E.; González-Olvera, R.; Angeles-Beltrán, D.; Herrera-Hernández, H.; Romero-Romo, M.; Palomar-Pardavé, M. Mild steel corrosion inhibition in HCl by di-alkyl and di-1,2,3-triazole derivatives of uracil and thymine. Mater. Chem. Phys. 2014, 145, 407–417. [Google Scholar] [CrossRef]
- Vargas-Arista, B.; Balvantin, A.; Baltazar, A.; Garcia Vazquez, F. On the use of ultrasonic spectra analysis for the characterization of artificially degrade API 5L X52 steel pipeline welded joints. Mater. Sci. Eng. A 2012, 550, 227–234. [Google Scholar] [CrossRef]
- Javidi, M.; Bahalaou Horeh, S. Investigating the mechanism of stress corrosion craking in near-neutral and high pH enviroments for API 5L X52 steel. Corros. Sci. 2014, 80, 213–220. [Google Scholar] [CrossRef]
- Heydari, M.; Javidi, M. Corrosion inhibition and adsorption behaviour of an amido-imidazoline derivative on API 5L X52steel in CO2-saturated solution and synergistic effect of iodide ions. Corros. Sci. 2012, 61, 148–155. [Google Scholar]
- Sastri, V.S. Green Corrosion Inhibitors: Theory and Practice; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Kappe, C.O. 100 Years of the Biginelli Dihydropyrimidine Synthesis. Tetrahedron 1993, 49, 6937–6963. [Google Scholar] [CrossRef]
- Kappe, C.O. Biologically active dihydropirimidones of the Biginelli-type—A literature survey. Eur. J. Med. Chem. 2000, 35, 1043–1052. [Google Scholar] [CrossRef]
- Kappe, C.O.; Stadler, A. The Biginelli dihydropyrimidine synthesis. Org. React. 2004, 63, 1–116. [Google Scholar]
- Jadhav, V.B.; Holla, H.V.; Tekale, S.U.; Pawar, R.P. Bioactive dihydropyrimidines: An overview. Chem. Sin. 2012, 3, 1213–1228. [Google Scholar]
- Suresh; Sandhu, J.S. Past, present and future of the Biginelli reaction: A critical perspective. ARKIVOC 2012, 1, 66–133. [Google Scholar]
- De Fátima, A.; Braga, T.C.; Neto, L.S.; Terra, B.S.; Oliveira, B.G.F.; da Silva, D.L.; Modolo, L.V. A mini-review on Biginelli adducts with notable pharmacological properties. J. Adv. Res. 2015, 6, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, N.; Akbas, E. The inhibition effect of some pyrimidine derivatives on austenitic stainless steel in acidic media. Mater. Chem. Phys. 2011, 126, 983–988. [Google Scholar] [CrossRef]
- Khanetskyy, B.; Dallinger, D.; Kappe, C.O. Combining Biginelli multicomponent and click chemistry: Generation of 6-(1,2,3-triazol-1-yl)-dihydropyrimidone libraries. J. Comb. Chem. 2004, 6, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-J.; Wang, J.-D. Ultrasound-assisted synthesis of novel 4-(2-phenyl-1,2,3-Triazol-4-yl)-3,4-dihydropyrimidin-2(1H)-(thio)ones Catalyzed by Sm(ClO4)3. Molecules 2010, 15, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Salehi, P.; Dabiri, M.; Koohshari, M.; Movahed, S.K.; Bararjanian, M. One-pot synthesis of 1,2,3-triazole linked dihydropyrimidinones via Huisgen 1,3-dipolar/Biginelli cycloaddition. Mol. Divers. 2011, 15, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, X.; Li, Y. An efficient protocol for the one-pot synthesis of 4-(2-(4-bromophenyl)-1,2,3-triazol-4-yl)-3,4-dihydropyrimidin-2(1H)-ones/thiones catalyzed by Mg(NO3)2. J. Heterocycl. Chem. 2011, 48, 92–95. [Google Scholar] [CrossRef]
- Quan, Z.-J.; Xu, Q.; Zhang, Z.; Da, Y.-X.; Wang, X.-C. Copper-catalyzed click synthesis of functionalized 1,2,3-triazoles with 3,4-dihydropyrimidinone or amide group via a one-pot four-component reaction. Tetrahedron 2013, 69, 881–887. [Google Scholar] [CrossRef]
- Dharma Rao, G.B.; Anjaneyulu, B.; Kaushik, M.P. A facile one-pot five-component synthesis of glycoside annulated dihydropyrimidinone derivatives with 1,2,3-triazol linkage via transesterification/Biginelli/click reactions in aqueous medium. Tetrahedron Lett. 2014, 55, 19–22. [Google Scholar] [CrossRef]
- Balan, B.; Bahulayan, D. A novel green synthesis of α/β-amino acid functionalized pyrimidinone peptidomimetics using triazole ligation through click-multi-component reactions. Tetrahedron Lett. 2014, 55, 227–231. [Google Scholar] [CrossRef]
- Sun, Z.-L.; Jiang, H. Synthesis and characterization of transition metal methanosulfonates and their catalytic behavior in Biginelli reactions. Trans. Metal. Chem. 2005, 30, 792–796. [Google Scholar]
- American Petroleum Institute. API 5L: Specification for Line Pipe; American Petroleum Institute: Washington, DC, USA, 2004; pp. 8–17. [Google Scholar]
- Kinfe, H.H.; Belay, Y.H. Synthesis and biological evaluation of novel thiosemicarbazone-triazole hybrid compounds as antimalarial agents. S. Afr. J. Chem. 2013, 66, 130–135. [Google Scholar]
- Chavan, P.V.; Pandit, K.S.; Desai, U.V.; Kulkarni, M.A.; Wadgaonkar, P.P. Cellulose supported cuprous iodide nanoparticles (Cell-CuI NPs): A new heterogeneous and recyclable catalyst for the one pot synthesis of 1,4-disubstituted-1,2,3-triazoles in water. RSC Adv. 2014, 4, 42137–42146. [Google Scholar] [CrossRef]
- Sample Availability: Samples of compounds 3–13 are available from the authors.

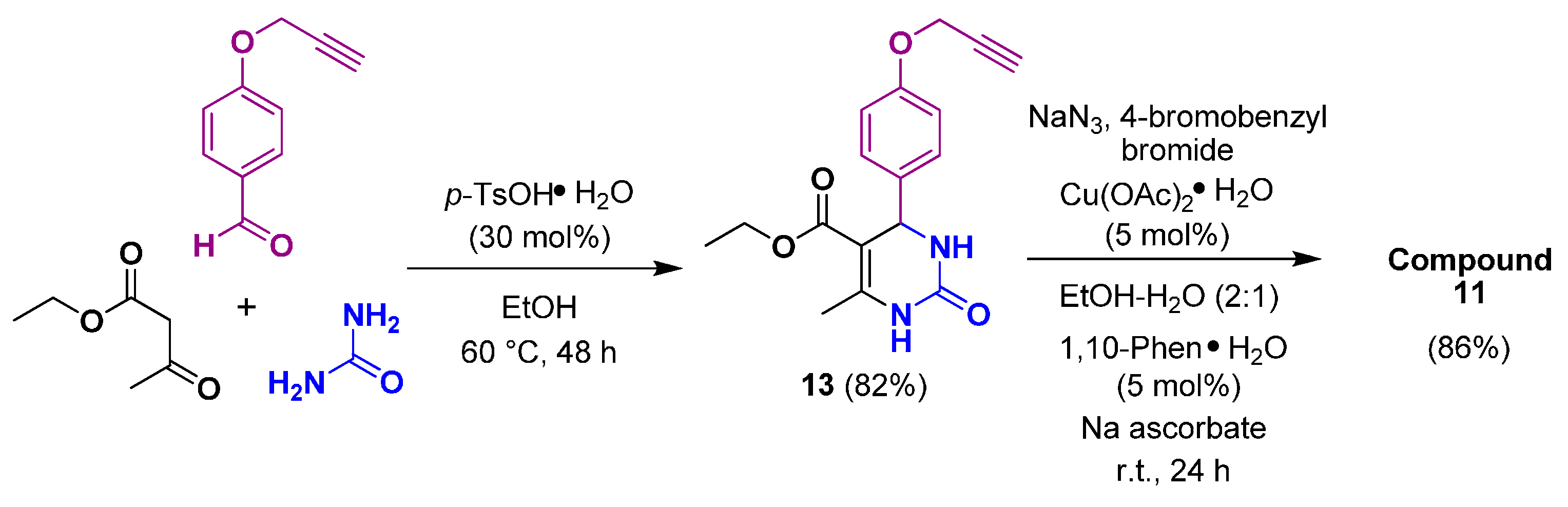
| Entry a | Compound | X | R | Yield c (%) |
|---|---|---|---|---|
| 1 b | 3 | Cl | H | 78 |
| 2 | 3 | Cl | H | 82 |
| 3 | 4 | Cl | F | 80 |
| 4 | 5 | Cl | Cl | 86 |
| 5 | 6 | Br | Br | 75 |
| 6 | 7 | Br | I | 72 |
| Entry a | Compound | R | Yield b (%) |
|---|---|---|---|
| 1 | 8 | H | 80 |
| 2 | 9 | F | 87 |
| 3 | 10 | Cl | 86 |
| 4 | 11 | Br | 85 |
| 5 | 12 | I | 84 |
| Compound | Cdl/µF·cm−2 | Rct/Ωcm2 | IE/% |
|---|---|---|---|
| Blank | 310.0 | 30.0 | - |
| 8 | 38.0 | 618.4 | 95.1 |
| 9 | 83.3 | 564.9 | 94.7 |
| 10 | 34.8 | 733.0 | 95.9 |
| 11 | 61.9 | 573.7 | 94.8 |
| 12 | 49.5 | 678.9 | 95.6 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Olvera, R.; Román-Rodríguez, V.; Negrón-Silva, G.E.; Espinoza-Vázquez, A.; Rodríguez-Gómez, F.J.; Santillan, R. Multicomponent Synthesis and Evaluation of New 1,2,3-Triazole Derivatives of Dihydropyrimidinones as Acidic Corrosion Inhibitors for Steel. Molecules 2016, 21, 250. https://doi.org/10.3390/molecules21020250
González-Olvera R, Román-Rodríguez V, Negrón-Silva GE, Espinoza-Vázquez A, Rodríguez-Gómez FJ, Santillan R. Multicomponent Synthesis and Evaluation of New 1,2,3-Triazole Derivatives of Dihydropyrimidinones as Acidic Corrosion Inhibitors for Steel. Molecules. 2016; 21(2):250. https://doi.org/10.3390/molecules21020250
Chicago/Turabian StyleGonzález-Olvera, Rodrigo, Viridiana Román-Rodríguez, Guillermo E. Negrón-Silva, Araceli Espinoza-Vázquez, Francisco Javier Rodríguez-Gómez, and Rosa Santillan. 2016. "Multicomponent Synthesis and Evaluation of New 1,2,3-Triazole Derivatives of Dihydropyrimidinones as Acidic Corrosion Inhibitors for Steel" Molecules 21, no. 2: 250. https://doi.org/10.3390/molecules21020250