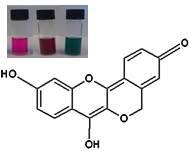
Peltomexicanin, a Peltogynoid Quinone Methide from Peltogyne Mexicana Martínez Purple Heartwood
Abstract
:1. Introduction
2. Results and Discussion
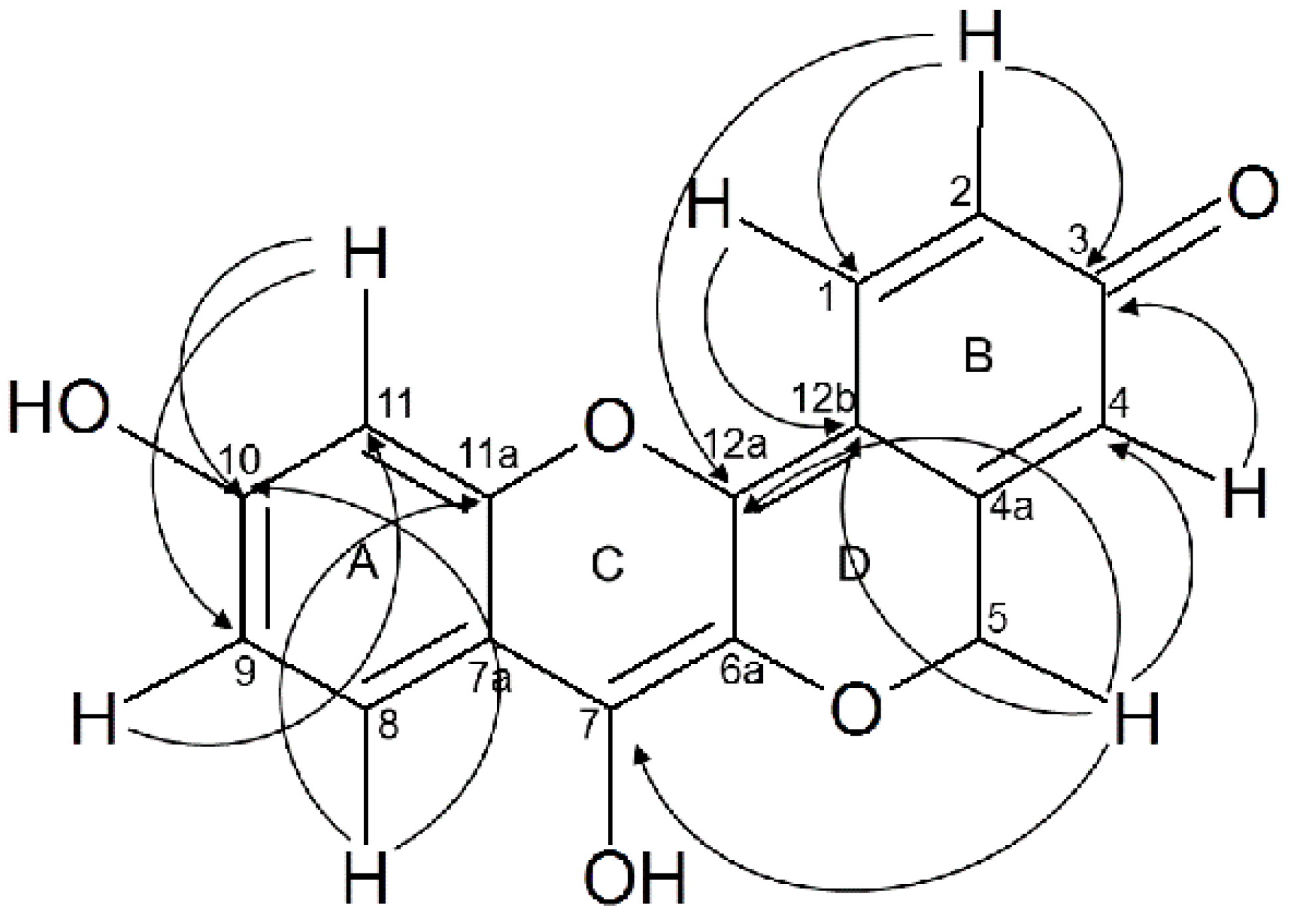
2.1. Structural Elucidation of the Isolated Compound
| Carbon Number | 13C δ | 1H δ, Multiplicity, J (Hz) | HMBC 1H-13C Correlations |
|---|---|---|---|
| 1 | 110.94 | 6.66, br s | C-12b |
| 2 | 108.76 | 7.16, br s | C-1, C-3, C-12a |
| 3 | 179.36 | ||
| 4 | 112.58 | 7.27, s | C-3 |
| 4a | 159.54 | ||
| 5 | 66.64 | 5.06 s | C-4, C-7 (W) C-12a C-12b |
| 6a | 113.16 | ||
| 7 | 147.0 | ||
| 7a | 133.47 | ||
| 8 | 131.74 | 7.94, d (10) | C-11a, C-10 |
| 9 | 107.52 | 6.26, dd (1, 10) | C-11 |
| 10 | 158.13 | ||
| 11 | 102.50 | 6.33, d (1) | C-9, C-10 |
| 11a | 152.58 | ||
| 12a | 146.06 | ||
| 12b | 121.81 |
2.2. Quantification by HPLC-DAD
2.3. Antioxidant Activity
3. Experimental
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Quantification by HPLC-DAD
3.5. Antioxidant Activity
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Navarro, M.J.; de la Borja, R.A.; Machuca, V.R. Características tecnológicas de la madera de Palo Morado (Peltogyne mexicana Martínez) de Tierra Colorada, Guerrero, México. Rev. Chapingo Ser. Cienc. 2005, 11, 73–82. [Google Scholar]
- Drewes, S.E.; Mashimbye, M.J.; Field, J.S.; Ramesar, N. 11,11-Dimethyl-1,3,8,10-tetrahydroxi-9-methoxypeltogynan and three pentacyclic triterpenes from Cassine transvaalensis. Phytochemistry 1991, 30, 3490–3493. [Google Scholar] [CrossRef]
- Ahmadu, A.; Abdulkarim, A.; Grougnet, R.; Myrianthopoulos, V.; Tillequin, F.; Magiatis, P.; Skaltsounis, A. Two new peltogynoids from Acacia nilotica Delile with kinase inhibitory activity. Planta Med. 2010, 76, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Brandt, E.V.; Roux, D.G. Metabolites from the purple heartwood of Mimosoideae I, Acacia peuce F. Muell: The first natural 2,3-cis-peltogynoid. J. Chem. Soc. Perkin Trans. 1 1979, 777, 777–780. [Google Scholar] [CrossRef]
- Brandt, E.V.; Ferreira, D.; Roux, D.G. Metabolites from purple heartwood of Mimosoideae. Part 2. Acacia carnei maiden: Isolation, synthesis, and reactions of crombeone. J. Chem. Soc. Perkin Trans. 1 1981, 2, 514–521. [Google Scholar] [CrossRef]
- Brandt, E.V.; Ferreira, D.; Roux, D.G. Metabolites from purple heartwood of Mimosoideae. Part 3. Acacia crombei C.T. White: Structure and Partial Synthesis of Crombein, a Natural Spiropeltogynoid. J. Chem. Soc. Perkin Trans. 1 1981, 7, 1879–1883. [Google Scholar] [CrossRef]
- Van Heerden, F.R.; Brandt, E.V.; Ferreira, D.; Roux, D.G. Metabolites from purple heartwoods of the Mimosoideae. Part 4. Acacia fasciculifera F. Muell ex. Benth: Fasciculiferin, Fasciculiferol, and the Synthesis of 7-Aryl and 7-Flavanyl-Peltogynoids. J. Chem. Soc. Perkin Trans. 1 1981, 2483–2490. [Google Scholar] [CrossRef]
- McPherson, D.D.; Cordell, G.A.; Soejarto, D.D.; Pezzuto, J.M.; Fong, H.; Havsteen, S. Peltogynoids and homoisoflavonoids from Caesalpinia pulcherrima. Phytochemistry 1983, 22, 2835–2838. [Google Scholar] [CrossRef]
- Robinson, G.M.; Robinson, R. Leuco-anthocyanins and Leucoanthocyanidins. Part I. The isolation of peltogynol and its molecular structure. J. Chem. Soc. 1935, 744, 744–752. [Google Scholar] [CrossRef]
- Hassall, C.H.; Weatherston, J. The absolute configuration of the leucoanthocyanidin, peltogynol. J. Chem. Soc. 1965, 2844, 2844–2849,. [Google Scholar] [CrossRef]
- Drewes, S.E.; Roux, D.G. Isolation of mopanine from Colophospermum mopane and interrelation of flavonoid components of Peltogyne spp. J. Chem. Soc. C Org. 1967, 1407, 1407–1410. [Google Scholar] [CrossRef]
- Lukeman, M. Photochemical generation and characterization of quinone methides. In Quinone Methides, 1st ed.; Rokita, S.E., Ed.; Wiley: Hoboken, NJ, USA, 2009; pp. 1–26. [Google Scholar]
- Bolton, J.L. Quinone methide bioactivation pathway: Contribution to Toxicity and/or Cytoprotection? Curr. Org. Chem. 2014, 18, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Toteva, M.M.; Richard, J.P. The generation and reactions of quinone methides. Adv. Phys. Org. Chem. 2011, 45, 39–91. [Google Scholar] [PubMed]
- Amouri, H. Metal-stabilized o-quinone methides, thioquinones, and selenoquinones: Trapping important reactive intermediates and beyond. Synlett 2011, 1357, 1357–1369. [Google Scholar] [CrossRef]
- Amouri, H.; le Bras, J. Taming reactive phenol tautomers and o-quinone methides with transition metals: A structure-reactivity relationship. Acc. Chem. Res. 2002, 35, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Amouri, H.; Besace, Y.; Bras, J.L.; Vaissermann, J. General synthesis, first crystal structure, and reactivity of stable o-quinone methide complexes of Cp* Ir. J. Am. Chem. Soc. 1998, 120, 6171–6172. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Nur-E-Alam, M.; Akhtar, F.; Ahmad, S.; Baig, I.; Ondognii, P.; Gombosurengyn, P.; Rahman, A. Five new peltogynoids from underground parts of Iris bungei: A mongolian medicinal plant. Chem. Pharm. Bull. 2001, 49, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mut. Res. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Rufino, M.; Alves, R.E.; de Brito, E.S.; de Morais, S.M.; Sampaio, C.D.G.; Pérez-Jimenez, J.; Saura-Calixto, F.D. Metodologia Científica: Determinação da Atividade Antioxidante Total en Frutas Pela Captura do Radical Livre ABTS+. Embrapa Agroind. Trop. Comun. Técn. 2007. Avaliable online: http://ainfo.cnptia.embrapa.br/digital/bitstream/CNPAT/10225/1/Cot_128.pdf (accessed on 3 February 2016).
- Sample Availability: Samples of peltomexicanin are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Macías, P.; Peralta-Cruz, J.; Borja-de-la-Rosa, A.; Barragán-Huerta, B.E. Peltomexicanin, a Peltogynoid Quinone Methide from Peltogyne Mexicana Martínez Purple Heartwood. Molecules 2016, 21, 186. https://doi.org/10.3390/molecules21020186
Gutiérrez-Macías P, Peralta-Cruz J, Borja-de-la-Rosa A, Barragán-Huerta BE. Peltomexicanin, a Peltogynoid Quinone Methide from Peltogyne Mexicana Martínez Purple Heartwood. Molecules. 2016; 21(2):186. https://doi.org/10.3390/molecules21020186
Chicago/Turabian StyleGutiérrez-Macías, Paulina, Javier Peralta-Cruz, Amparo Borja-de-la-Rosa, and Blanca E. Barragán-Huerta. 2016. "Peltomexicanin, a Peltogynoid Quinone Methide from Peltogyne Mexicana Martínez Purple Heartwood" Molecules 21, no. 2: 186. https://doi.org/10.3390/molecules21020186