Synthesis and Characterization of Macrocyclic Polyether N,N′-Diallyl-7,16-diaza-1,4,10,13-tetraoxa-dibenzo-18-crown-6
Abstract
:1. Introduction
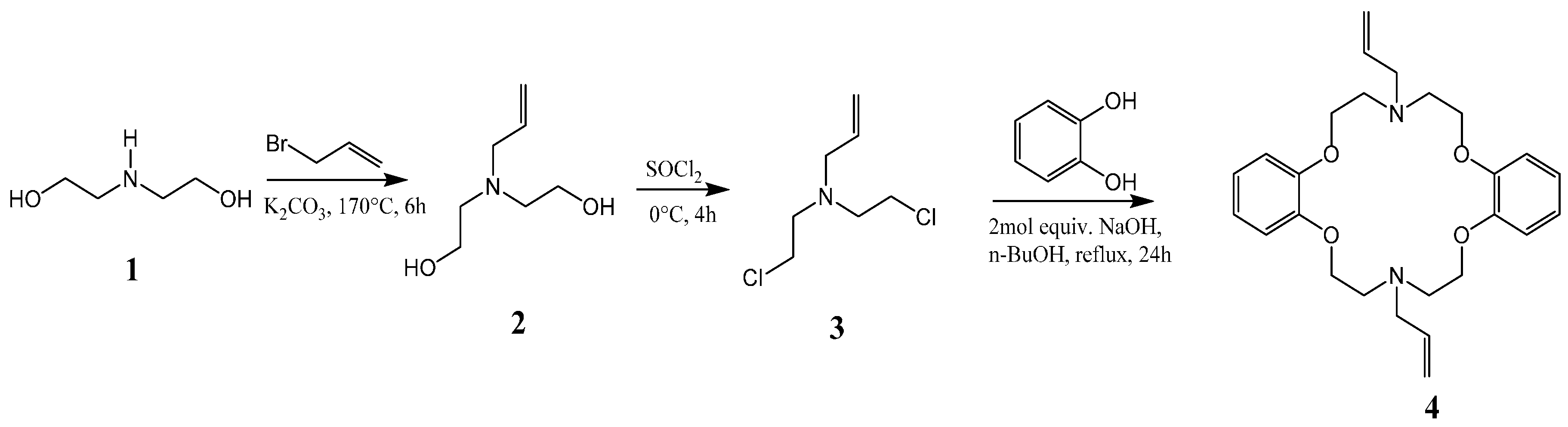
2. Results and Discussion
| Catechol (Moles) | Moles of 3 | Method of Adding NaOH | Yield (%) |
|---|---|---|---|
| 11.0 g (0.10 mol.) | 0.10 | One step 2-mol equivalent | 5 ± 1 |
| 110.11 g (1.00 mol.) | 1.00 | One step 2-mol equivalent | 3–6 |
| 11.0 g (0.10 mol.) | 0.10 | Two step 1-mol equivalent | 36 |
| 110.11 g (1.00 mol.) | 1.00 | Two step 1-mol equivalent | 42 |
2.1. Spectroscopic Characterization of the Azarown Polyether
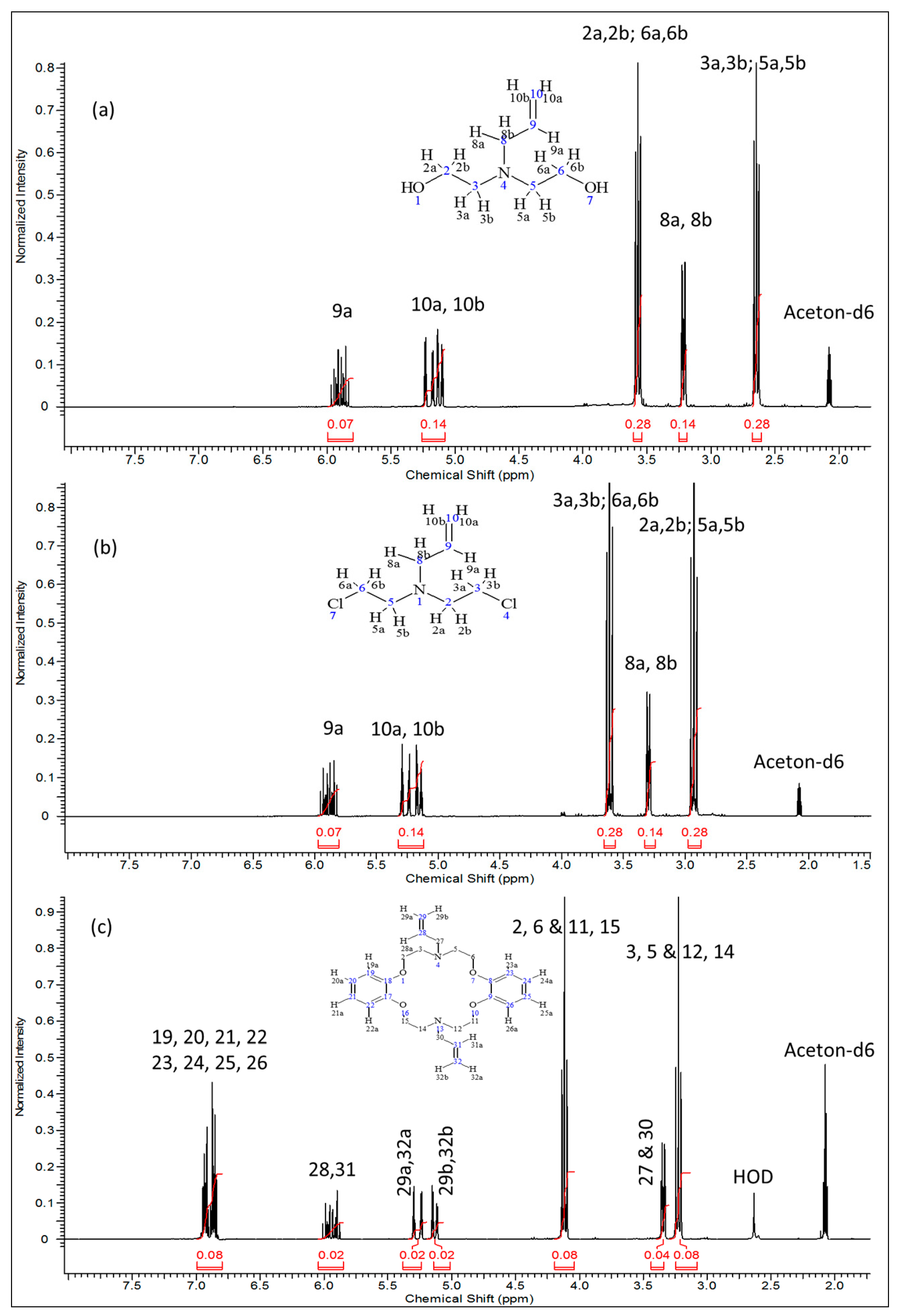
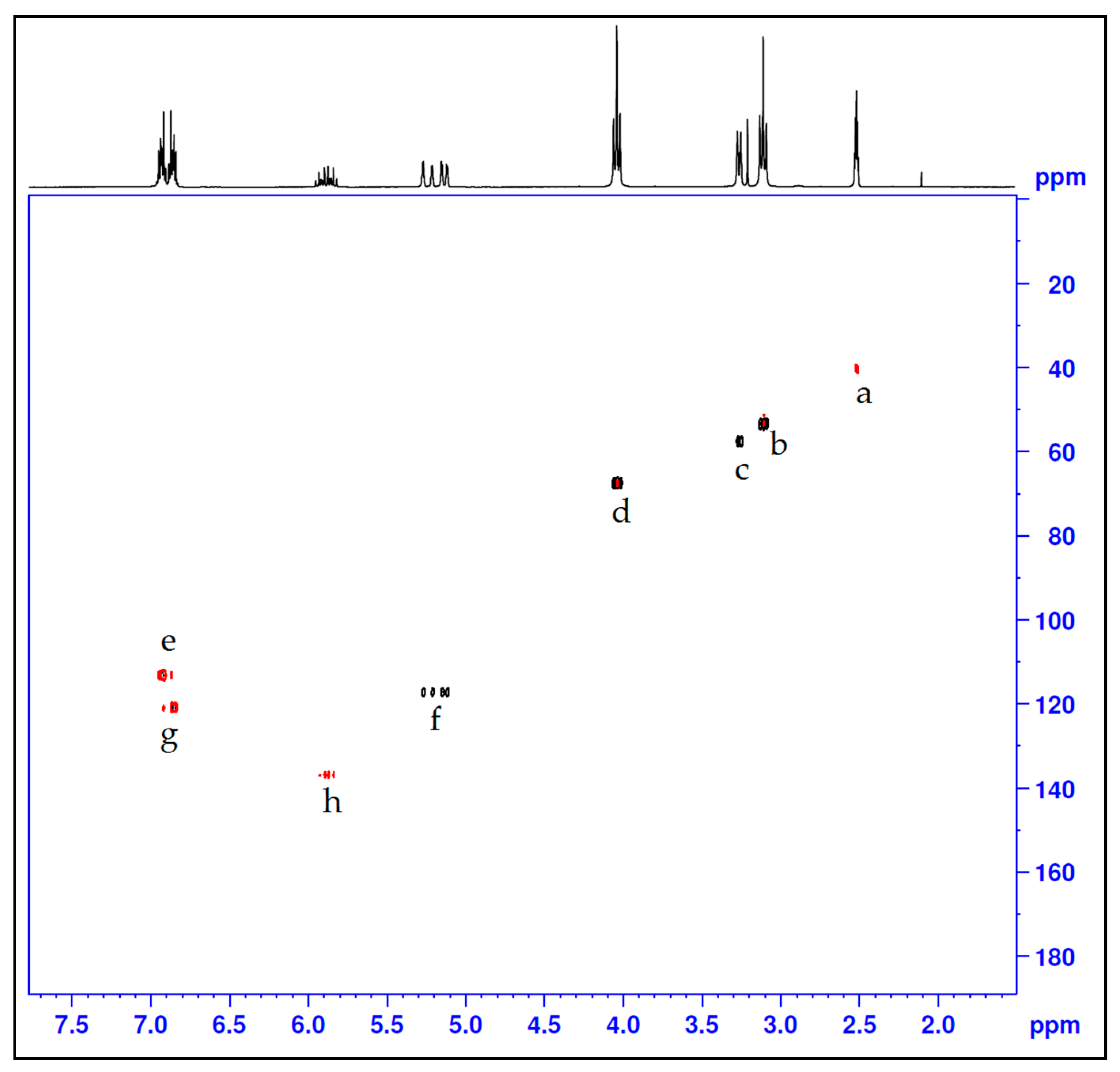
| Compound | Proton Position | Integral | Multiplicity |
|---|---|---|---|
| 2 | 9a | 0.07 | 1 |
| 10a & 10b | 0.14 | 2 | |
| 2a, 2b, 6a, & 6b | 0.28 | 4 | |
| 8a & 8b | 0.14 | 2 | |
| 3a, 3b, 5a & 5b | 0.28 | 4 | |
| 3 | 9a | 0.07 | 1 |
| 10a & 10b | 0.14 | 2 | |
| 3a, 3b, 6a & 6b | 0.28 | 4 | |
| 2a, 2b, 5a & 5b | 0.28 | 4 | |
| 4 | 19, 20, 21, 22, 23, 24, 25 & 26 | 0.08 | 8 |
| 28, 31 | 0.02 | 2 | |
| 29a, 32a | 0.02 | 2 | |
| 29b, 32b | 0.02 | 2 | |
| 2, 6, 11, & 15 | 0.08 | 8 | |
| 27 & 30 | 0.04 | 4 | |
| 3, 5, 12 & 14 | 0.08 | 8 |
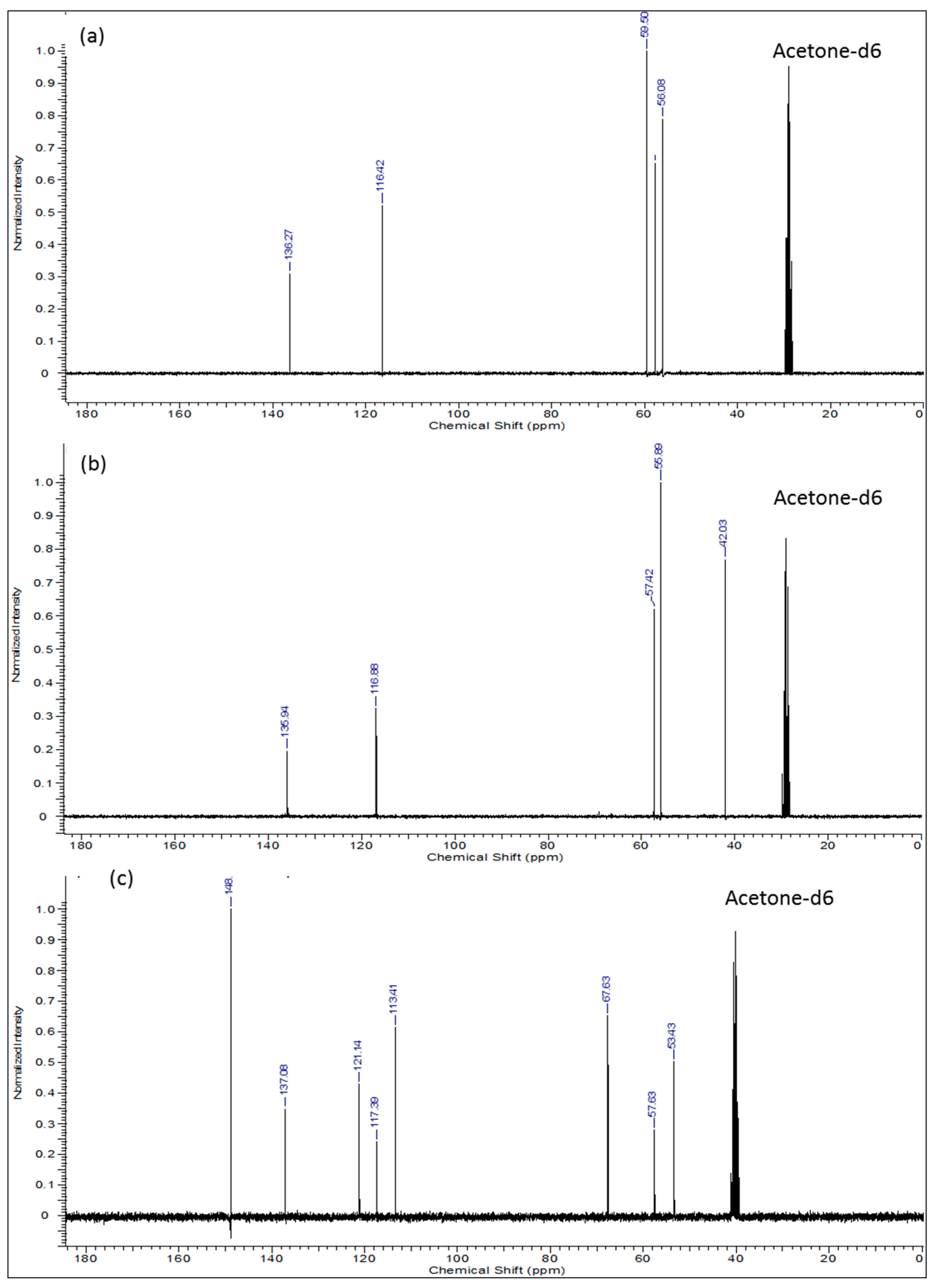
| Entry | Carbon Position | Chemical Shift (ppm) |
|---|---|---|
| a | Solvent (Acetone) | 40.00 |
| b | C3, C5, C12, C14 | 53.43 |
| c | C27, C30 | 57.63 |
| d | C2, C6, C11, C15 | 67.63 |
| e | C20, C21, C24, C25 | 113.41 |
| f | C29, C32 | 117.39 |
| g | C19, C22, C23, C26 | 121.14 |
| h | C28, C31 | 137.08 |
| i | C8, C9, C17 and C18 | 148.88 |
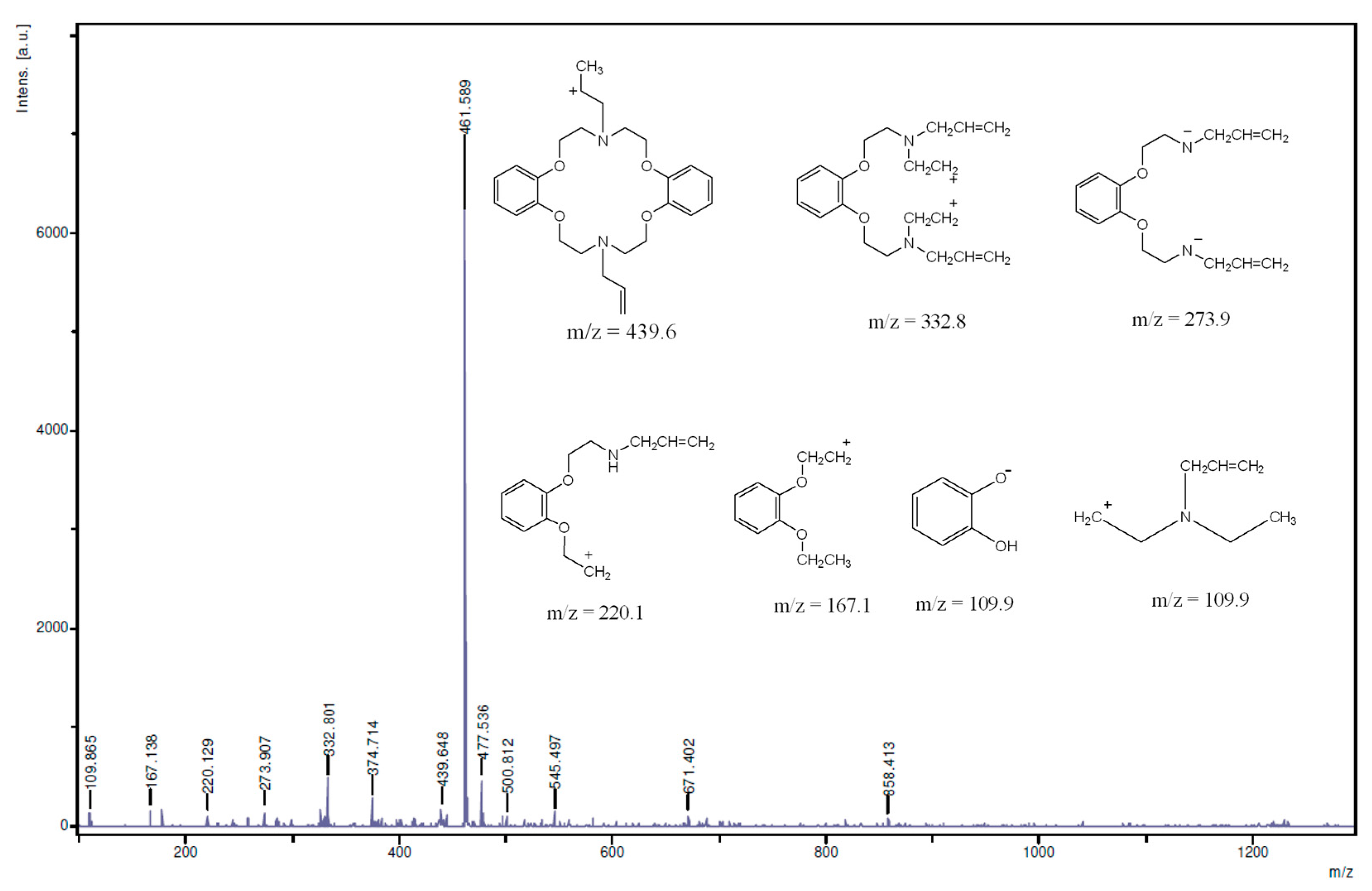
2.2. Thermal Analysis of the Azacrown Polyether Macrocycle
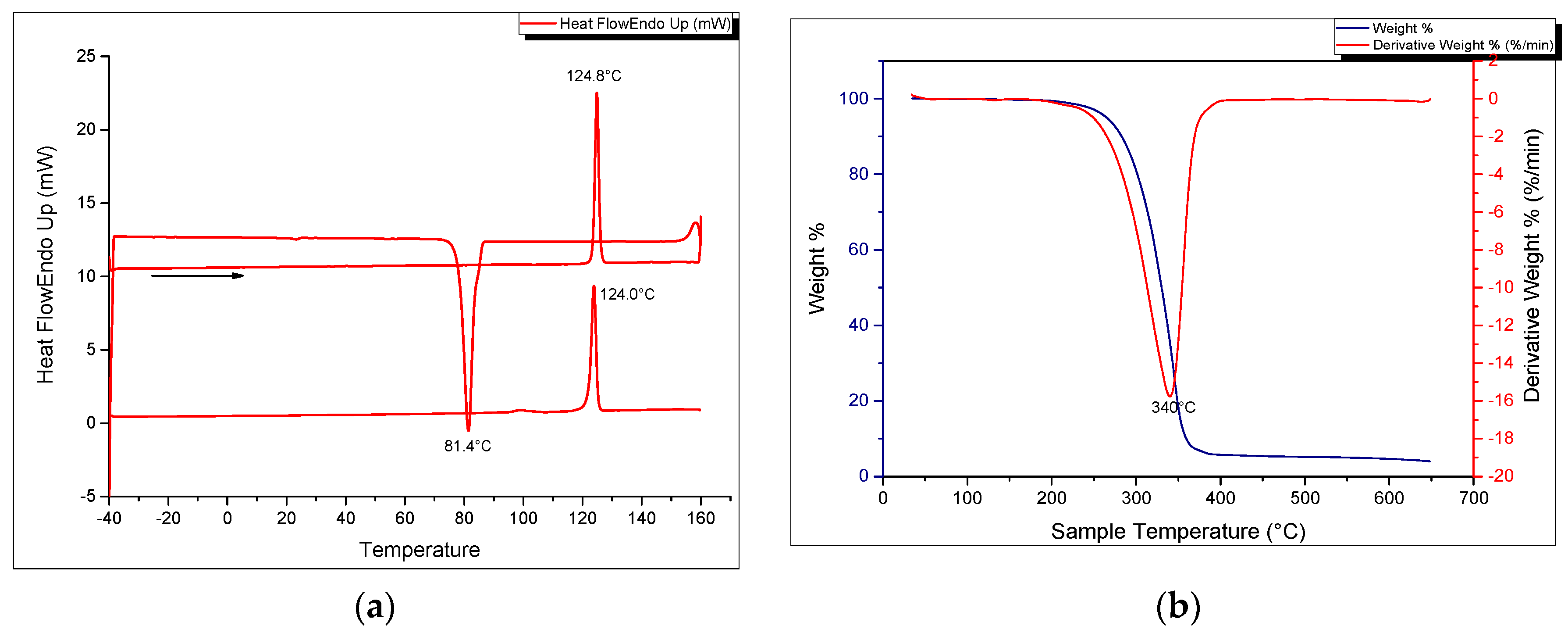
3. Experimental Section
3.1. General Information
3.2. Synthesis of the Azacrown Polyether Macrocycle
3.2.1. Synthesis of 2,2′-(Prop-2-en-1-ylimino)diethanol (2)
3.2.2. Synthesis of N,N′-Bis(2-chloroethyl)prop-2-en-1-amine (3)
3.2.3. Synthesis of N,N′-Diallyl-7,16-diaza-1,4,10,13-tetraoxa-dibenzo-18-crown-6 (4)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bradshaw, J.S.; Krakowiak, K.E.; Izatt, R.M. Aza-Crown Macrocycles; Wiley: New York, NY, USA, 1993. [Google Scholar]
- Puntoriero, F.; Bergamini, G.; Ceroni, P.; Balzani, V.; Vögtle, F. Azacrown Ethers with Naphthyl Branches. Fluorescence Properties, Protonation and Metal Coordination. J. Inorg. Organomet. Polym. Mater. 2007, 18, 189–194. [Google Scholar] [CrossRef]
- Krakowiak, K.E.; Bradshaw, J.S.; Zamecka-Krakowiak, D.J. Synthesis of aza-crown ethers. Chem. Rev. 1989, 89, 929–972. [Google Scholar] [CrossRef]
- Izatt, R.M.; Pawlak, K.; Bradshaw, J.S.; Bruening, R.L. Thermodynamic and Kinetic Data for Macrocycle Interaction with Cations, Anions, and Neutral Molecules. Chem. Rev. 1995, 95, 2529–2586. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Belyakov, S.A.; Sorochinsky, A.E.; Steel, P.J.; Schall, O.F.; Gokel, G.W. Novel Syntheses of N-Pivot Lariat Diaza-Crown Ethers from 4, 13-Bis(benzotriazolylmethyl)-4,13-diaza-1,7,10,16-tetraoxacyclooctadecane. J. Org. Chem. 1996, 61, 7585–7592. [Google Scholar] [CrossRef] [PubMed]
- Gatto, V.J.; Gokel, G.W. Syntheses of calcium-selective, substituted diaza-crown ethers: A novel, one-step formation of bibracchial lariat ethers (BiBLES). J. Am. Chem. Soc. 1984, 106, 8240–8244. [Google Scholar] [CrossRef]
- Gao, B.; Wang, S.; Zhang, Z. Study on complexation adsorption behavior of dibenzo-18-crown-6 immobilized on CPVA microspheres for metal ions. J. Incl. Phenom. Macrocycl. Chem. 2010, 68, 475–483. [Google Scholar] [CrossRef]
- Izatt, R.M.; Bradshaw, J.S.; Nielsen, S.A.; Lamb, J.D.; Christensen, J.J.; Sen, D. Thermodynamic and kinetic data for cation-macrocycle interaction. Chem. Rev. 1985, 85, 271–339. [Google Scholar] [CrossRef]
- Alexandratos, S.D.; Stine, C.L. Synthesis of ion-selective polymer-supported crown ethers: A review. React. Funct. Polym. 2004, 60, 3–16. [Google Scholar] [CrossRef]
- Su, N.; Bradshaw, J.S.; Zhang, X.X.; Savage, P.B.; Krakowiak, K.E.; Izatt, R.M. Diaza-18-Crown-6 Ligands Containing Two Aminophenol Side Arms: New Heterobinuclear Metal Ion Receptors. J. Org. Chem. 1999, 64, 3825–3829. [Google Scholar] [CrossRef]
- Malkondu, S.; Kocak, A.; Yilmaz, M. Immobilization of Two Azacrown Ethers on Chitosan: Evaluation of Selective Extraction Ability Toward Cu(II) and Ni(II). J. Macromol. Sci. A 2009, 46, 745–750. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Tang, Y. Preparation and adsorption properties of metal ions of crosslinked chitosan azacrown ethers. J. Appl. Polym. Sci. 1999, 74, 3053–3058. [Google Scholar] [CrossRef]
- Yang, Z.; Yuan, Y.; Wang, Y. Synthesis and evaluation of chitosan aryl azacrown ethers as adsorbents for metal ions. J. Appl. Polym. Sci. 2000, 77, 3093–3098. [Google Scholar] [CrossRef]
- Yang, Z.; Yuan, Y. Studies on the synthesis and properties of hydroxyl azacrown ether-grafted chitosan. J. Appl. Polym. Sci. 2001, 82, 1838–1843. [Google Scholar] [CrossRef]
- Rivas, B.L.; Sánchez, J.; Urbano, B.F. Polymers and nanocomposites: Synthesis and metal ion pollutant uptake. Polym. Int. 2015. [Google Scholar] [CrossRef]
- De Jong, F.; van Zon, A.; Reinhoudt, D.N.; Torny, G.J.; Tomassen, H.P.M. Chemistry of crown ethers XIX. Functionalized crown ethers for the solubilization of barium sulfate. Recl. Trav. Chim. Pays Bas 1983, 102, 164–173. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, X.; Feng, X.; Wang, Y.; Ma, S.; Peng, Q.; Zhang, W. Synthesis of N,N′-diallyl dibenzo 18-crown-6 crown ether crosslinked chitosan and their adsorption properties for metal ions. React. Funct. Polym. 2006, 66, 357–363. [Google Scholar] [CrossRef]
- Pedersen, C.J. Macrocyclic polyethers: Dibenzo-18-crown-6 polyether and dicyclo-18-crown-6 polyether. Org. Synth. 1972, 52, 66. [Google Scholar] [CrossRef]
- Izatt, R. Synthetic Multidentate Macrocyclic Compounds; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Greene, R.N. 18-crown-6: A strong complexing agent for alkali metal cations. Tetrahedron Lett. 1972, 13, 1793–1796. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Affolter, C. Structure Determination of Organic Compounds: Tables of Spectral Data, 3rd ed.; Springer: Berlin, Germany; New York, NY, USA, 2000. [Google Scholar]
- Silverstein, R.M.; Webster, F.X. Spectrometric Identification of Organic Compounds, 6th ed.; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Correa, W.H.; Scott, J.L. Synthesis and Characterisation of Macrocyclic Diamino Chiral Crown Ethers. Molecules 2004, 9, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Hayvalı, Z.; Hayvali, M.; Dal, H. Synthesis and Spectroscopic Characterization of New Schiff Bases Containing the Benzo-15-Crown-5 Moiety. Molecules 2004, 9, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 2−4 are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toeri, J.; Laborie, M.-P. Synthesis and Characterization of Macrocyclic Polyether N,N′-Diallyl-7,16-diaza-1,4,10,13-tetraoxa-dibenzo-18-crown-6. Molecules 2016, 21, 171. https://doi.org/10.3390/molecules21020171
Toeri J, Laborie M-P. Synthesis and Characterization of Macrocyclic Polyether N,N′-Diallyl-7,16-diaza-1,4,10,13-tetraoxa-dibenzo-18-crown-6. Molecules. 2016; 21(2):171. https://doi.org/10.3390/molecules21020171
Chicago/Turabian StyleToeri, Julius, and Marie-Pierre Laborie. 2016. "Synthesis and Characterization of Macrocyclic Polyether N,N′-Diallyl-7,16-diaza-1,4,10,13-tetraoxa-dibenzo-18-crown-6" Molecules 21, no. 2: 171. https://doi.org/10.3390/molecules21020171







