Constructing a MoS2 QDs/CdS Core/Shell Flowerlike Nanosphere Hierarchical Heterostructure for the Enhanced Stability and Photocatalytic Activity
Abstract
:1. Introduction
2. Results and Discussion
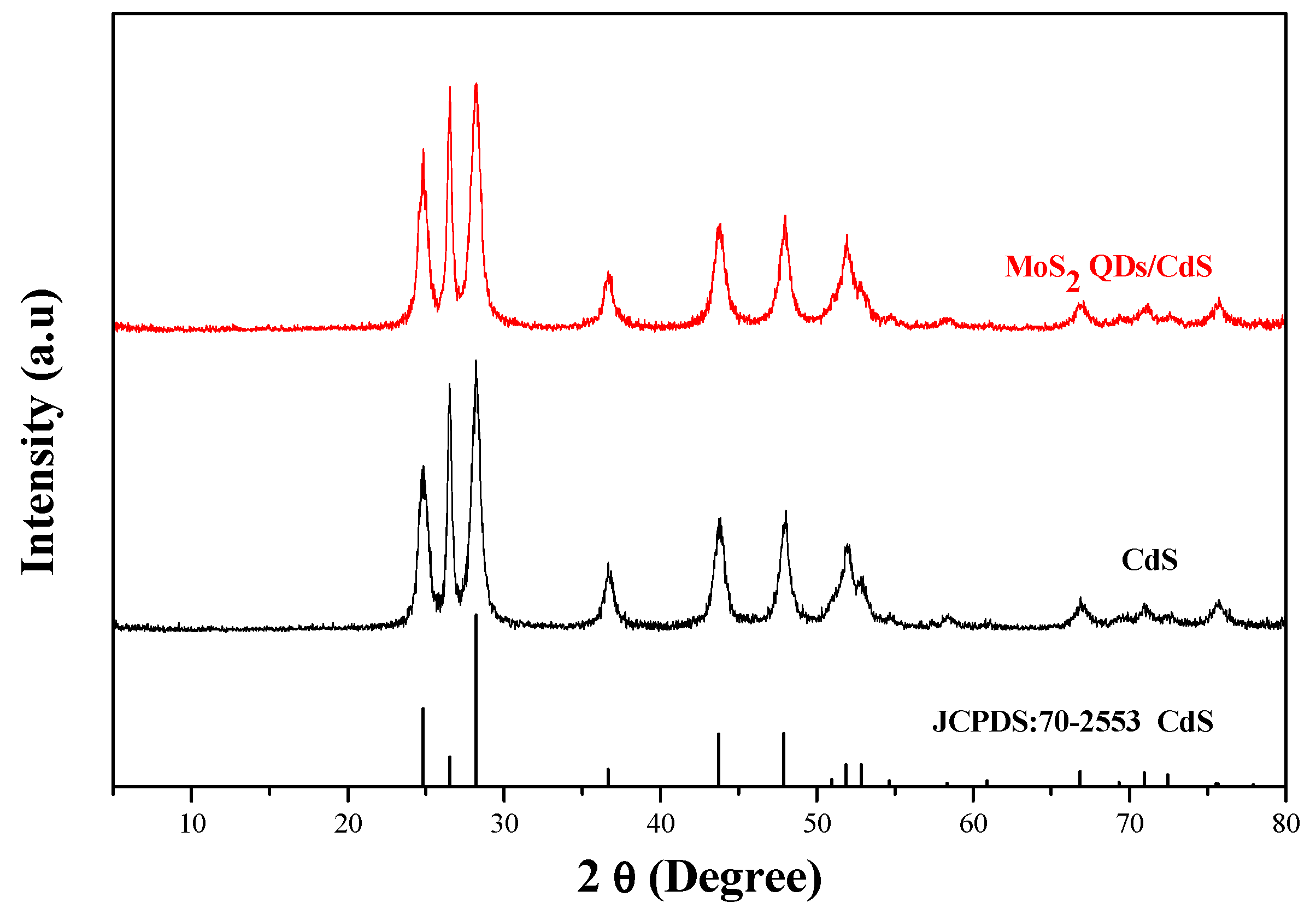
2.1. Phase Structure and Morphology
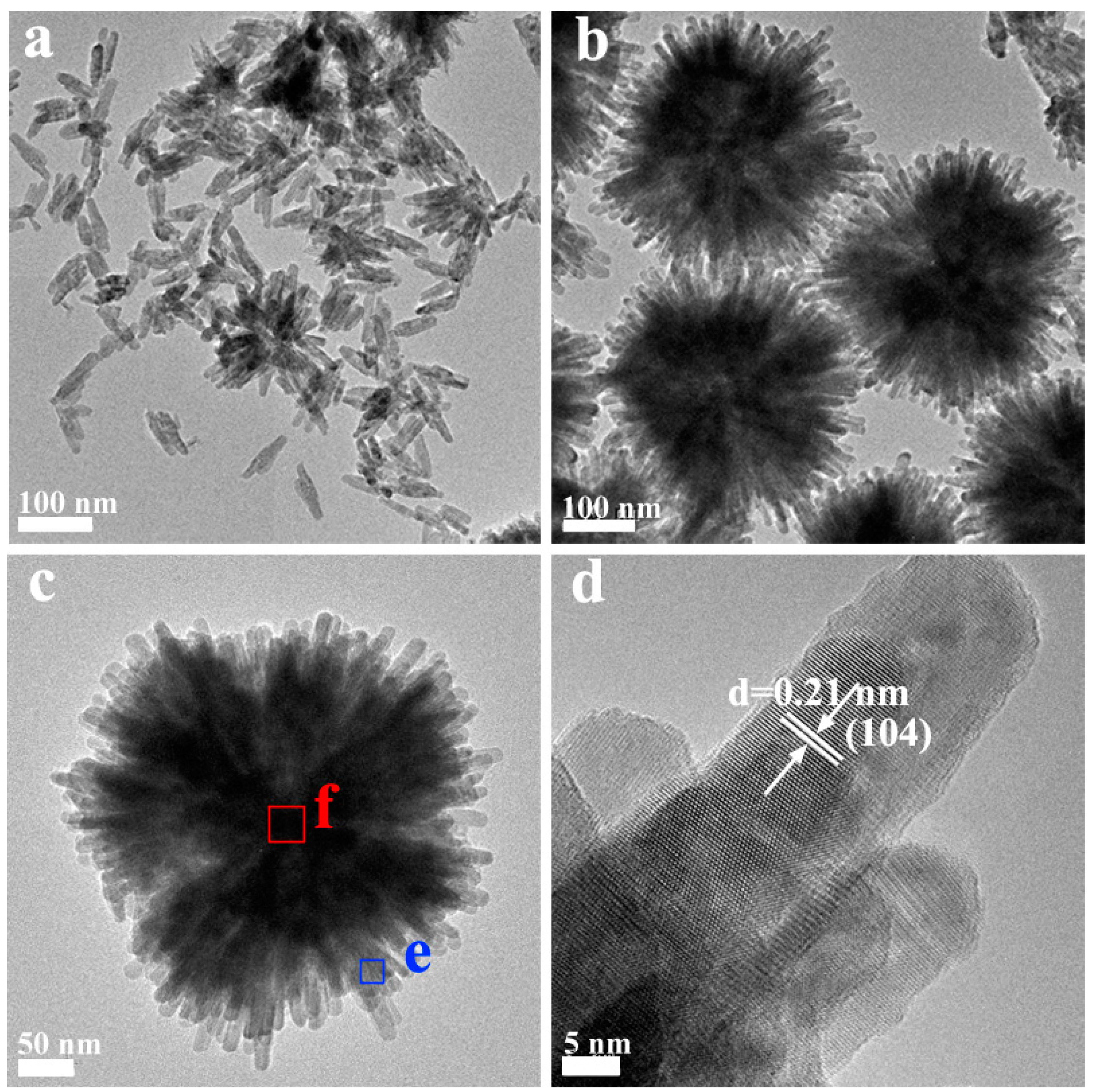
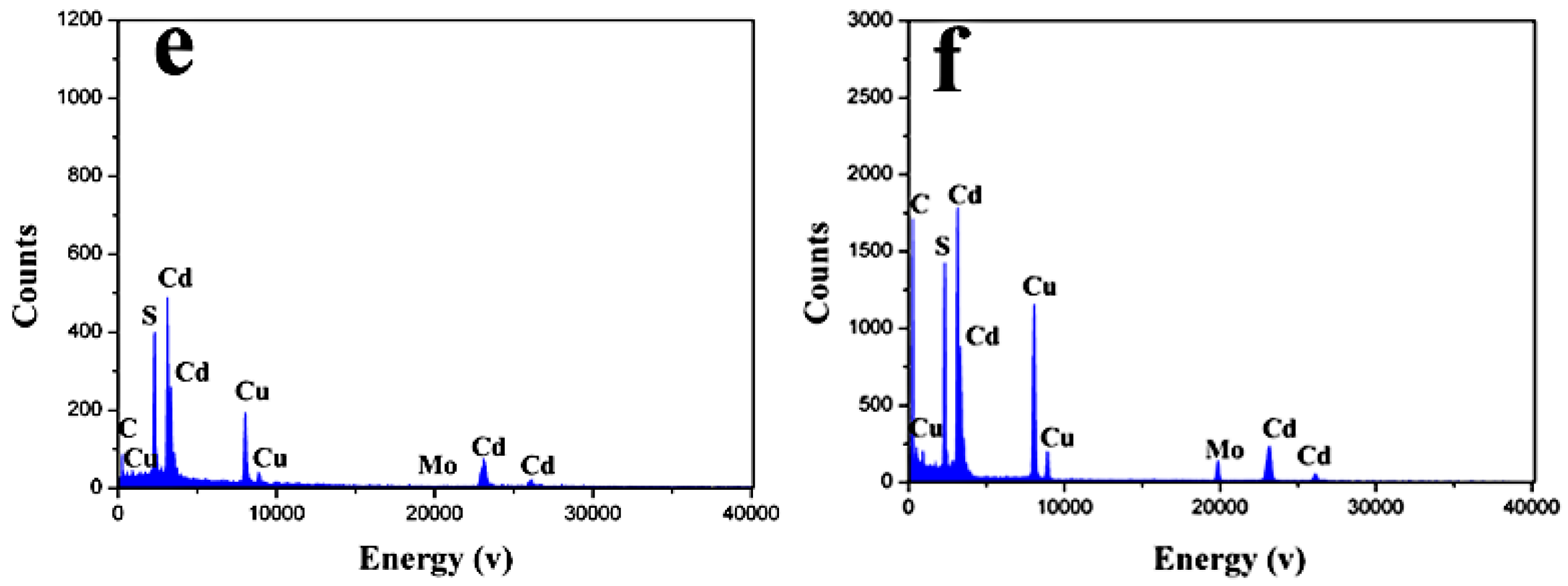
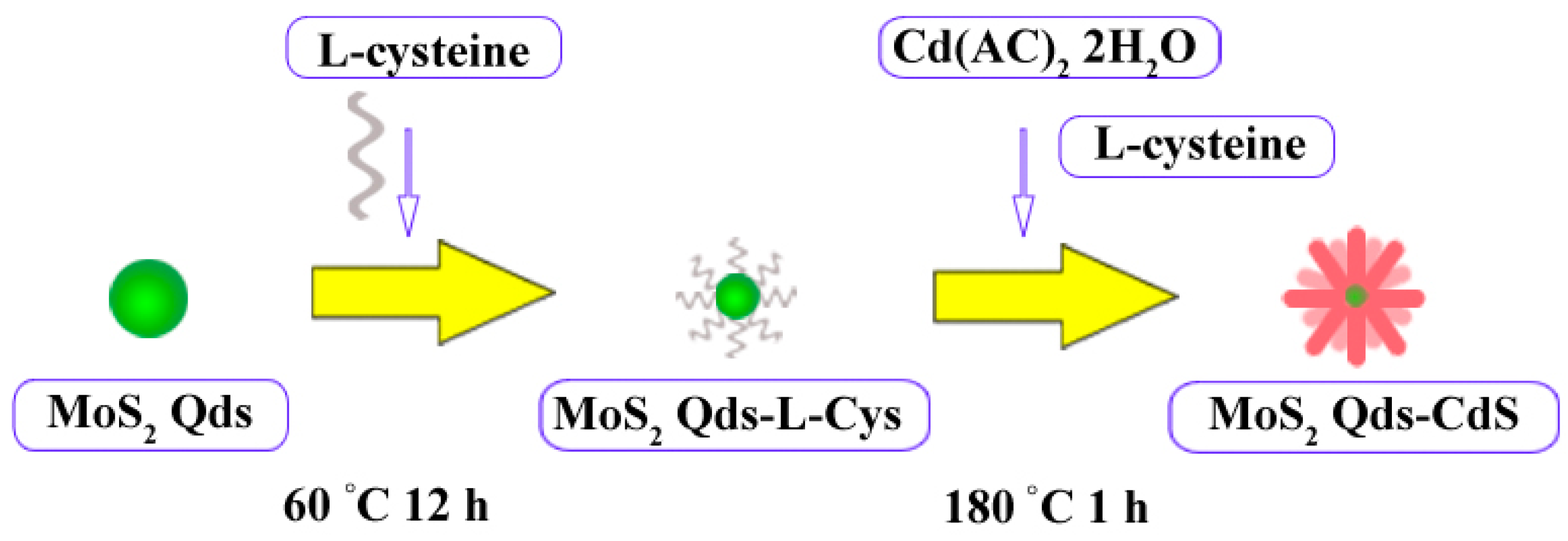
2.2. The Possible Formation Process of the Flowerlike Nanospheres

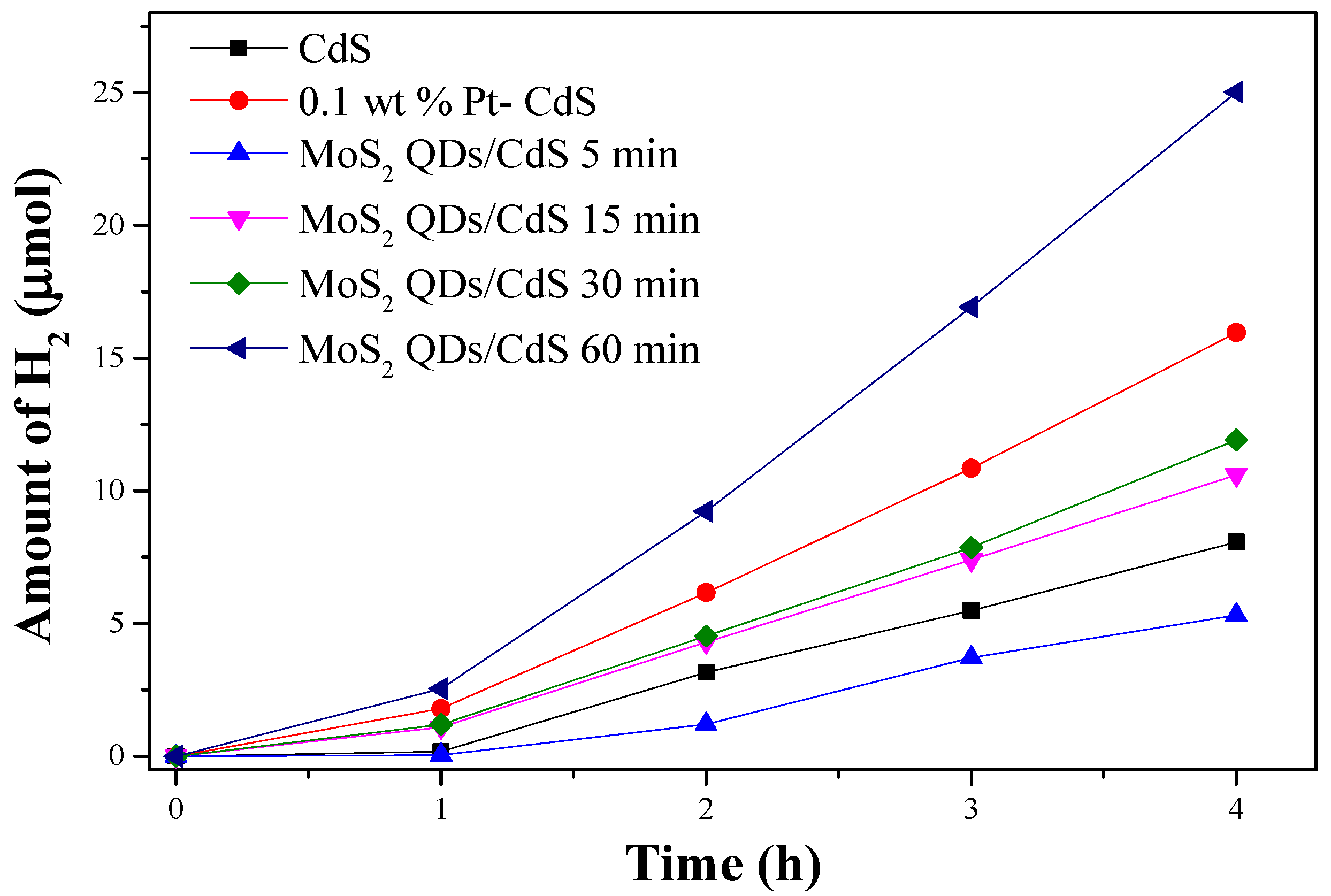
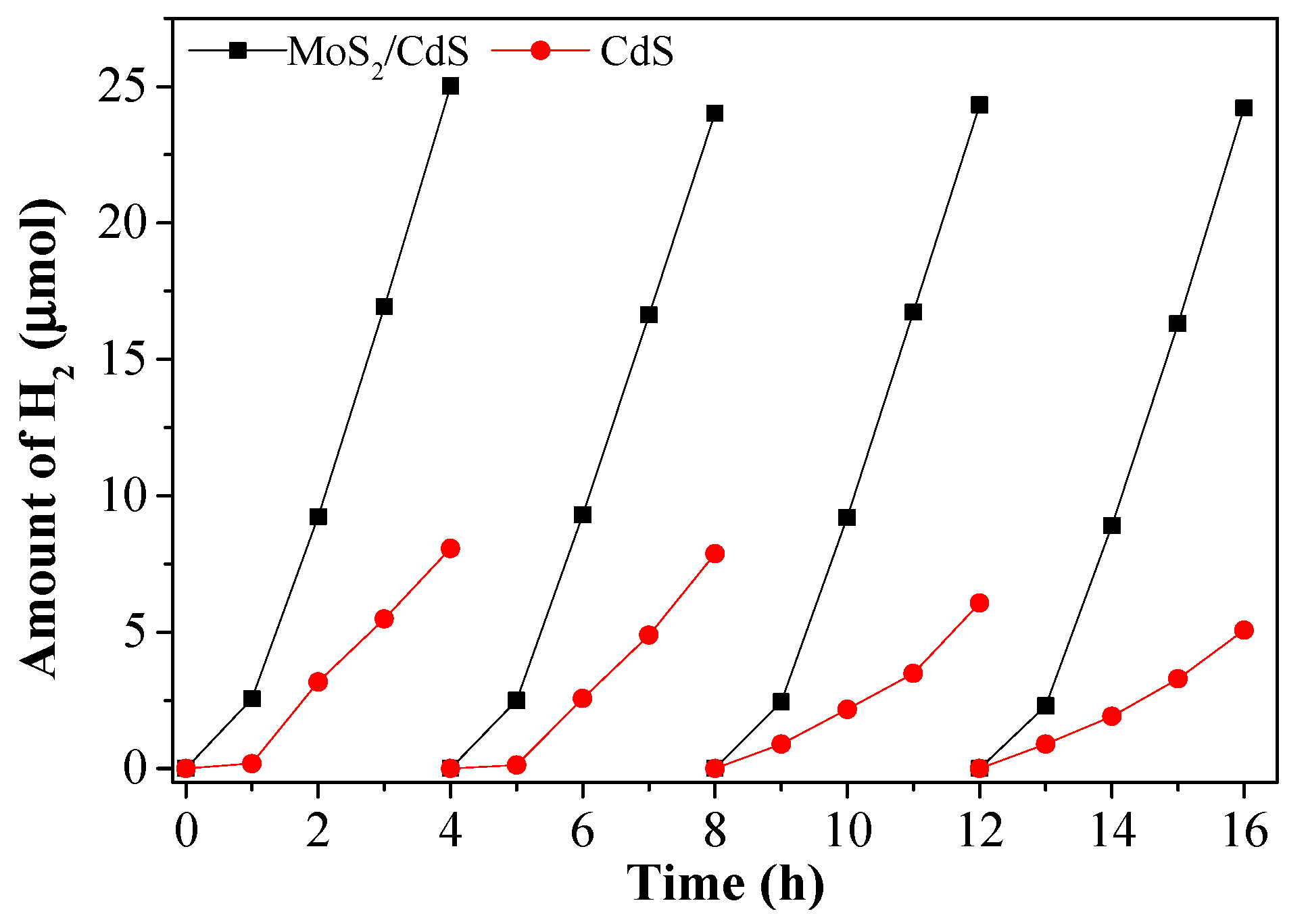
2.3. Photocatalytic Properties
2.4. Photoabsorption Performance and BET Surface Area

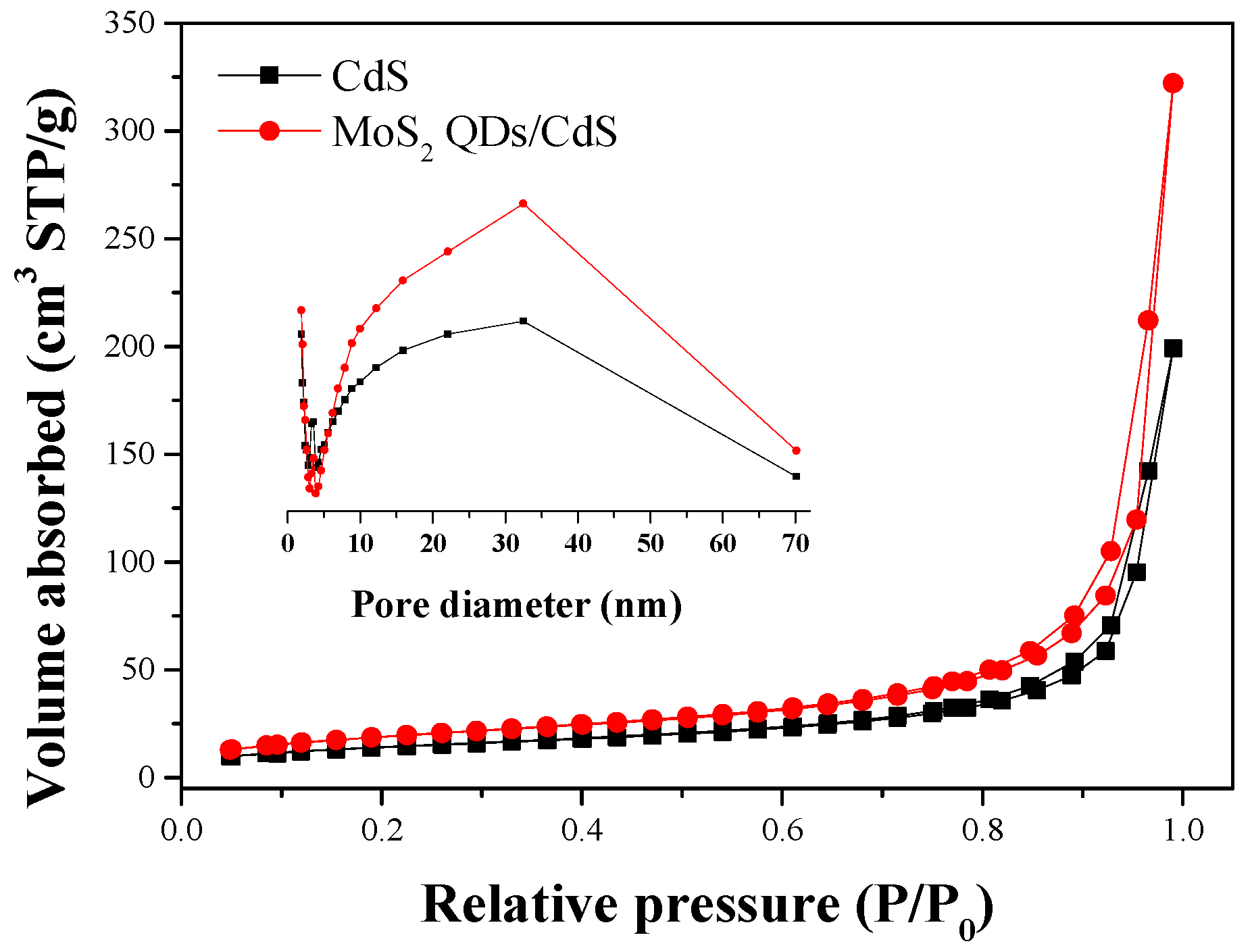
| Sample | Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Size (nm) | H2 Evolution Rate (μmol·h−1·g−1) |
|---|---|---|---|---|
| CdS | 50.7 | 10.3 | 25.13 | 100.8 |
| MoS2 QDs/CdS | 68.97 | 10.49 | 29.67 | 312.75 |
2.5. Photoelectrochemical Performance

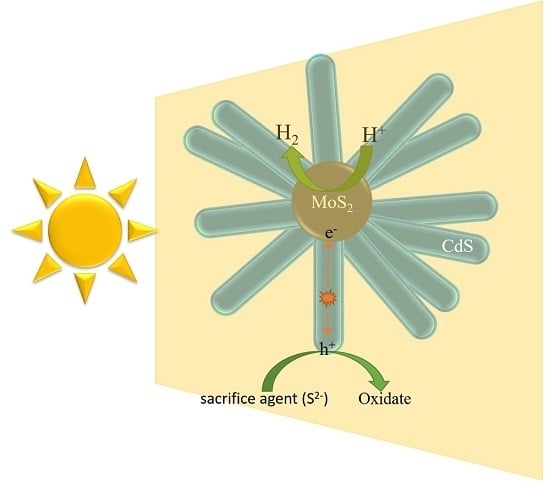
2.6. Possible Photocatalytic Mechanism

3. Experimental Section
3.1. Synthesis of Photocatalysts
3.2. Characterization
3.3. Photoelectrochemical Measurements
3.4. Photocatalytic Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zheng, Y.; Lin, L.; Wang, B.; Wang, X. Graphitic carbon nitride polymers toward sustainable photoredox catalysis. Angew. Chem. Int. Ed. 2015, 54, 12868–12884. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Sun, S.; Wang, W.; Liu, J.; Liu, Z.; Yu, J.C. A black-red phosphorus heterostructure for efficient visible-light-driven photocatalysis. J. Mater. Chem. A 2015, 3, 3285–3288. [Google Scholar] [CrossRef]
- Liu, G.; Yu, J.C.; Lu, G.Q.; Cheng, H.M. Crystal facet engineering of semiconductor photocatalysts: Motivations, advances and unique properties. Chem. Commun. 2011, 47, 6763–6783. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Yu, J.C. Graphene-based photocatalytic composites. RSC Adv. 2011, 1, 1426–1434. [Google Scholar] [CrossRef]
- Liu, Y.; Xiong, J.; Luo, S.; Liang, R.; Qin, N.; Liang, S.; Wu, L. Ultrathin HNbWO6 nanosheets: Facile synthesis and enhanced hydrogen evolution performance from photocatalytic water splitting. Chem. Commun. 2015, 51, 15125–15128. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Li, L.; Liang, S.; Liu, M.; Bi, J.; Wu, L. Efficient synthesis of monolayer carbon nitride 2D nanosheet with tunable concentration and enhanced visible-light photocatalytic activities. Appl. Catal. B 2015, 163, 135–142. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, Y.; Cao, C.; Shen, L.; Wu, W.; Liang, S.; Liang, R.; Wu, L. An architecture of CdS/H2Ti5O11 ultrathin nanobelt for photocatalytic hydrogenation of 4-nitroaniline with highly efficient performance. J. Mater. Chem. A 2015, 3, 6935–6942. [Google Scholar] [CrossRef]
- Liang, S.; Wen, L.; Lin, S.; Bi, J.; Feng, P.; Fu, X.; Wu, L. Monolayer HNb3O8 for selective photocatalytic oxidation of benzylic alcohols with visible light response. Angew. Chem. Int. Ed. 2014, 53, 2951–2955. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Zhu, S.; Chen, Y.; Wu, W.; Wang, X.; Wu, L. Rapid template-free synthesis and photocatalytic performance of visible light-activated SnNb2O6 nanosheet. J. Mater. Chem. 2012, 22, 2670–2678. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. CdS/Graphene Nanocomposite Photocatalysts. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Zhai, T.; Fang, X.; Li, L.; Bando, Y.; Golberg, D. One-dimensional CdS nanostructures: Synthesis, properties, and applications. Nanoscale 2010, 2, 168–187. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.P.; Yu, Z.B.; Liu, G.; Ma, X.L.; Cheng, H.M. CdS-mesoporous ZnS core-shell particles for efficient and stable photocatalytic hydrogen evolution under visible light. Energy Environ. Sci. 2014, 7, 1895–1901. [Google Scholar] [CrossRef]
- Wu, K.; Chen, Z.; Lv, H.; Zhu, H.; Hill, C.L.; Lian, T. Hole removal rate limits photodriven H2 generation efficiency in CdS-Pt and CdSe/CdS-Pt semiconductor nanorod–metal tip heterostructures. J. Am. Chem. Soc. 2014, 136, 7708–7716. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yu, J.C.; Fu, X. Characterization and photocatalytic mechanism of nanosized CdS coupled TiO2 nanocrystals under visible light irradiation. J. Mol. Catal. A Chem. 2006, 244, 25–32. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.S.; Joshi, U.A.; Lee, J.S. Solvothermal synthesis of CdS nanowires for photocatalytic hydrogen and electricity production. J. Phys. Chem. C 2007, 111, 13280–13287. [Google Scholar] [CrossRef]
- Xiang, Q.; Cheng, B.; Yu, J. Hierarchical porous CdS nanosheet-assembled flowers with enhanced visible-light photocatalytic H2-production performance. Appl. Catal. B 2013, 138–139, 299–303. [Google Scholar] [CrossRef]
- Chen, C.C.; Lin, J.J. Controlled growth of cubic cadmium sulfide nanoparticles using patterned self-assembled monolayers as a template. Adv. Mater. 2001, 13, 136–139. [Google Scholar] [CrossRef]
- Li, L.; Wu, P.C.; Fang, X.S.; Zhai, T.Y.; Dai, L.; Liao, M.Y.; Koide, Y.; Wang, H.; Bando, Y.; Golberg, D. Single-crystalline CdS nanobelts for excellent field-emitters and ultrahigh quantum-efficiency photodetectors. Adv. Mater. 2010, 22, 3161–3165. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gao, X.H.; Yu, L.; Wang, Y.R.; Ning, J.Q.; Xu, S.J.; Lou, X.W. Carbon-coated CdS petalous nanostructures with enhanced photostability and photocatalytic activity. Angew. Chem. Int. Ed. Engl. 2013, 25, 5636–5639. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Yifeng, E.F.; Fan, L.Z.; Wang, Z.H.; Liu, H.B.; Li, Y.L.; Yang, S.; Li, Y. Directed assembly of hierarchical CdS nanotube arrays from CdS nanoparticles: Enhanced solid state electro-chemiluminescence in H2O2 solution. Adv. Mater. 2007, 19, 3677–3681. [Google Scholar] [CrossRef]
- Shen, L.; Liang, S.; Wu, W.; Liang, R.; Wu, L. CdS-decorated UiO-66(NH2) nanocomposites fabricated by a facile photodeposition process: An efficient and stable visible-light-driven photocatalyst for selective oxidation of alcohols. J. Mater. Chem. A 2013, 1, 11473–11482. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of Cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Yan, H.; Wu, G.; Ma, G.; Wen, F.; Wang, L.; Li, C. Enhancement of photocatalytic H2 evolution on CdS by loading MoS2 as cocatalyst under visible light irradiation. J. Am. Chem. Soc. 2008, 130, 7176–7177. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Yu, J.; Jaroniec, M. Synergetic effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 6575–6578. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Li, M.; Wang, T.; Ouyang, S.; Li, P.; Liu, L.; Ye, J. Drastic layer-number-dependent activity enhancement in photocatalytic H2 evolution over nMoS2/CdS (n ≥ 1) under visible light. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Hou, Y.; Laursen, A.B.; Zhang, J.; Zhang, G.; Zhu, Y.; Wang, X.; Dahl, S.; Chorkendorff, I. Layered nanojunctions for hydrogen-evolution catalysis. Angew. Chem. Int. Ed. 2013, 52, 3621–3625. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Liu, Y.; Wang, D.; Liang, S.; Wu, W.; Wu, L. An efficient cocatalyst of defect-decorated MoS2 ultrathin nanoplates for the promotion of photocatalytic hydrogen evolution over CdS nanocrystal. J. Mater. Chem. A 2015, 3, 12631–12635. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; Li, S.; Grote, F.; Zhang, X.; Zhang, H.; Wang, R.; Lei, Y.; Pan, B.; Xie, Y. Controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. J. Am. Chem. Soc. 2013, 135, 17881–17888. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.; Yu, J.C.; Lin, J.; Yu, J.; Li, P. Preparation and photocatalytic behavior of MoS2 and WS2 nanocluster sensitized TiO2. Langmuir 2004, 20, 5865–5869. [Google Scholar] [CrossRef]
- Zhou, W.J.; Yin, Z.Y.; Du, Y.P.; Huang, X.; Zeng, Z.Y.; Fan, Z.X.; Liu, H.; Wang, J.Y.; Zhang, H. Synthesis of few-layer MoS2 nanosheet-coated TiO2 nanobelt heterostructures for enhanced photocatalytic activities. Small 2013, 9, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, X.-J.; Yin, L.; Li, B.; Hong, X.; Fan, Z.; Chen, B.; Xue, C.; Zhang, H. One-pot synthesis of CdS nanocrystals hybridized with single-layer transition-metal dichalcogenide nanosheets for efficient photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2015, 54, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Wu, G.P.; Yan, H.J.; Ma, G.J.; Shi, J.Y.; Wen, F.Y.; Wang, L.; Li, C. Photocatalytic H2 evolution on MoS2/CdS catalysts under visible light irradiation. J. Phys. Chem. C 2010, 114, 1963–1968. [Google Scholar] [CrossRef]
- Li, H.L.; Li, T.D.; Liu, H.X.; Huang, B.B.; Zhang, Q. Hierarchical flower-like nanostructures of anatase TiO2 nanosheets dominated by {001} facets. J. Alloy Compd. 2016, 657. [Google Scholar] [CrossRef]
- Xu, L.; Ji, X.; Jiang, J.G.; Han, L.; Che, S.; Wu, P. Intergrown zeolite MWW polymorphs prepared by the rapid dissolution–recrystallization route. Chem. Mater. 2015, 27, 7852–7860. [Google Scholar] [CrossRef]
- Yang, F.L.; Liu, Y.F.; Lu, Y.; Song, F.; Qian, H.; Yuan, Z. Citric acid-mediated microwave-assisted hydrothermal synthesis and luminescence property of NaSm(MoO4)2 submicro-crystals. J. Mater. Sci. 2015, 26, 8595–8602. [Google Scholar] [CrossRef]
- Yu, J.G.; Jin, J.; Cheng, B.; Jaroniec, M. A noble metal-free reduced graphene oxide–CdS nanorod composite for the enhanced visible-light photocatalytic reduction of CO2 to solar fuel. J. Mater. Chem. A 2014, 2, 3407–3416. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination ofsurface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Jin, J.; Yu, J.; Liu, G.; Wong, P.K. Single crystal CdS nanowires with high visible-light photocatalytic H2-production performance. J. Mater. Chem. A 2013, 1, 10927–10934. [Google Scholar] [CrossRef]
- Min, Y.L.; He, G.Q.; Xu, Q.J.; Chen, Y.C. Dual-functional MoS2 sheet-modified CdS branch-like heterostructures with enhanced photostability and photocatalytic activity. J. Mater. Chem. A 2014, 2, 2578–2584. [Google Scholar] [CrossRef]
- Xu, Y.X.; Bai, H.; Lu, G.W.; Li, C.; Shi, G.Q. Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of MoS2 QDs and MoS2/CdS are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.; Zhou, Z.; Wu, X.; Zhu, S.; Bi, J.; Zhou, L.; Liu, M.; Wu, L. Constructing a MoS2 QDs/CdS Core/Shell Flowerlike Nanosphere Hierarchical Heterostructure for the Enhanced Stability and Photocatalytic Activity. Molecules 2016, 21, 213. https://doi.org/10.3390/molecules21020213
Liang S, Zhou Z, Wu X, Zhu S, Bi J, Zhou L, Liu M, Wu L. Constructing a MoS2 QDs/CdS Core/Shell Flowerlike Nanosphere Hierarchical Heterostructure for the Enhanced Stability and Photocatalytic Activity. Molecules. 2016; 21(2):213. https://doi.org/10.3390/molecules21020213
Chicago/Turabian StyleLiang, Shijing, Zhouming Zhou, Xiuqin Wu, Shuying Zhu, Jinhong Bi, Limin Zhou, Minghua Liu, and Ling Wu. 2016. "Constructing a MoS2 QDs/CdS Core/Shell Flowerlike Nanosphere Hierarchical Heterostructure for the Enhanced Stability and Photocatalytic Activity" Molecules 21, no. 2: 213. https://doi.org/10.3390/molecules21020213