Carbon Nanodots as Peroxidase Nanozymes for Biosensing
Abstract
:1. Introduction
2. Fundamentals of C-Dots: An Overview
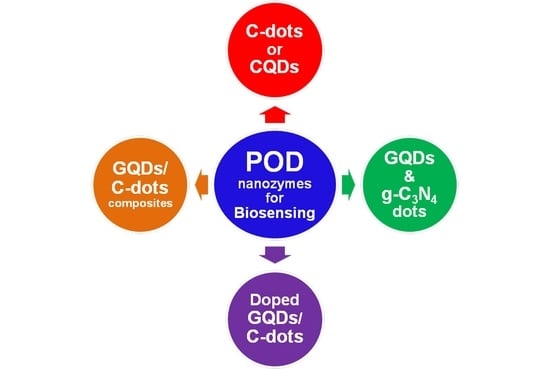
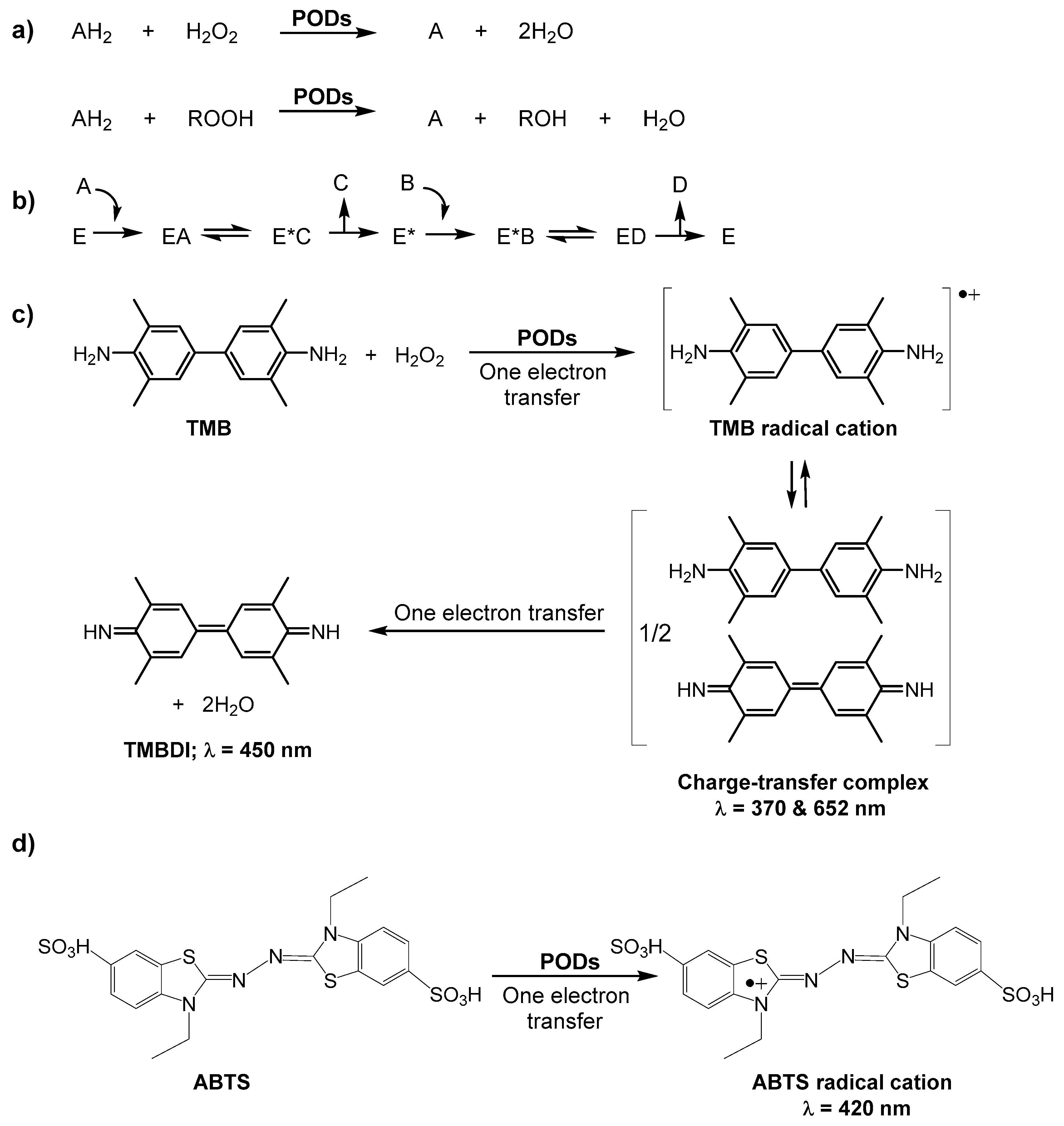
3. Carbon Nanodots (C-Dots) as POD Nanozymes
4. Graphene Quantum Dots (GQDs) as POD Nanozymes
5. Doped GQDs/C-Dots as POD Nanozymes
6. GQDs/C-Dots Conjugates and/or Nanocomposites as POD Nanozymes
7. Carbon Nitride Dots (CN-Dots) as POD Nanozymes
8. Advantages, Challenges and Future Perspectives
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Breslow, R.; Overman, L.E. Artificial enzyme combing a metal catalytic group and a hydrophobic binding cavity. J. Am. Chem. Soc. 1970, 92, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Bhabak, K.P.; Mugesh, G. Functional mimics of glutathione peroxidase: Bioinspired synthetic antioxidants. Acc. Chem. Res. 2010, 43, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Friedle, S.; Reisner, E.; Lippard, S.J. Current challenges of modeling diiron enzyme active sites for dioxygen activation by biomimetic synthetic complexes. Chem. Soc. Rev. 2010, 39, 2768–2779. [Google Scholar] [CrossRef] [PubMed]
- Manea, F.; Houillon, F.B.; Pasquato, L.; Scrimin, P. Nanozymes: Gold-nanoparticle-based transphosphorylation catalysts. Angew. Chem. Int. Ed. 2004, 43, 6165–6169. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Asati, A.; Santra, S.; Kaittanis, C.; Nath, S.; Perez, J.M. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 2308–2312. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ren, J.; Qu, X. Catalytically active nanomaterials: A promising candidate for artificial enzymes. Acc. Chem. Res. 2014, 47, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wamer, W.; Xia, Q.; Yin, J.-J.; Fu, P.P. Enzyme-like activity of nanomaterials. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2014, 32, 186–211. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, X.; Zhao, C.; Qu, K.; Ren, J.; Qu, X. Label-free colorimetric detection of single nucleotide polymorphism by using single-walled carbon nanotube intrinsic peroxidase-like activity. Chem. Eur. J. 2010, 16, 3617–3621. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene oxide: Intrinsic peroxidase catalytic activity and its application to glucose detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Ling, Y.-C. Versatilities of graphene-based catalysts in organic transformations. Green Mater. 2013, 1, 47–61. [Google Scholar] [CrossRef]
- Garg, B.; Bisht, T.; Ling, Y.-C. Graphene-based nanomaterials as heterogeneous acid catalysts: A comprehensive perspective. Molecules 2014, 19, 14582–14614. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Bisht, T.; Ling, Y.-C. Sulfonated graphene as highly efficient and reusable acid carbocatalyst for the synthesis of ester plasticizers. RSC Adv. 2014, 4, 57297–57307. [Google Scholar] [CrossRef]
- Garg, B.; Sung, C.-H.; Ling, Y.-C. Graphene-based nanomaterials as molecular imaging agents. WIREs Nanomed. Nanobiotechnol. 2015, 7, 737–758. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Bisht, T.; Ling, Y.-C. Graphene-based nanomaterials as efficient peroxidase mimetic catalysts for biosensing applications: An overview. Molecules 2015, 20, 14155–14190, and references there in. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Bisht, T.; Ling, Y.-C. Graphene-based nanomaterials: Versatile catalysts for carbon-carbon bond forming reactions. Curr. Org. Chem. 2016, 20, 1547–1566. [Google Scholar] [CrossRef]
- Xu, X.Y.; Ray, R.; Gu, Y.L.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ohulchanskyy, T.Y.; Liu, R.L.; Koynov, K.; Wu, D.Q.; Best, A.; Kumar, R.; Bonoiu, A.; Prasad, P.N. Photoluminescent carbon dots as biocompatible nanoprobes for targeting cancer cells in vitro. J. Phys. Chem. C 2010, 114, 12062–12068. [Google Scholar] [CrossRef]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.-T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939, and references there in. [Google Scholar] [CrossRef]
- Miao, P.; Han, K.; Tang, Y.; Wang, B.; Lin, T.; Cheng, W. Recent advances in carbon nanodots: Synthesis, properties and biomedical applications. Nanoscale 2015, 7, 1586–1595, and references there in. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Chen, Z.; Zhao, C.; Gao, N.; Ren, J.; Qu, X. Recent advances in bioapplications of C-dots. Carbon 2015, 85, 309–327. [Google Scholar] [CrossRef]
- Liu, H.; Ye, T.; Mao, C. Fluorescent carbon nanoparticles derived from candle soot. Angew. Chem. Int. Ed. 2007, 46, 6473–6475. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Chen, P.-C.; Periasamy, A.P.; Chen, Y.-N.; Chang, H.-T. Photoluminescent carbon nanodots: Synthesis, physicochemical properties and analytical applications. Mater. Today 2015, 18, 447–458, and references there in. [Google Scholar] [CrossRef]
- Veitch, N.C. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M. Khalil-ur-Rehman Potential applications of peroxidases. Food Chem. 2009, 115, 1177–1186. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Wei, H. Nanozymes in bionanotechnology: From sensing to therapeutics and beyond. Inorg. Chem. Front. 2016, 3, 41–60. [Google Scholar] [CrossRef]
- Shinde, D.B.; Pillai, V.K. Electrochemical preparation of luminescent graphene quantum dots from multiwalled carbon nanotubes. Chem. Eur. J. 2012, 18, 12522–12528. [Google Scholar] [CrossRef] [PubMed]
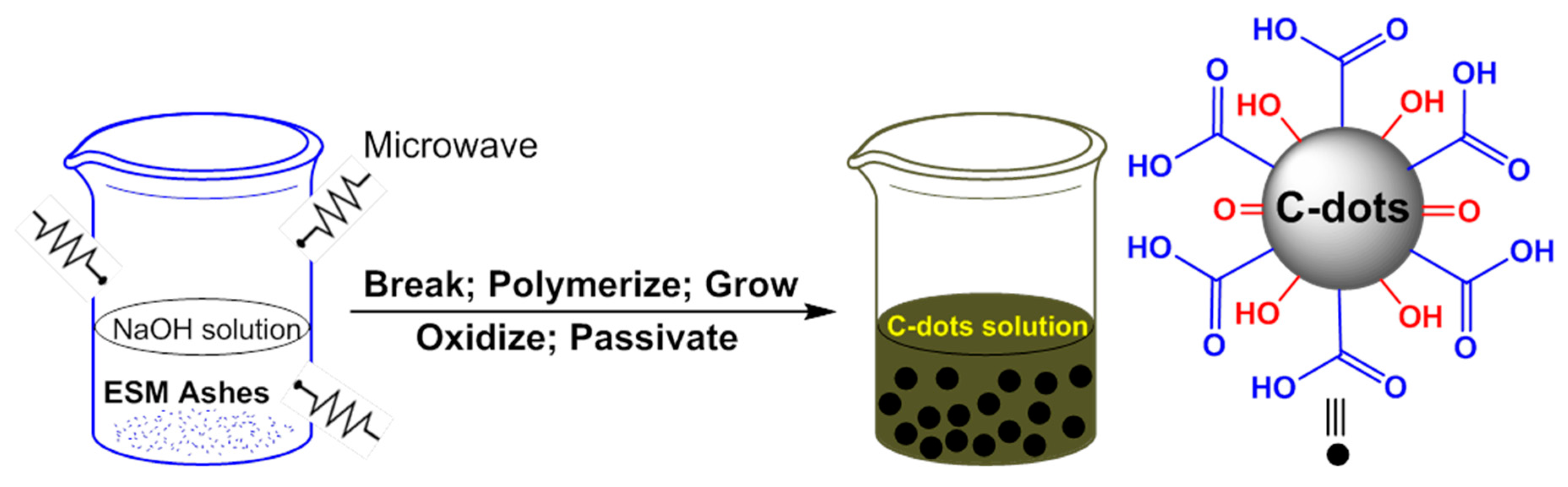
- Wang, Q.; Liu, X.; Zhang, L.; Lv, Y. Microwave assisted synthesis of carbon nanodots through an eggshell membrane and their fluorescent application. Analyst 2012, 137, 5392–5397. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Garg, B.; Ling, Y.-C. Waste chicken eggshell as low-cost precursor for efficient synthesis of nitrogen-doped fluorescent carbon nanodots and their multi-functional applications. RSC Adv. 2014, 4, 58329–58336. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Zhao, Y.; Shi, G.; Deng, L.; Hou, Y.; Qu, L. An electrochemical avenue to green-luminescent graphene quantum dots as potential electron-acceptors for photovoltaics. Adv. Mater. 2011, 23, 776–780. [Google Scholar]
- Wang, W.; Li, Y.M.; Cheng, L.; Cao, Z.Q.; Liu, W.G. Water-soluble and phosphorus-containing carbon dots with strong green fluorescence for cell labeling. J. Mater. Chem. B 2014, 2, 46–48. [Google Scholar] [CrossRef]
- Dong, Y.; Pang, H.; Yang, H.B.; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon based dots co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew. Chem. Int. Ed. 2013, 52, 7800–7804. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Anilkumar, P.; Cao, L.; Liu, J.H.; Luo, P.G.; Tackett, K.N., 2nd; Sahu, S.; Wang, P.; Wang, X.; Sun, Y.P. Carbon dots of different composition and surface functionalization: Cytotoxicity issues relevant to fluorescence cell imaging. Exp. Biol. Med. (Maywood) 2011, 236, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, Q.; Long, Y.; Cheng, Z.; Chen, S.; Zheng, H.; Huang, Y. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem. Commun. 2011, 47, 6695–6697. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qu, K.; Xu, B.; Ren, J.; Qu, X. Multicolor luminescent carbon nanoparticles: Synthesis, supramolecular assembly with porphyrin, intrinsic peroxidase-like catalytic activity and applications. Nano Res. 2011, 4, 908–920. [Google Scholar] [CrossRef]
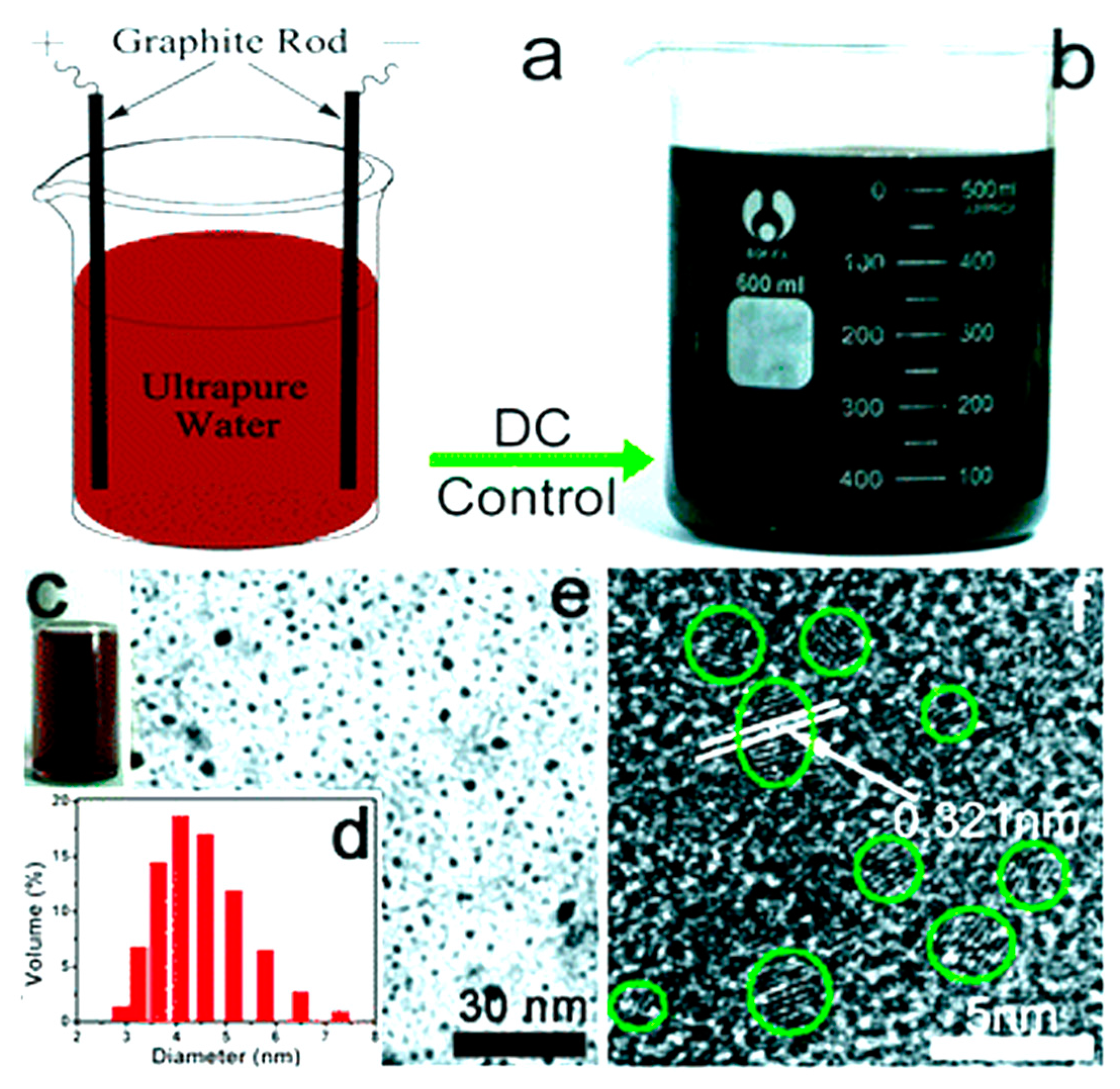
- Ming, H.; Ma, Z.; Liu, Y.; Pan, K.; Yu, H.; Wang, F.; Kang, Z. Large scale electrochemical synthesis of high quality carbon nanodots and their photocatalytic property. Dalton Trans. 2012, 41, 9526–9531. [Google Scholar] [CrossRef] [PubMed]
- Safavi, A.; Sedaghati, F.; Shahbaazi, H.; Farjami, E. Facile approach to the synthesis of carbon nanodots and their peroxidase mimetic function in azo dyes degradation. RSC Adv. 2012, 2, 7367–7370. [Google Scholar] [CrossRef]
- Shamsipur, M.; Safavi, A.; Mohammadpour, Z. Indirect colorimetric detection of glutathione based on its radical restoration ability using carbon nanodots as nanozymes. Sens. Actuators B Chem. 2014, 199, 463–469. [Google Scholar] [CrossRef]
- Mohammadpour, Z.; Safavi, A.; Shamsipur, M. A new label free colorimetric chemosensor for detection of mercury ion with tunable dynamic range using carbon nanodots as enzyme mimics. Chem. Eng. J. 2014, 255, 1–7. [Google Scholar] [CrossRef]
- Wu, D.; Deng, X.; Huang, X.; Wang, K.; Liu, Q. Low-cost preparation of photoluminescent carbon nanodots and application as peroxidase mimetics in colorimetric detection of H2O2 and glucose. J. Nanosci. Nanotechnol. 2013, 13, 6611–6616. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, J.; Jiang, Z.; Wang, W.; Liu, X. High-quality carbon dots: Synthesis, peroxidase-like activity and their application in the detection of H2O2, Ag+ and Fe3+. RSC Adv. 2014, 4, 17387–17392. [Google Scholar] [CrossRef]
- Long, Y.; Wang, X.; Shen, D.; Zheng, H. Detection of glucose based on the peroxidase-like activity of reduced state carbon dots. Talanta 2016, 159, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, L.; Wei, W.; Qu, X. Recent advances in graphene quantum dots in sensing. Mater. Today 2013, 16, 433–442. [Google Scholar] [CrossRef]
- Zheng, A.-X.; Cong, Z.-X.; Wang, J.-R.; Li, J.; Yang, H.-H.; Chen, G.-N. Highly-efficient peroxidase-like catalytic activity of graphene dots for biosensing. Biosens. Bioelectron. 2013, 49, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Nirala, N.R.; Abraham, S.; Kumar, V.; Bansal, A.; Srivastava, A.; Saxena, P.S. Colorimetric detection of cholesterol based on highly efficient peroxidase mimetic activity of graphene quantum dots. Sens. Actuators B Chem. 2015, 218, 42–50. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, A.; Gao, N.; Li, K.; Ren, J.; Qu, X. Deciphering a nanocarbon-based artificial peroxidase: Chemical identification of the catalytically active and substrate-binding sites on graphene quantum dots. Angew. Chem. Int. Ed. 2015, 54, 7176–7180. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.L.; Song, X.; Chen, Y.; Rong, M.; Zhao, T.; Wang, Y.; Jiang, Y.; Chen, X. Intrinsic peroxidase-like catalytic activity of nitrogen-doped graphene quantum dots and their application in the colorimetric detection of H2O2 and glucose. Anal. Chim. Acta 2015, 869, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Garg, B.; Ling, Y.-C. A novel graphene-based label-free fluorescence ‘turn-on’ nanosensor for selective and sensitive detection of phosphorylated species in biological samples and living cells. Nanoscale 2016, 8, 4547–4556. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.F.; Allen, B.L.; Kauffman, D.R.; Star, A. Electrocatalytic activity of nitrogen-doped carbon nanotube cups. J. Am. Chem. Soc. 2009, 131, 13200–13201. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, Y.; Wu, Y.; Weng, B.; Liu, Y.; Li, C.M. Synthesis of nitrogen- and iron-containing carbon dots, and their application to colorimetric and fluorometric determination of dopamine. Microchem. Acta 2016, 183, 2491–2500. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Zhou, X.; Wu, X.; Yang, Y.; Wu, H.; Guo, S.; Zhang, J. Graphene quantum dots/gold electrode and its application in living cell H2O2 detection. Nanoscale 2013, 5, 1816–1819. [Google Scholar] [CrossRef] [PubMed]
- Essner, J.B.; McCay, R.N.; Smith, C.J., II; Cobb, S.M.; Laber, C.H.; Baker, G.A. A switchable peroxidase mimic derived from the reversible co-assembly of cytochrome c and carbon dots. J. Mater. Chem. B 2016, 4, 2163–2170. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, X.; Wang, X.; Iqbal, A.; Yang, C.; Liu, W.; Qin, W. Carbon dot/NiAl-layered double hydroxide hybrid material: Facile synthesis, intrinsic peroxidase-like catalytic activity and its application. RSC Adv. 2015, 5, 95495–95503. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y.; Han, T.; Wu, H.; Guo, S.; Zhang, J. Composite of graphene quantum dots and Fe3O4 nanoparticles: Peroxidase activity and application in phenolic compound removal. RSC Adv. 2014, 4, 3299–3305. [Google Scholar] [CrossRef]
- Liu, W.; Yang, H.; Ma, C.; Ding, Y.; Ge, S.; Yu, J.; Yan, M. Graphene-palladium nanowires based electrochemical sensor using ZnFe2O4-graphene quantum dots as an effective peroxidase mimic. Anal. Chim. Acta 2014, 852, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, J.; Jiang, P.; Wang, G.; Wu, X.; Zhao, H.; Zhang, C. Superior peroxidase mimetic activity of carbon dots-Pt nanocomposites relies on synergistic effects. New J. Chem. 2015, 39, 4141–4146. [Google Scholar] [CrossRef]
- Zheng, C.; Ke, W.; Yin, T.; An, X. Intrinsic peroxidase-like activity and the catalytic mechanism of gold@carbon dots nanocomposites. RSC Adv. 2016, 6, 35280–35286. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Wang, L.; Luo, Y.; Sun, X. A general strategy for the production of photoluminescent carbon nitride dots from organic amines and their application as novel peroxidase-like catalysts for colorimetric detection of H2O2 and glucose. RSC Adv. 2012, 2, 411–413. [Google Scholar] [CrossRef]
- Yang, L.; Liu, X.; Lu, Q.; Huang, N.; Liu, M.; Zhang, Y.; Yao, S. Catalytic and peroxidase-like activity of carbon based-AuPd bimetallic nanocomposite produced using carbon dots as the reductant. Anal. Chim. Acta 2016, 930, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, H.; Ma, Y.; Tong, J. Highly sensitive fluorescent detection of dihydroxybenzene based on graphene quantum dots. Sens. Actuators B Chem. 2014, 205, 227–233. [Google Scholar] [CrossRef]
- Huang, H.; Feng, Z.; Li, Y.; Liu, Z.; Zhang, L.; Ma, Y.; Tong, J. Highly sensitive detection of bisphenol A in food packaging based on graphene quantum dots and peroxidase. Anal. Methods 2015, 7, 2928–2935. [Google Scholar] [CrossRef]






| Nanomaterial(s) | Synthesis Method | Substrate(s) | Application(s) | LOD | Reference |
|---|---|---|---|---|---|
| C-dots b | Oxidation with HNO3 | TMB, OPD, and THB | H2O2 detection | 0.2 μM | [36] |
| Glucose detection | 0.4 μM | ||||
| CNPs c | Oxidation with HNO3 | TMB and DAB | H2O2 detection | 1.0 μM | [37] |
| Glucose detection | 20.0 μM | ||||
| C-dots | Electrochemical | − | Degradation of MO azo dye | − | [38] |
| CDs d | MW-IL method | − | Degradation of MO and MR azo dyes | − | [39] |
| CDs d | Hydrothermal | TMB | GSH detection | 0.3 μM | [40] |
| CDs d | Hydrothermal | TMB | Hg2+ detection | 23 nM | [41] |
| CDs d | Hydrothermal | TMB | H2O2 detection | 0.6 μM | [42] |
| Glucose detection | 5.2 μM | ||||
| CDs d | Calcination followed by oxidation with HNO3 | TMB | H2O2 detection | 1.0 μM | [43] |
| Fe3+ detection | 0.8 μM | ||||
| Ag+ detection | 0.5 μM | ||||
| r-CDs d | Oxidation with HNO3 | TMB | Glucose detection | 2.0 μM | [44] |
| Nanoconjugate(s)/nanocomposite(s) | Synthesis Method | Substrate(s) | Application(s) | LOD | Reference |
|---|---|---|---|---|---|
| GQDs/Au | Photo-Fenton reaction and covalent assembly | TMB | H2O2 detection | 0.7 μM | [53] |
| C-dots/cyt c | Thermal pyrolysis and electrostatic assembly | ABTS | Enzyme modulation | − | [54] |
| C-dots/NiAl-LDH | Electrostatic assembly | TMB | H2O2 detection | 0.11 μM | [55] |
| GQDs-Fe3O4 | Co-precipitation | TMB | Removal of phenolics | − | [56] |
| ZnFe2O4-GQDs | Hydrothermal + photo-Fenton reaction | TMB | DNA detection | 62 aM | [57] |
| CDs-Pt b | Hydrothermal + mechanical stirring | TMB | H2O2 detection | 0.8 μM | [58] |
| Glucose detection | 1.67 μM | ||||
| Au NPs@CDs b | MW-assisted synthesis + chemical reaction | TMB | − | − | [59] |
| CNDs c | MW-assisted synthesis | TMB | H2O2 detection | 0.4 μM | [60] |
| Glucose detection | 0.5 μM |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garg, B.; Bisht, T. Carbon Nanodots as Peroxidase Nanozymes for Biosensing. Molecules 2016, 21, 1653. https://doi.org/10.3390/molecules21121653
Garg B, Bisht T. Carbon Nanodots as Peroxidase Nanozymes for Biosensing. Molecules. 2016; 21(12):1653. https://doi.org/10.3390/molecules21121653
Chicago/Turabian StyleGarg, Bhaskar, and Tanuja Bisht. 2016. "Carbon Nanodots as Peroxidase Nanozymes for Biosensing" Molecules 21, no. 12: 1653. https://doi.org/10.3390/molecules21121653