Long-Term Stability of New Co-Amorphous Drug Binary Systems: Study of Glass Transitions as a Function of Composition and Shelf Time
Abstract
:1. Introduction
2. Results
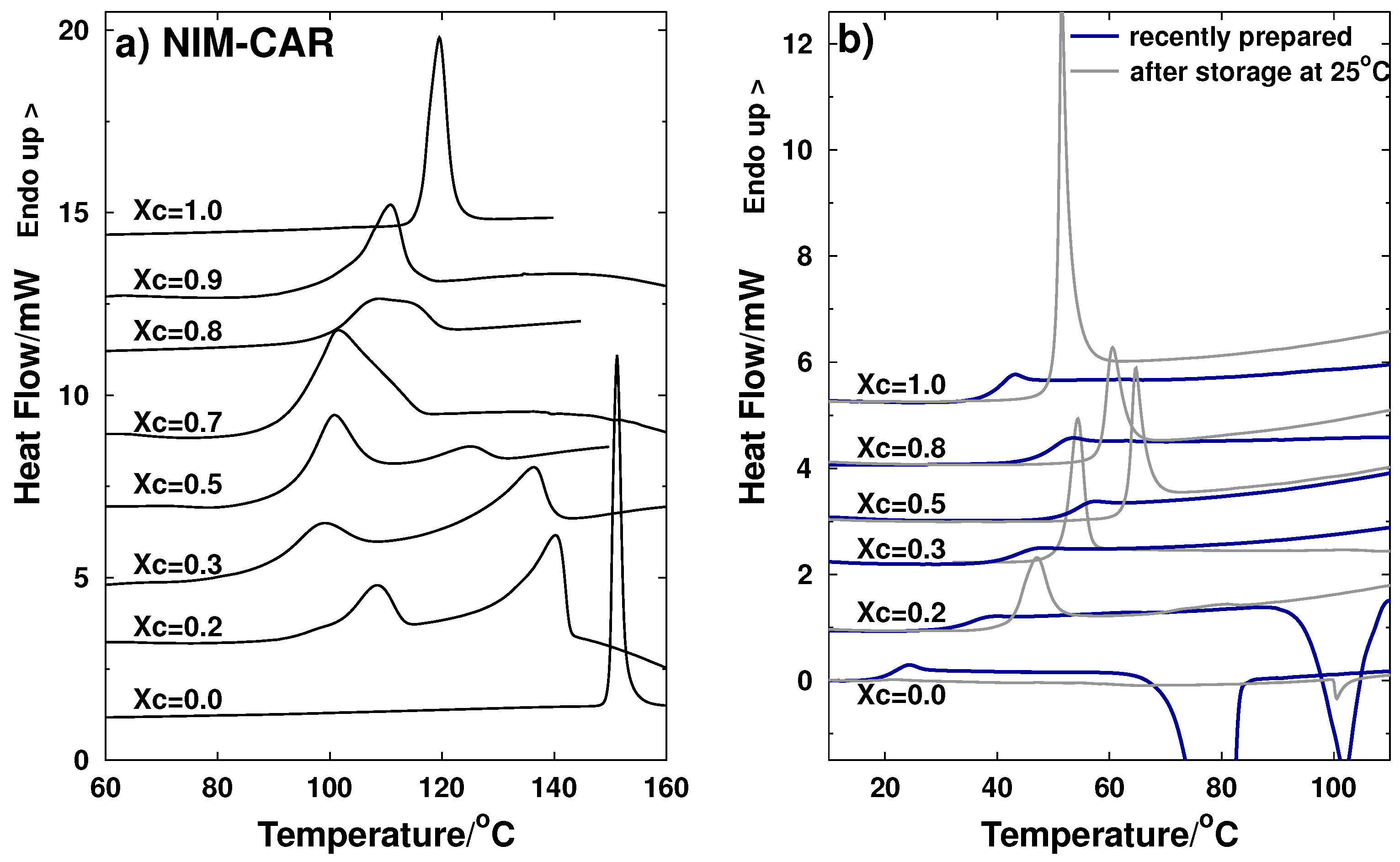
2.1. Thermal Characterization
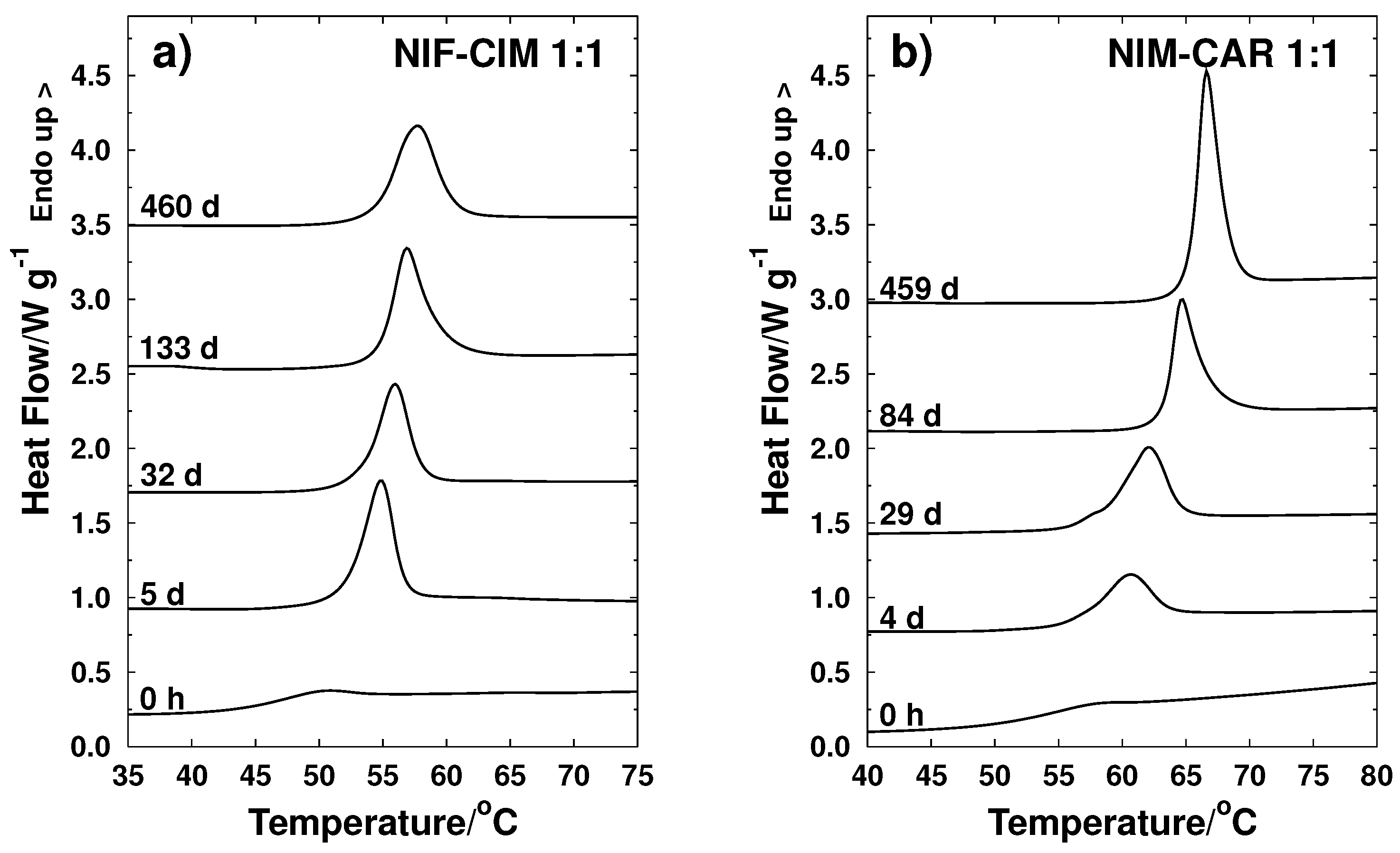
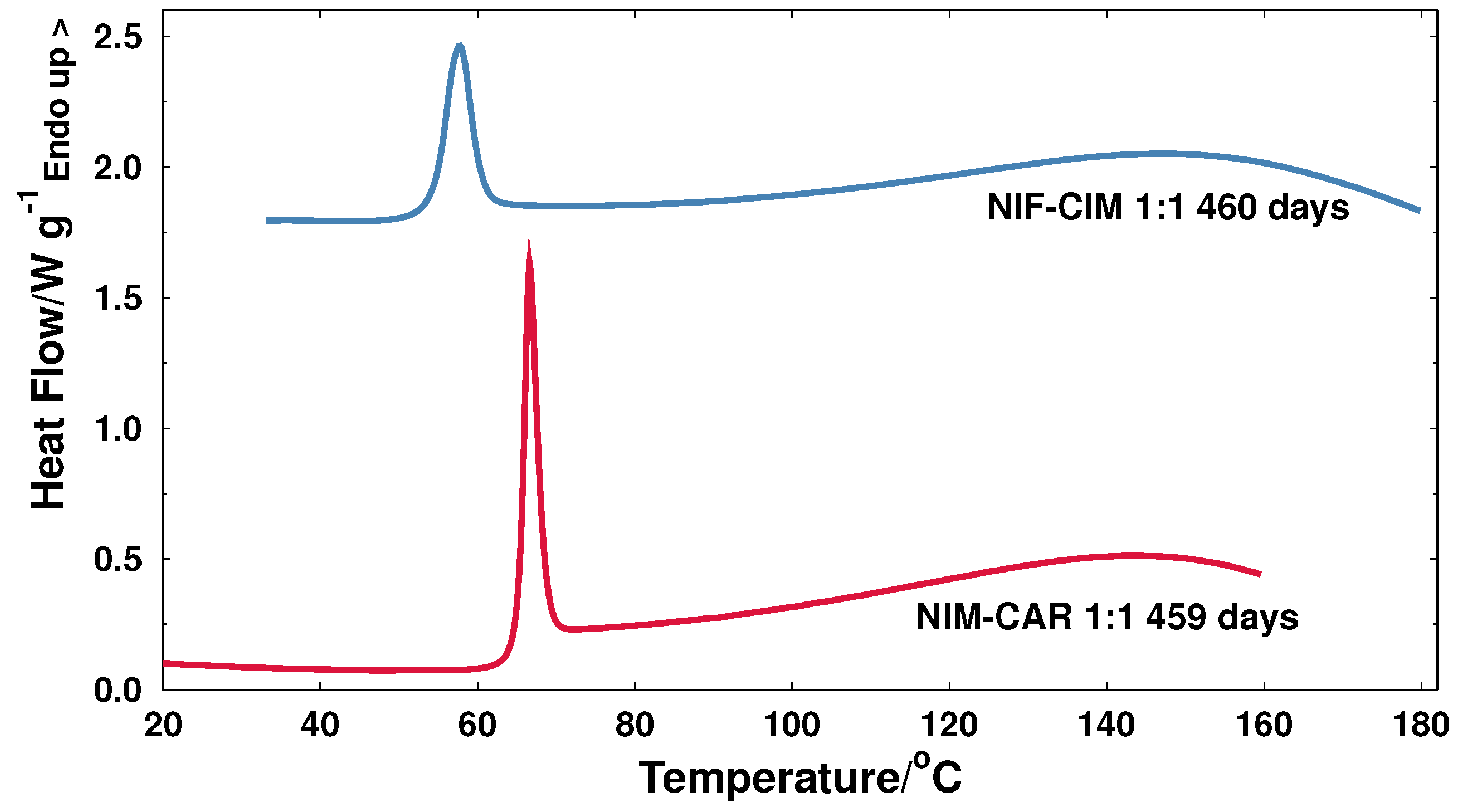
2.1.1. The NIF-CIM System
2.1.2. NIM-CAR System
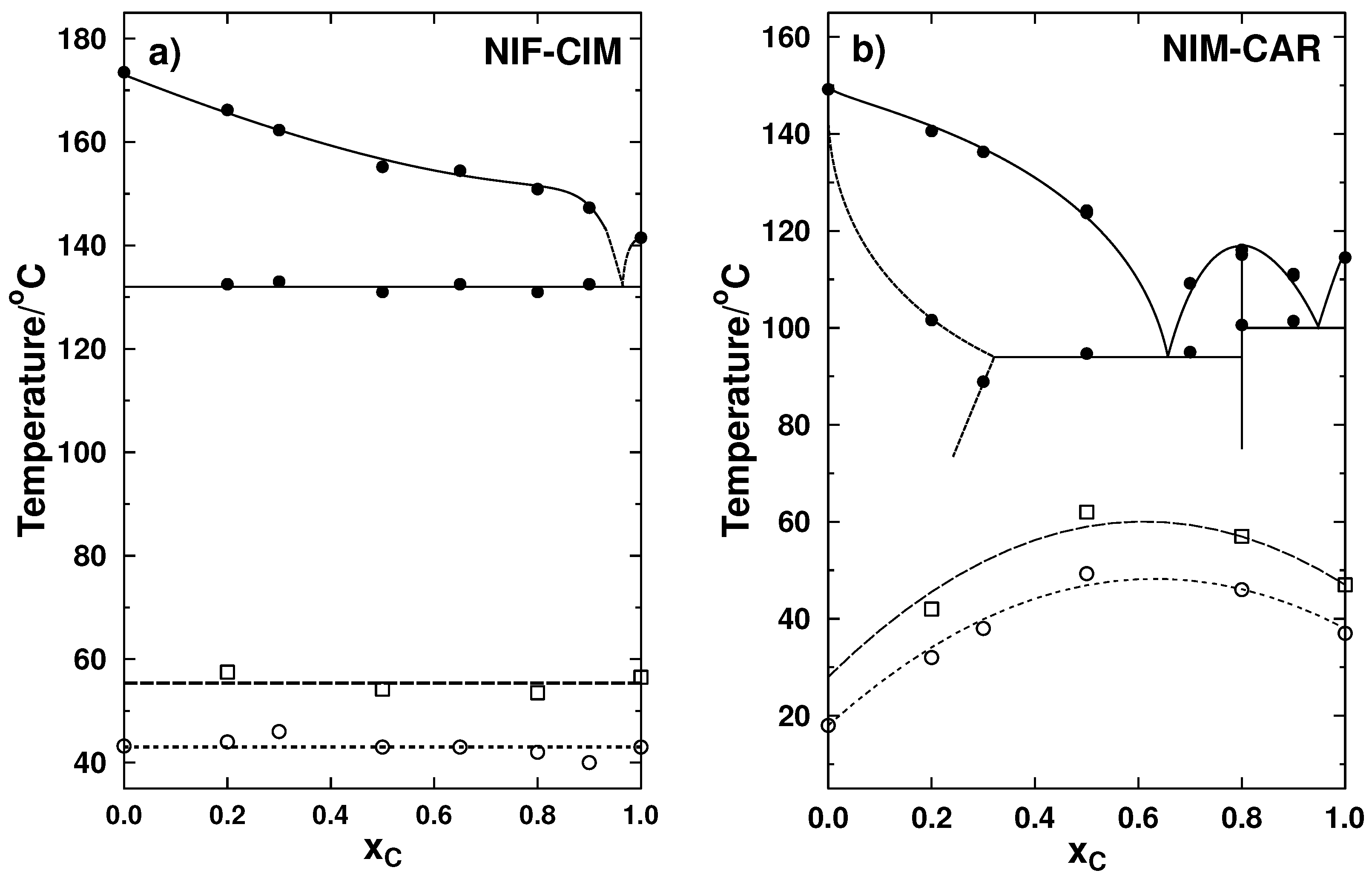
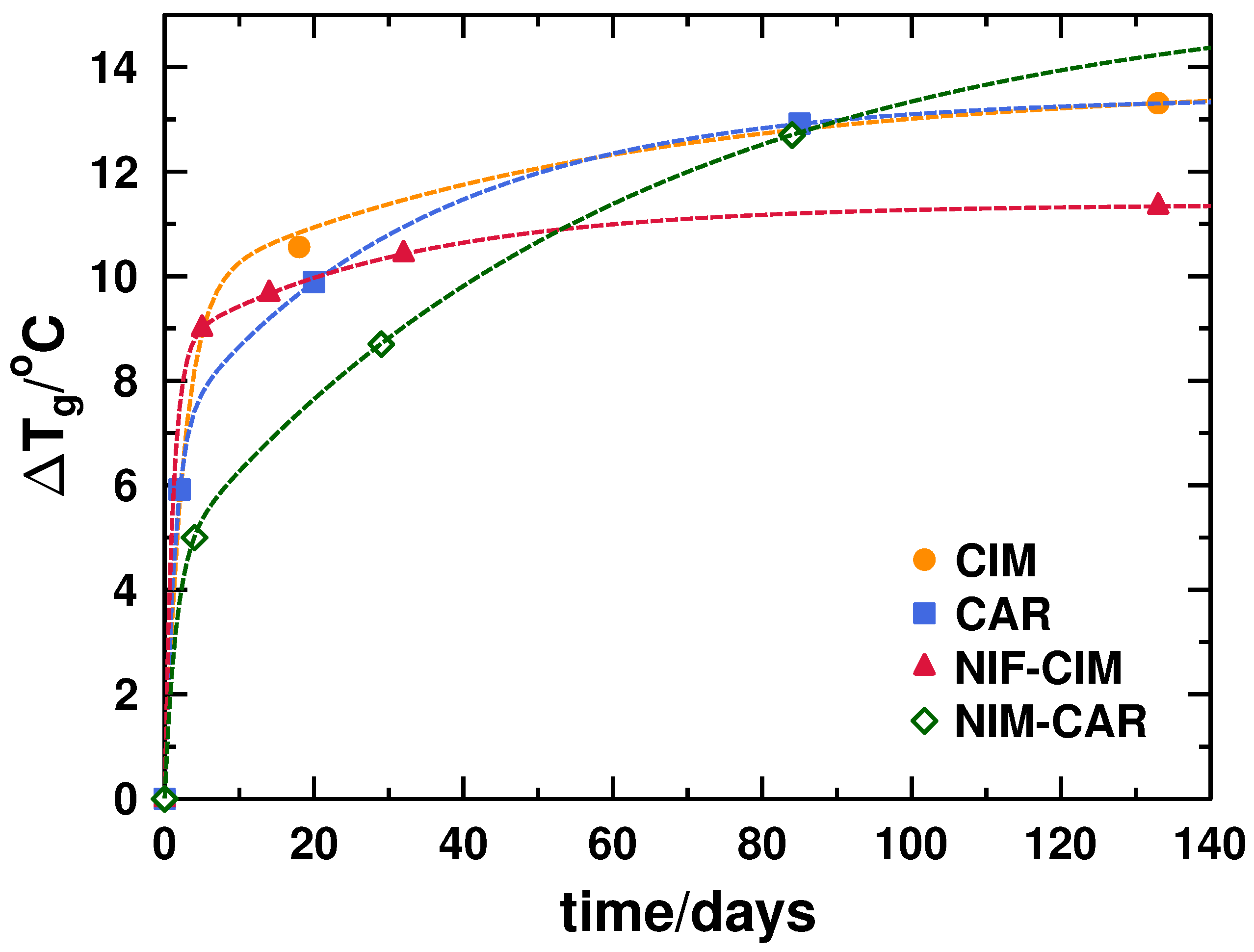
2.1.3. Phase Transition Diagrams
3. Discussion
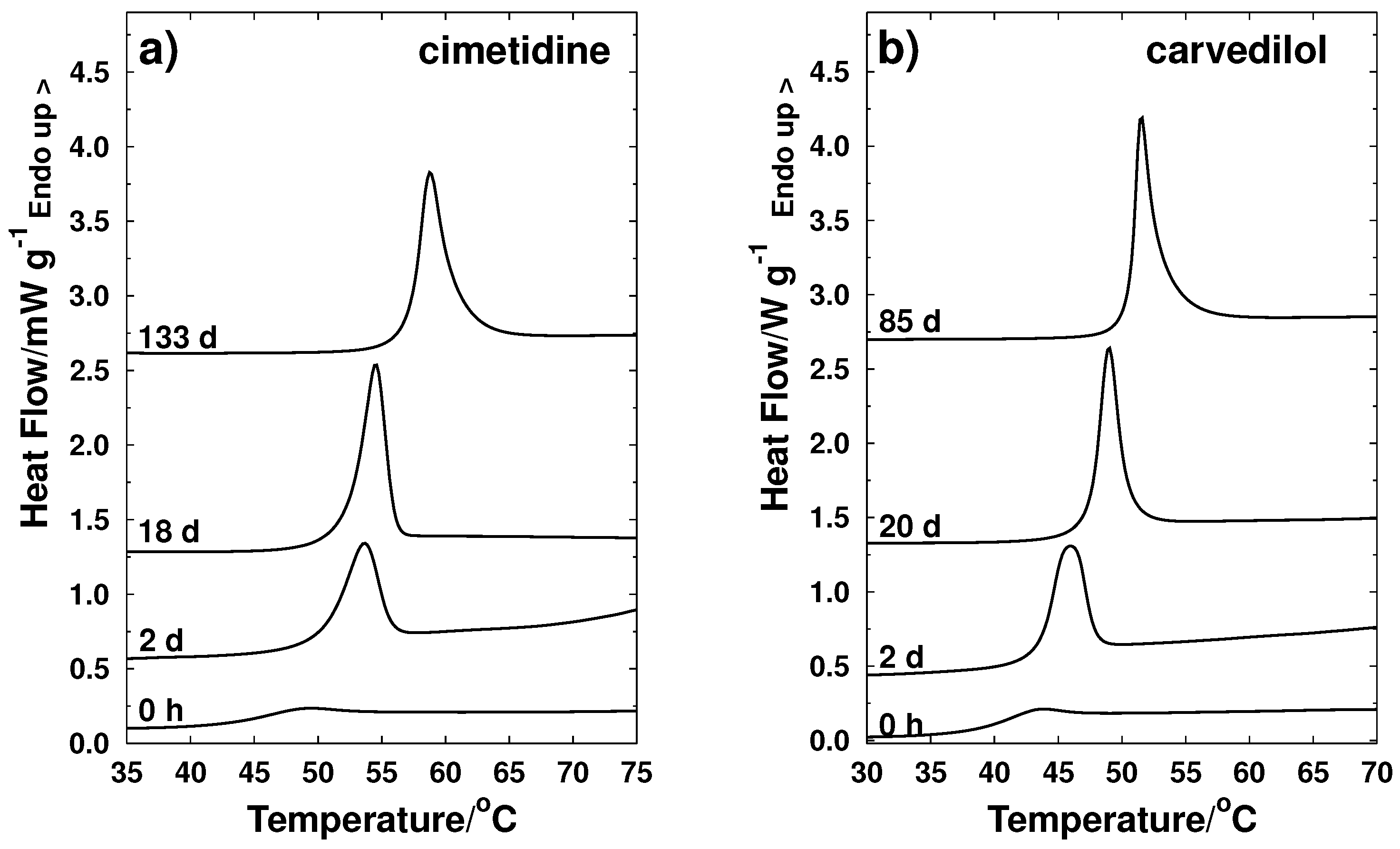
3.1. Behavior of Glass Transition during Storage
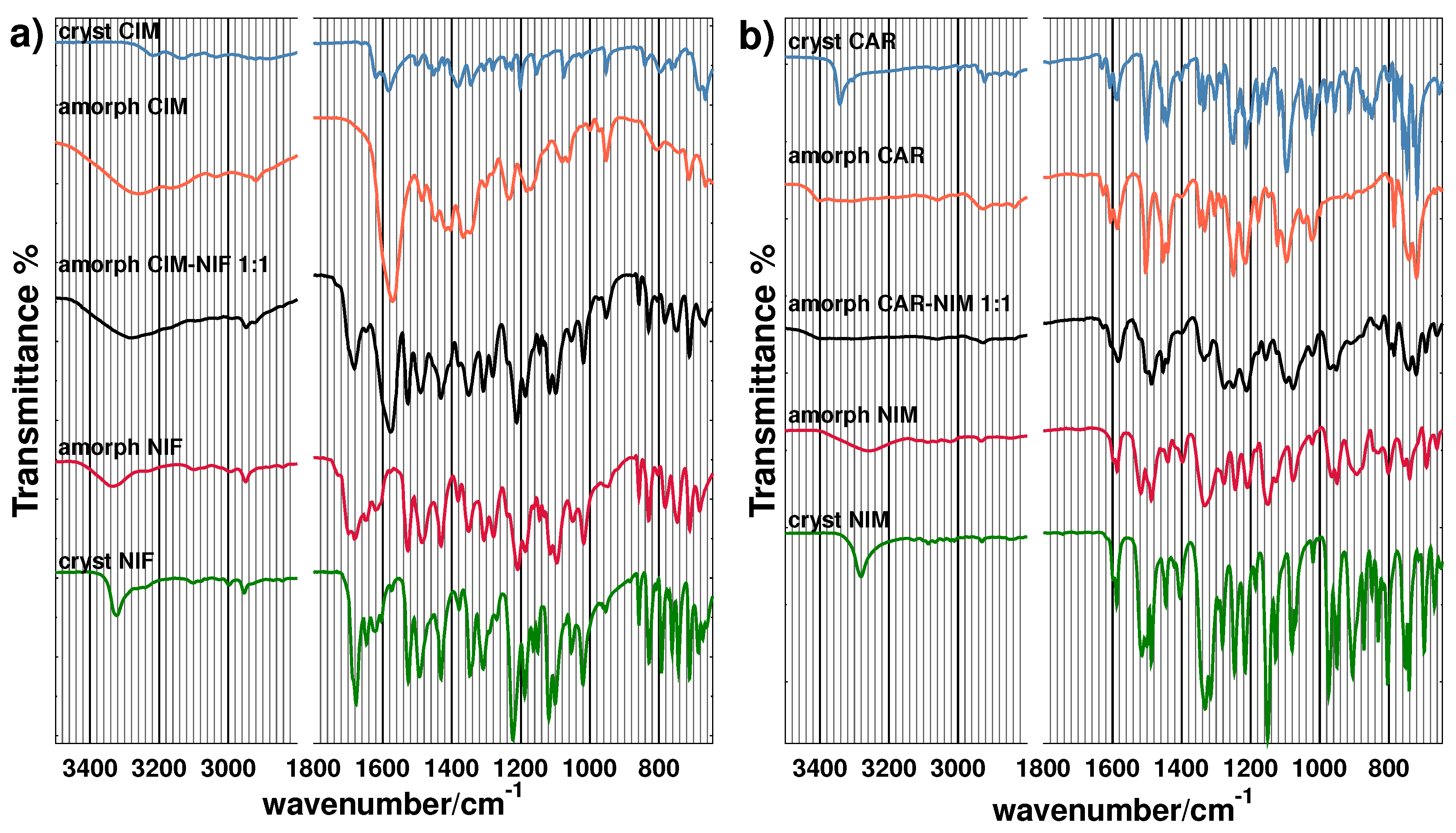
3.2. Spectroscopic Analysis
4. Materials and Methods
4.1. Materials
4.2. Determination of Phase Transitions by Thermal Analysis
4.3. FTIR Spectroscopic Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| API | Active pharmaceutical ingredient |
| ATR | Attenuated total reflectance |
| CAR | carvedilol |
| CIM | cimethidine |
| DSC | Differential scanning calorimetry |
| FTIR | Fourier transform infrared spectroscopy |
| NIF | nifedipine |
| NIM | nimesulide |
| NIR | near infrared |
| USP | United States Pharmacopeia |
References
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed]
- Dengale, S.J.; Grohganz, H.; Rades, T.; Löbmann, K. Recent advances in co-amorphous drug formulations. Adv. Drug Deliv. Rev. 2016, 100, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, O.; Pajula, K.; Laitinen, R. Rational excipient selection for co-amorphous formulations. Expert Opin. Drug Deliv. 2016, 5247, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, R.; Löbmann, K.; Strachan, C.J.; Grohganz, H.; Rades, T. Emerging trends in the stabilization of amorphous drugs. Int. J. Pharm. 2013, 453, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Chawla, G.; Bansal, A.K. Physical Stability and Solubility Advantage from Amorphous Celecoxib: The Role of Thermodynamic Quantities and Molecular Mobility. Mol. Pharm. 2004, 1, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Hancock, B.C.; Parks, M. What is the True Solubility Advantage for Amorphous Pharmaceuticals? Pharm. Res. 2000, 17, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Ida, Y.; Kadota, K.; Tozuka, Y. Raman mapping for kinetic analysis of crystallization of amorphous drug based on distributional images. Int. J. Pharm. 2014, 462, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Yoshioka, S.; Aso, Y.; Kawanishi, T. Crystallization rate of amorphous nifedipine analogues unrelated to the glass transition temperature. Int. J. Pharm. 2007, 336, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Kato, F.; Matsuda, Y. Physicochemical stability of cimetidine amorphous forms estimated by isothermal microcalorimetry. AAPS PharmSciTech 2002, 3, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.M.; Videa, M.; López-Silva, G.A.; de Los Reyes, C.A.; Cruz-Angeles, J.; González, N. Stabilization of amorphous paracetamol based systems using traditional and novel strategies. Int. J. Pharm. 2014, 477, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Babu, N.J.; Nangia, A. Solubility Advantage of Amorphous Drugs and Pharmaceutical Cocrystals. Cryst. Growth Des. 2011, 11, 2662–2679. [Google Scholar] [CrossRef]
- Allesø, M.; Chieng, N.; Rehder, S.; Rantanen, J.; Rades, T.; Aaltonen, J. Enhanced dissolution rate and synchronized release of drugs in binary systems through formulation: Amorphous naproxen-cimetidine mixtures prepared by mechanical activation. J. Controll. Release 2009, 136, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Löbmann, K.; Laitinen, R.; Grohganz, H.; Strachan, C.; Rades, T.; Gordon, K.C. A theoretical and spectroscopic study of co-amorphous naproxen and indomethacin. Int. J. Pharm. 2013, 453, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Kubecova, M.; Kolostova, K.; Pinterova, D.; Kacprzak, G.; Bobek, V. Cimetidine: An anticancer drug? Eur. J. Pharm. Sci. 2011, 42, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Langley, S.J.; Mullins, F.G.; Dixon, J.S.; Toon, S. The pharmacokinetics and pharmacodynamics of nifedipine at steady state during concomitant administration of cimetidine or high dose ranitidine. Br. J. Clin. Pharmacol. 1991, 32, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Piel, G.; Pirotte, B.; Delneuville, I.; Neven, P.; Llabres, G.; Delarge, J.; Delattre, L. Study of the Influence of Both Cyclodextrins and L-Lysine on the Aqueous Solubility of Nimesulide; Isolation and Characterization of Nimesulide-Lysine-Cyclodextrin Complexes. J. Pharm. Sci. 1997, 86, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A. Overview of nimesulide. Rheumatology 1999, 38, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Kamiya, K.; Kodama, I. Carvedilol: Molecular and Cellular Basis for Its Multifaceted Therapeutic Potential. Cardiovasc. Drug Rev. 2001, 19, 152–171. [Google Scholar] [CrossRef] [PubMed]
- Bauer-Brandl, A. Polymorphic transitions of cimetidine during manufacture of solid dosage forms. Int. J. Pharm. 1996, 140, 195–206. [Google Scholar] [CrossRef]
- Keratichewanun, S.; Yoshihashi, Y.; Sutanthavibul, N.; Terada, K.; Chatchawalsaisin, J. An investigation of nifedipine miscibility in solid dispersions using raman spectroscopy. Pharm. Res. 2015, 32, 2458–2473. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.M.V.; Prado, L.D.; Rocha, H.V.A. Evaluation and correlation of the physicochemical properties of carvedilol. Pharm. Dev. Technol. 2015, 7450, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pokharkar, V.B.; Mandpe, L.P.; Padamwar, M.N.; Ambike, A.A.; Mahadik, K.R.; Paradkar, A. Development, characterization and stabilization of amorphous form of a low Tg drug. Powder Technol. 2006, 167, 20–25. [Google Scholar] [CrossRef]
- Moneghini, M.; Zingone, G.; De Zordi, N. Influence of the microwave technology on the physical-chemical properties of solid dispersion with Nimesulide. Powder Technol. 2009, 195, 259–263. [Google Scholar] [CrossRef]
- Sanphui, P.; Sarma, B.; Nangia, A. Phase transformation in conformational polymorphs of nimesulide. J. Pharm. Sci. 2011, 100, 2287–2299. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.L.A.; Fleming, O.S.; Kazarian, S.G.; Vassou, D.; Chryssikos, G.D.; Gionis, V. Polymorphism and devitrification of nifedipine under controlled humidity: A combined FT-Raman, IR and Raman microscopic investigation. J. Raman Spectrosc. 2004, 35, 353–359. [Google Scholar] [CrossRef]
- Hecq, J.; Deleers, M.; Fanara, D.; Vranckx, H.; Amighi, K. Preparation and characterization of nanocrystals for solubility and dissolution rate enhancement of nifedipine. Int. J. Pharm. 2005, 299, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, L.; Johari, G.P.; Shanker, R.M. Structural relaxation of acetaminophen glass. Pharm. Res. 2006, 23, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Luthra, S.A.; Hodge, I.M.; Pikal, M.J. Investigation of the impact of annealing on global molecular mobility in glasses: Optimization for stabilization of amorphous pharmaceuticals. J. Pharm. Sci. 2008, 97, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Pajula, K.; Taskinen, M.; Lehto, V.P.; Ketolainen, J.; Korhonen, O. Predicting the formation and stability of amorphous small molecule binary mixtures from computationally determined flory-huggins interaction parameter and phase diagram. Mol. Pharm. 2010, 7, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.W.; Löbmann, K.; Grohganz, H.; Rades, T.; Chieng, N. Investigation of physical properties and stability of indomethacin–cimetidine and naproxen–cimetidine co-amorphous systems prepared by quench cooling, coprecipitation and ball milling. J. Pharm. Pharmacol. 2016, 68, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: The compounds were purchased from commercial sources and are not currently available from the authors.
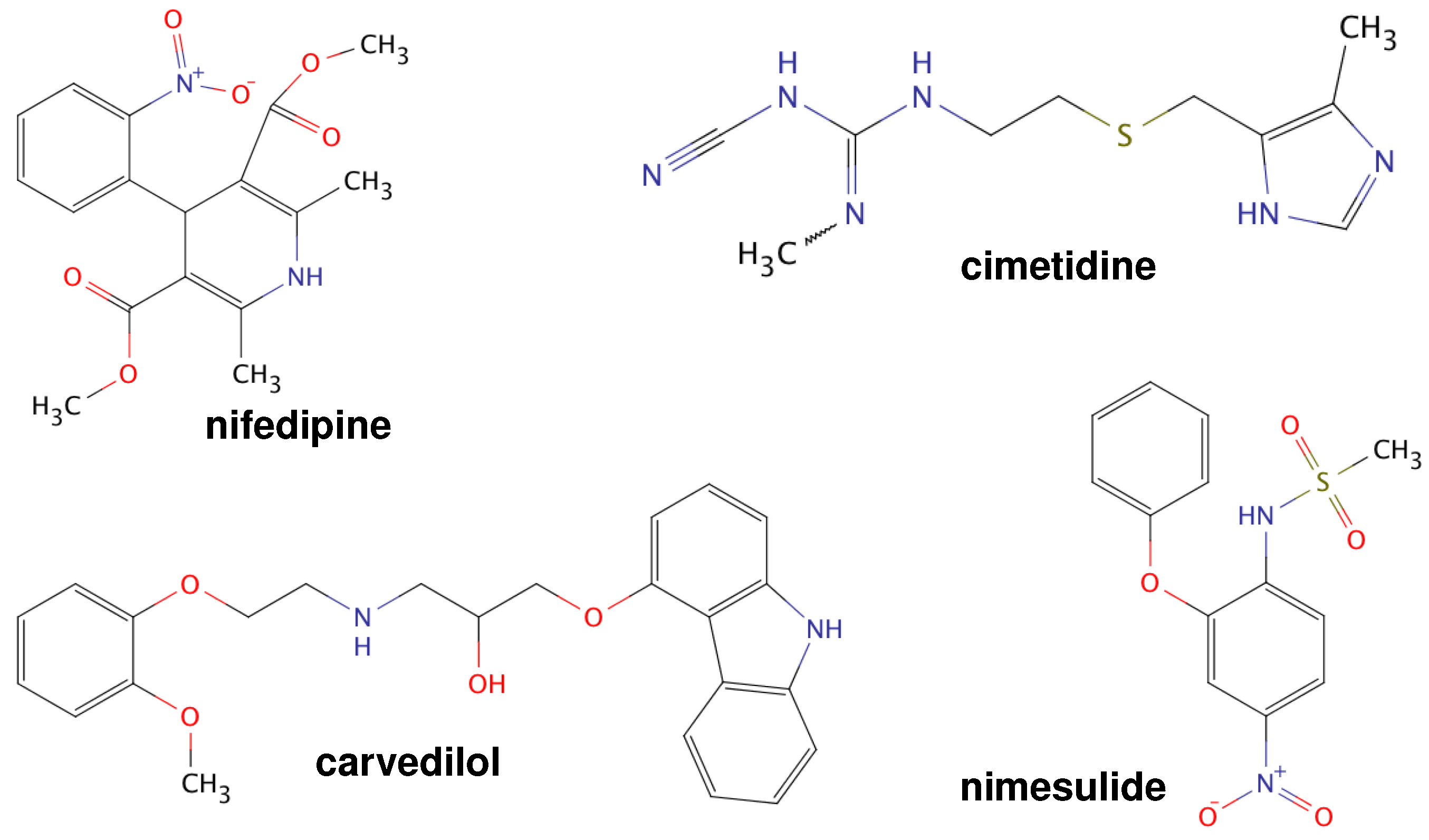

| Pharmaceutical | /C | Polymorph | /C | Therapeutic | Long-Term Stability | References |
|---|---|---|---|---|---|---|
| Ingredient | Activity | (Amorphous State) | ||||
| Cimetidine | 36 | Form A or D | 141–143 | anti-ulcer agent | not studied | [12,19] |
| CHNS | Form C | 83 | ||||
| Nifedipine | 45 | Form | 172 | antianginal/ | unstable | [20] |
| CHNO | Form | 170 | antihypertensive | |||
| Carvedilol | 39 | Form I | 123–126 | antihypertensive/ | stable | [21,22] |
| CHNO | Form II | 114–115 | heart failure treatment | |||
| Nimesulide | 20 | Form I | 144 | anti-inflammatory | unstable | [23,24] |
| CHNOS | Form II | 140 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, L.M.; Videa, M.; Sosa, N.G.; Ramírez, J.H.; Castro, S. Long-Term Stability of New Co-Amorphous Drug Binary Systems: Study of Glass Transitions as a Function of Composition and Shelf Time. Molecules 2016, 21, 1712. https://doi.org/10.3390/molecules21121712
Martínez LM, Videa M, Sosa NG, Ramírez JH, Castro S. Long-Term Stability of New Co-Amorphous Drug Binary Systems: Study of Glass Transitions as a Function of Composition and Shelf Time. Molecules. 2016; 21(12):1712. https://doi.org/10.3390/molecules21121712
Chicago/Turabian StyleMartínez, Luz María, Marcelo Videa, Nahida González Sosa, José Héctor Ramírez, and Samuel Castro. 2016. "Long-Term Stability of New Co-Amorphous Drug Binary Systems: Study of Glass Transitions as a Function of Composition and Shelf Time" Molecules 21, no. 12: 1712. https://doi.org/10.3390/molecules21121712








