Miracle Fruit (Synsepalum dulcificum) Exhibits as a Novel Anti-Hyperuricaemia Agent
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antioxidant Effect and Xanthine Oxidase Suppression Following Miracle Fruit Treatment in Vitro
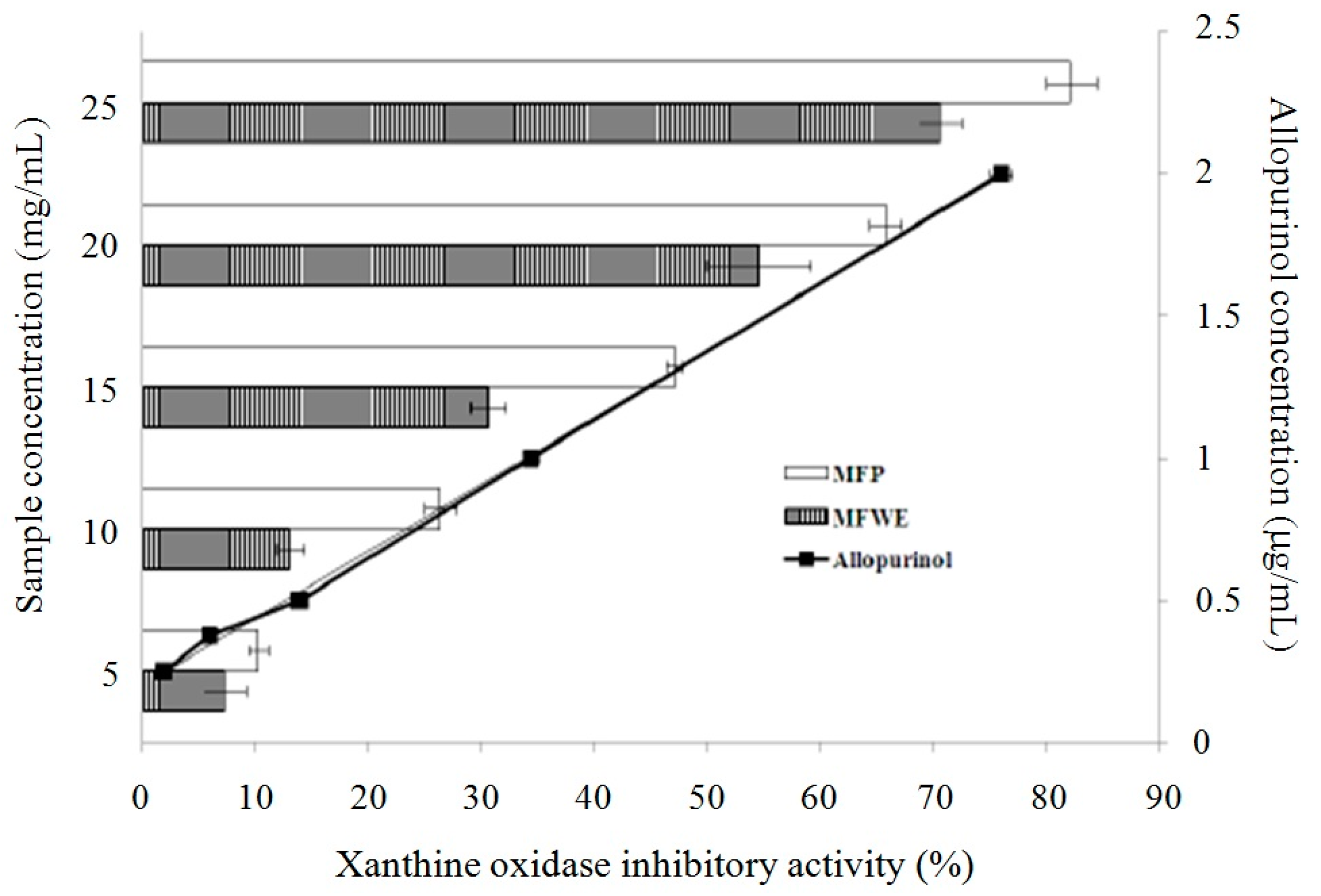


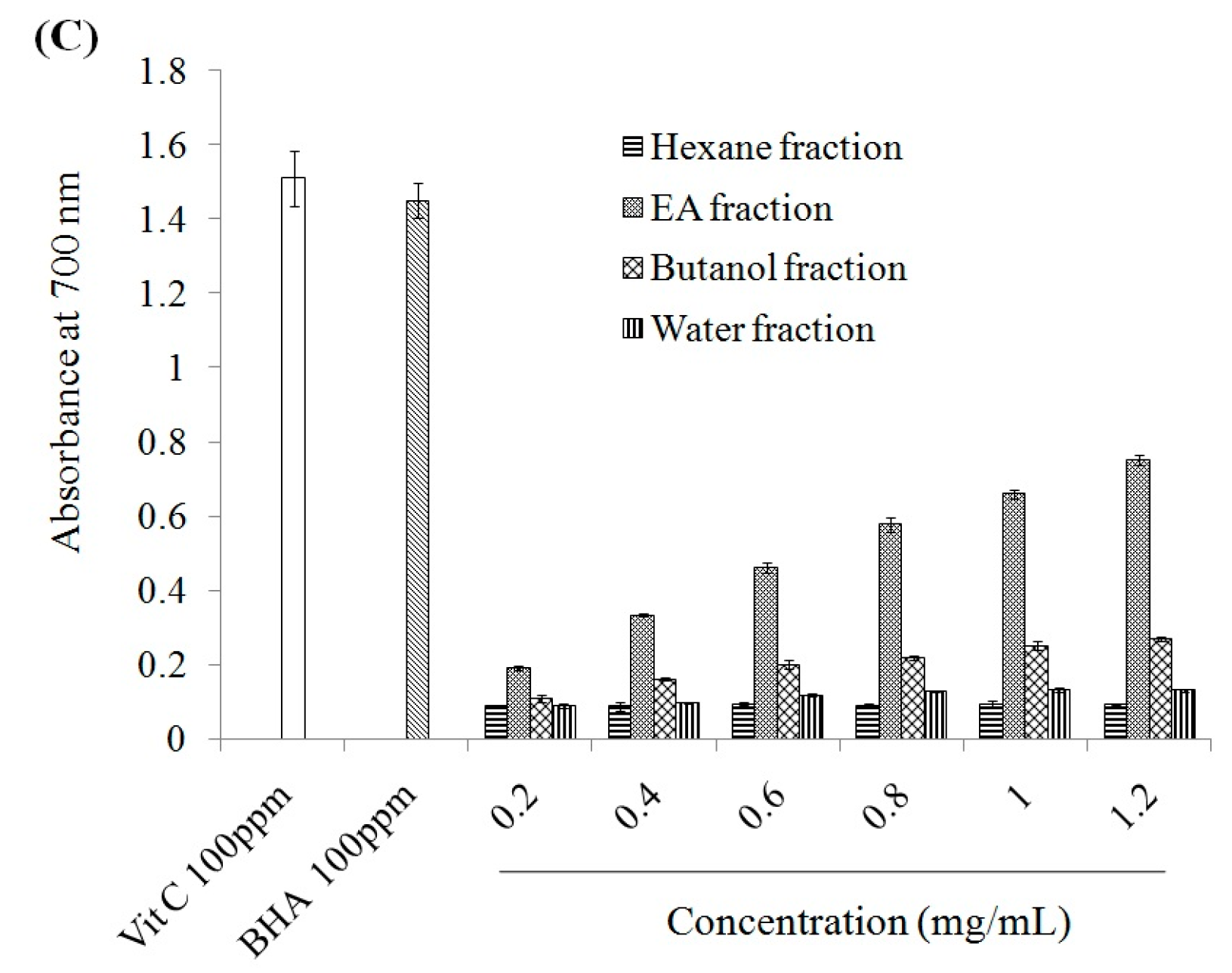
| Fractions | Total Phenolics | Flavonoids |
|---|---|---|
| Water Fraction | 0.91 ± 0.16 c | 1.12 ± 0.14 b |
| Butanol Fraction | 4.15 ± 0.36 b | 1.28 ± 0.13 b |
| EA Fraction | 12.91 ± 1.15 a | 5.38 ± 0.52 a |
| Hexane Fraction | 1.03 ± 0.99 c | 1.32 ± 0.17 b |
2.2. Inhibition of Oxidative Stress in MSU-Treated RAW264.7 Macrophages

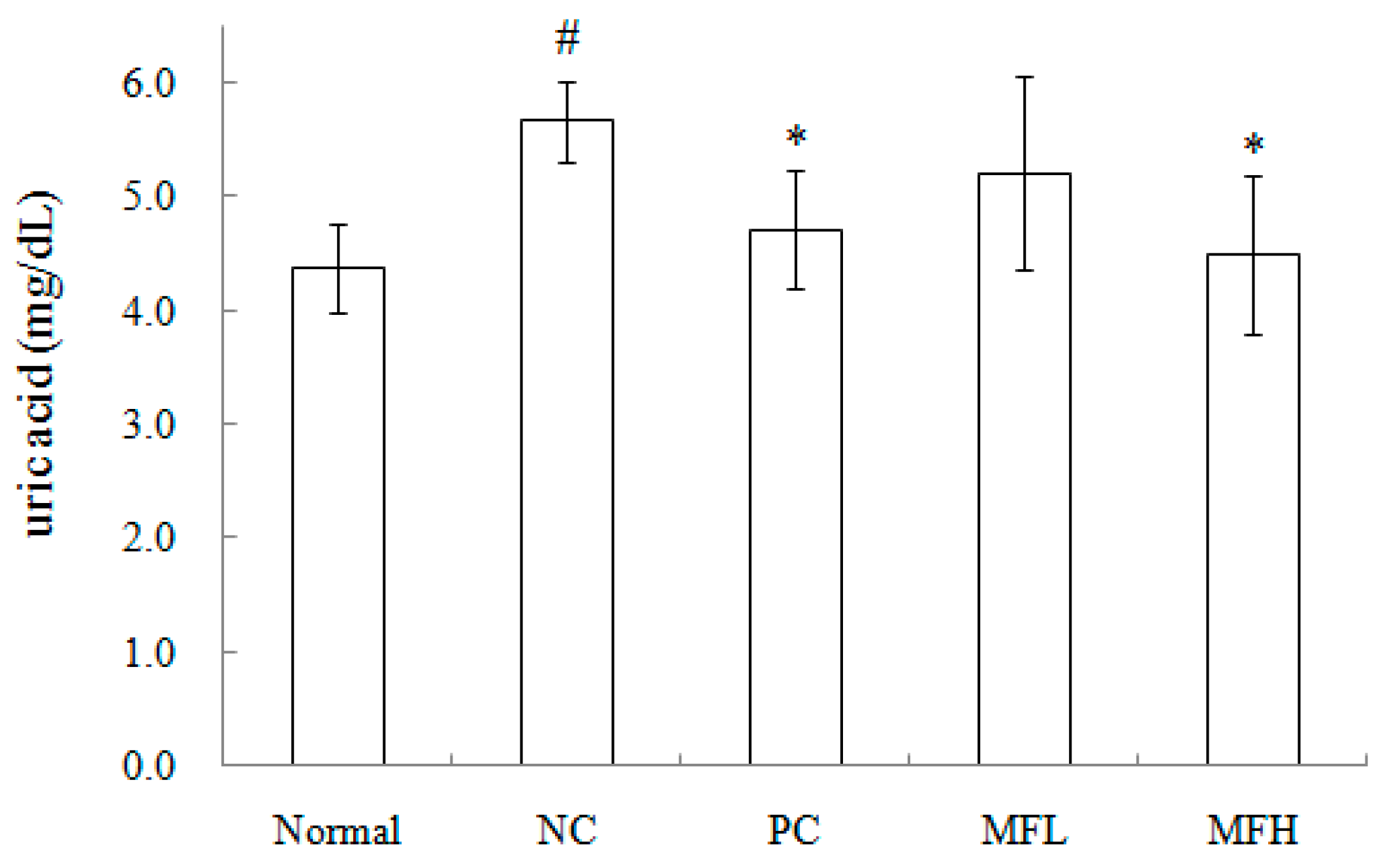
2.3. Miracle Fruit Downregulates Uric Acid Levels in Hyperuricaemic Mice
| Groups | Relative Organ Weight (g/100 g of bw) | |
|---|---|---|
| Liver | Kidney | |
| Normal | 4.84 ± 0.51 | 1.57 ± 0.14 |
| NC | 5.12 ± 0.62 | 1.58 ± 0.19 |
| PC | 4.96 ± 0.36 | 1.59 ± 0.14 |
| MFL | 5.01 ± 0.51 | 1.61 ± 0.16 |
| MFH | 5.22 ± 0.28 | 1.67 ± 0.2 |
| Groups | CRE | BUN |
|---|---|---|
| mg/dL | ||
| Normal | 0.55 ± 0.06 | 19.6 ± 1.1 |
| NC | 0.65 ± 0.21 | 26.3 ± 3.6 |
| PC | 0.48 ± 0.14 | 22.4 ± 5.9 |
| MFL | 0.48 ± 0.07 | 22.5 ± 7.1 |
| MFH | 0.46 ± 0.13 | 22.4 ± 4.0 |
| Groups | Xanthine Oxidase Activity |
|---|---|
| (nmol/min/mg/protein) | |
| Normal | 1.07 ± 0.31 ab |
| NC | 1.35 ± 0.40 a |
| PC | 0.75 ± 0.11 b |
| MFL | 1.02 ± 0.27 ab |
| MFH | 0.82 ± 0.30 b |
3. Materials and Methods
3.1. Chemicals
3.2. Sample Preparation
3.3. Antioxidation
3.4. Inhibition of Xanthine Oxidase Activity in Vitro
3.5. Cell Culture
3.6. Animal Model
3.7. Measurement of Hepatic Xanthine Oxidase Activity
3.8. Measurement of Oxidative Stress
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis (3-ethylbenzthiasoline-6-sulfonic acid) |
| BHA | butylated hydroxyanisole |
| BUN | blood urea nitrogen |
| CRE | creatinine |
| DCHF-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| DPPH | 1,1-diphenyl-2-pichryl hydrazyl |
| MFP | miracle fruit powder |
| MFWE | miracle fruit water extract |
| MSU | monosodiumurate |
| NBT | p-nitroblue tetrazolium chloride |
| NLRP3 | nucleotide-binding oligomerization domain-like receptor pyrindomain containing 3 |
| ROS | oxygen species |
References
- Inglett, G.E.; Chen, D. Contents of phenolics and flavonoids and antioxidant activities in skin, pulp, and seeds of miracle fruit. J. Food Sci. 2011, 76, C479–C482. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Shen, Y.; Zhang, X.; Prinyawiwatkul, W.; Xu, Z. Antioxidant-rich phytochemicals in miracle berry (Synsepalum dulcificum) and antioxidant activity of its extracts. Food Chem. 2014, 153, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; Kern, M. Miracle fruit improves sweetness of a low-calorie dessert without promoting subsequent energy compensation. Appetite 2011, 56, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.J.; Walton, M.; Harper, J. Resident macrophages initiating and driving inflammation in a monosodium urate monohydrate crystal-induced murineperitoneal model of acute gout. Arthritis Rheum. 2009, 60, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Shi, Y.; Hearn, A.; Fitzgerald, K.; Golenbock, D.; Reed, G. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J. Clin. Investig. 2006, 116, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Jhang, J.J.; Cheng, Y.T.; Ho, C.Y.; Yen, G.C. Monosodium urate crystals trigger Nrf2- and heme oxygenase-1-dependent inflammation in THP-1 cells. Cell Mol. Immunol. 2014, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Struthers, A.D. Role of urate, xanthine oxidase and the effects of allopurinol in vascular oxidative stress. Vasc. Health Risk Manag. 2009, 5, 265–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.; Li, G.; Yang, Y.; Shi, L.; Xu, L.; Yin, L. A network pharmacology approach to determine active ingredients and rationality of herb combinations of modified-simiaowan for treatment of gout. J. Ethnopharmacol. 2015, 168, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F. Signaling by ROS drives inflammasome activation. Eur. J. Immunol. 2010, 40, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, S.; Zhu, M.; Chen, J.; Zhu, X. Effects of genistein, apigenin, quercetin, rutin, and astilbein on serum uric acid levels and xanthine oxidase activities in normal and hyperuricemic mice. Food Chem. Toxicol. 2011, 49, 1943–1947. [Google Scholar] [CrossRef] [PubMed]
- Ramallo, I.A.; Zacchino, S.A.; Furlan, R.L. A rapid TLC autographic method for the detection of xanthine oxidase inhibitors and superoxide scavengers. Phytochem. Anal. 2006, 17, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Unno, T.; Sugimoto, A.; Kakuda, T. Xanthine oxidase inhibitors from the leaves of Lagerstroemia speciosa (L). Pers. J. Ethnopharmacol. 2004, 93, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.G.; Gaze, D.C.; Davison, G.W.; George, T.W.; Scotter, M.J.; Howatson, G. Montmorency tart cherry (Prunuscerasus L.) concentrate lowers uric acid, independent of plasma cyaniding-3-O-glucosiderutinoside. J. Funct. Food 2014, 11, 82–90. [Google Scholar] [CrossRef]
- Chen, L.; Mola, M.; Deng, X.; Mei, Z.; Huang, X.; Shu, G. Dolichos falcate Klein attenuated the inflammation induced by monosodium urate crystals in vivo and in vitro. J. Ethnopharmacol. 2013, 150, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Govardhan Singh, R.S.; Negi, P.S.; Radha, C. Phenolic composition, antioxidant and antimicrobial activities of free and bound phenolic extracts of Moringaoleifera seed flour. J. Funct. Food 2013, 5, 1883–1891. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.X.; Kong, L.D.; Yang, C.; Cheng, C.H.; Zhang, X. Administration of procyanidins from grape seeds reduces serum uric acid levels and decreases hepatic xanthine dehydrogenase/oxidase activities in oxonate-treated mice. Basic Clin. Pharmacol. Toxicol. 2004, 94, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.X.; Kong, L.D.; Ye, W.C.; Cheng, C.H.K.; Tan, R.X. Inhibition of xanthine and monoamine oxidases by stilbenoids from Veratrum taliense. Planta Med. 2001, 67, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Yen, J.H.; Lin, L.C.; Chen, M.C.; Sarang, Z.; Leong, P.Y.; Chang, I.C. The metastatic tumor antigen 1-transglutaminase-2 pathway in involved in self-limitation of monosodium urate crystal-induced inflammation by upregulating TGF-beta1. Arthritics Res. Ther. 2015, 17. [Google Scholar] [CrossRef]
- Ives, A.; Nomura, J.; Martinon, F.; Roger, T.; LeRoy, D.; Miner, J.N. Xanthine oxidoreductase regulates macrophage IL1beta secretion upon NLRP3 inflammasome activation. Nat. Comm. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. The relative contributions of ascorbic acid and phenolic antioxidants to the total antioxidant activity of orange and apple fruit juices and black currant drink. Food Chem. 1997, 60, 331–337. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 50, 6929–6934. [Google Scholar] [CrossRef]
- Abu Bakar, M.F.; Mohamed, M.; Rahmat, A.; Fry, J. Phytochemicals and antioxidant activity of different parts of bambangan (Mangiferapajang) and tarap (Artocarpusodoratissimus). Food Chem. 2009, 113, 479–483. [Google Scholar] [CrossRef]
- Hsu, W.H.; Lee, B.H.; Pan, T.M. Red mold dioscorea-induced G2/M arrest and apoptosis in human oral cancer cells. J. Sci. Food Agric. 2010, 90, 2709–2715. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.T.; Awale, S.; Tezuka, Y.; Tran, Q.L.; Watanabe, H.; Kadota, S. Xanthine oxidase inhibitory activity of Vietnamese medicinal plants. Biol. Pharm. Bull. 2004, 27, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Orallo, F.; Álvarez, E.; Camiña, M.; Leiro, J.M.; Gómez, E.; Fernández, P. The possible implication of trans-resveratrol in the cardioprotective effects of long-term moderate wine consumption. Mol. Pharmacol. 2002, 61, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Hsu, W.H.; Huang, T.; Chang, Y.Y.; Hsu, Y.W.; Pan, T.M. Effects of monascin on anti-inflammation mediated by Nrf2 activation in advanced glycation end product-treated THP-1 monocytes and methylglyoxal-treated Wistar rats. J. Agric. Food Chem. 2013, 61, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Nestby, R.; Krogstad, T.; Joner, E.; Vohnik, M. The effect of NP fertilization on European blueberry (Vaccinium myrtillus L.) development on cultivated land in mid-Norway. J. Berry Res. 2014, 4, 147–157. [Google Scholar]
- Pissard, A.; Lateur, M.; Baeten, V.; Magein, H.; Dupont, P.; Tabart, J.; Pincemail, J.; Kevers, C. Determination of total phenolic compound content and antioxidant activity in cherry species and cultivars. J. Berry Res. 2015. [Google Scholar] [CrossRef]
- Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Bompadre, S.; Mezzetti, B.; Quiles, J.L.; Giampien, F.; Battino, M. The healthy effects of strawberry polyphenols: Which strategy behind antioxidant capacity? Crit. Rev. Food Sci. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Gonzalez-Paramas, A.M.; Santos-Buelga, C.; Quiles, J.L.; Bompadre, S.; Mezzetti, B.; et al. An anthocyanin-rich strawberry extract protects against oxidative stress damage and improves mitochondrial functionality in human dermal fibroblasts exposed to an oxidizing agent. Food Funct. 2014, 5, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.-C.; Lin, K.-S.; Jhai, Y.-F.; Lee, B.-H.; Han, Y.; Cui, Z.; Hsu, W.-H.; Wu, S.-C. Miracle Fruit (Synsepalum dulcificum) Exhibits as a Novel Anti-Hyperuricaemia Agent. Molecules 2016, 21, 140. https://doi.org/10.3390/molecules21020140
Shi Y-C, Lin K-S, Jhai Y-F, Lee B-H, Han Y, Cui Z, Hsu W-H, Wu S-C. Miracle Fruit (Synsepalum dulcificum) Exhibits as a Novel Anti-Hyperuricaemia Agent. Molecules. 2016; 21(2):140. https://doi.org/10.3390/molecules21020140
Chicago/Turabian StyleShi, Yeu-Ching, Kai-Sian Lin, Yi-Fen Jhai, Bao-Hong Lee, Yifan Han, Zhibin Cui, Wei-Hsuan Hsu, and She-Ching Wu. 2016. "Miracle Fruit (Synsepalum dulcificum) Exhibits as a Novel Anti-Hyperuricaemia Agent" Molecules 21, no. 2: 140. https://doi.org/10.3390/molecules21020140






