The Draft Genome Sequence of Actinokineospora bangkokensis 44EHWT Reveals the Biosynthetic Pathway of the Antifungal Thailandin Compounds with Unusual Butylmalonyl-CoA Extender Units
Abstract
:1. Introduction
2. Results
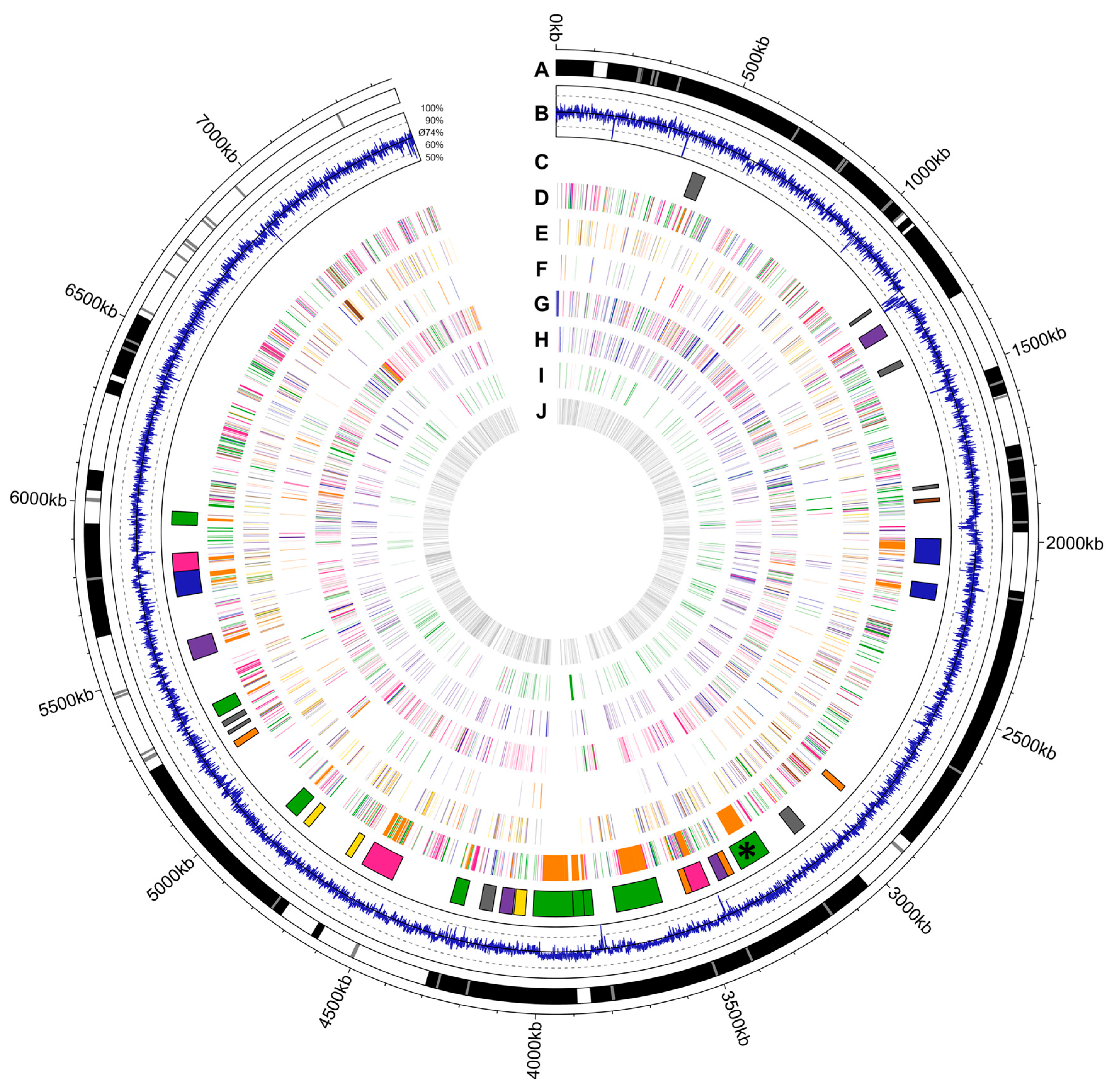
2.1. Draft Genome Sequence of Actinokineospora bangkokensis 44EHWT
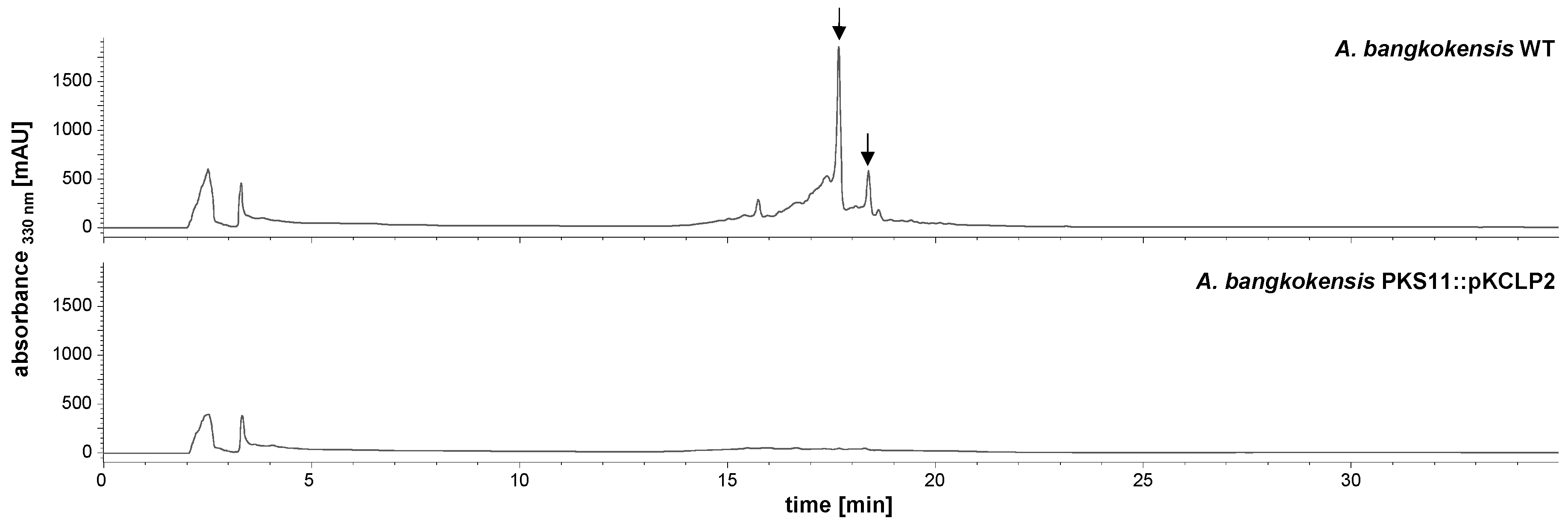
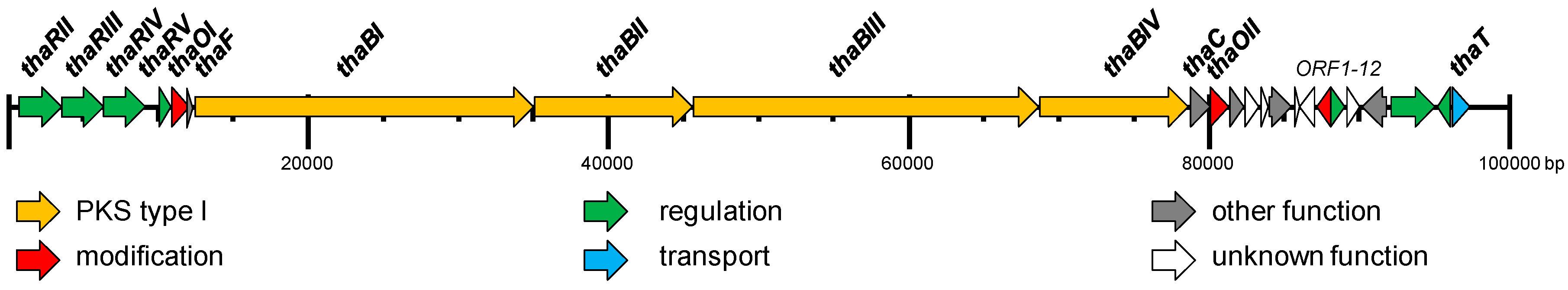
2.2. Identification and Verification of the Thailandin Biosynthetic Gene Cluster
2.3. Proposed Thailandin Biosynthetic Pathway
3. Discussion
4. Materials and Methods
4.1. Bacterial Growth Condition
4.2. Extraction of Secondary Metabolites
4.3. Analysis of Secondary Metabolite Production by HPLC/MS
4.4. Isolation of Genomic DNA
4.5. Genome Sequencing of A. bangkokensis 44EHWT
4.6. Genome Annotation and Identification of the Thailandin Biosynthetic Gene Cluster
4.7. Visualization of the Genome of A. bangkokensis 44EHWT
4.8. Cloning of Single Crossover Vector pKCLP2_PKS11
4.9. Genetic Manipulation of A. bangkokensis 44EHWT
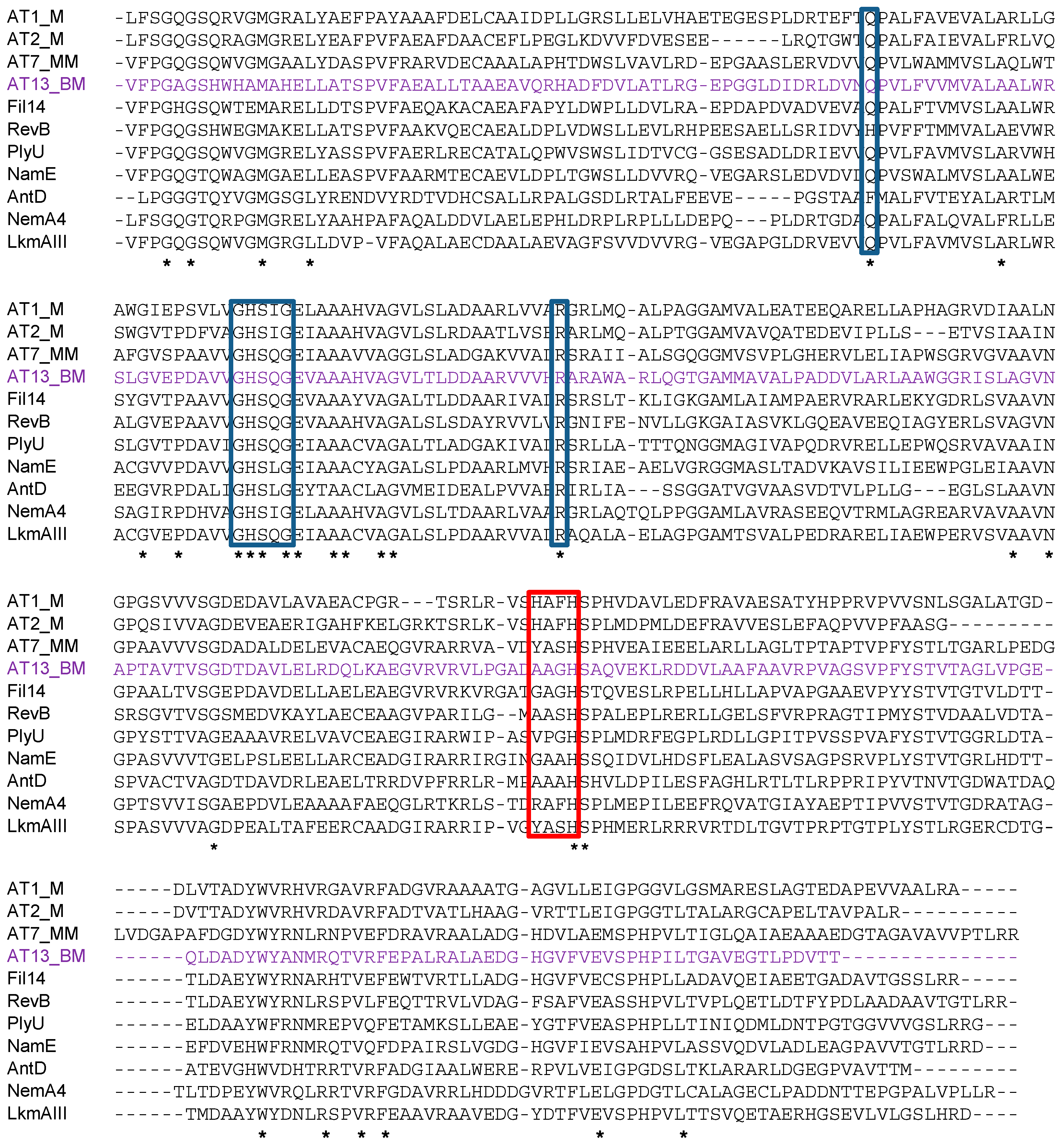
4.10. Alignment of Sequences
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
| Cluster # | Type Secondary Metabolite | Scaffold | from [bp] | to [bp] |
|---|---|---|---|---|
| 1 | other | 5 | 222,242 | 266,147 |
| 2 | butyrolactone | 9 | 112,144 | 123,172 |
| 3 | PKS II/ectoine | 9 | 173,085 | 217,111 |
| 4 | nucleosid | 10 | 89,791 | 111,566 |
| 5 | bacteriocin | 13 | 79,417 | 91,126 |
| 6 | siderophore | 13 | 123,673 | 135,433 |
| 7 | trans AT-PKS/NRPS/other | 14 | 21,256 | 106,054 |
| 8 | NRPS/PKS I | 15 | 12,027 | 65,701 |
| 9 | terpene | 16 | 2354 | 23,460 |
| 10 | other | 17 | 25,281 | 68,715 |
| 11 * | PKS I THAILANDIN CLUSTER | 17 | 179,992 | 276,120 |
| 12 | terpene | 17 | 304,613 | 326,544 |
| 13 | PKS III | 17 | 319,674 | 361,110 |
| 14 | NRPS/ladderane/arylpolyene | 17 | 392,681 | 462,423 |
| 15 | terpene | 17 | 454,050 | 475,513 |
| 16 * | oligosaccharide/PKS I | 17 | 557,011 | 710,221 |
| 17 | PKS I | 17 | 785,384 | 815,172 |
| 18 | PKS I | 18 | 1 | 35,881 |
| 19 * | PKS I/oligosaccharide | 19 | 1 | 133,613 |
| 20 | lantipeptide | 19 | 158,947 | 196,476 |
| 21 | PKS II | 19 | 200,846 | 243,937 |
| 22 | other | 19 | 266,838 | 310,212 |
| 23 | PKS I | 19 | 361,716 | 408,222 |
| 24 | NRPS/lantipeptide | 20 | 198,722 | 311,653 |
| 25 | lassopeptide | 22 | 18,493 | 40,971 |
| 26 | lantipeptide | 23 | 93,884 | 117,519 |
| 27 | other PKS/PKS I | 23 | 147,485 | 196,457 |
| 28 | terpene | 23 | 444,400 | 466,643 |
| 29 | butyrolactone | 23 | 494,036 | 505,109 |
| 30 | indole | 24 | 1 | 18,253 |
| 31 | PKS I | 24 | 37,982 | 83,864 |
| 32 | other PKS | 24 | 239,959 | 308,466 |
| 33 | NRPS/PKS I | 25 | 71,292 | 162,064 |
| 34 | ladderane/NRPS | 25 | 153,235 | 214,305 |
| 35 | PKS I | 26 | 1 | 44,677 |
| Strain | Product | Genome Sequence * | Literature |
|---|---|---|---|
| A. auranticolor IFO 16518 | ? | - | [55] |
| A. baliensis ID03-0561T | ? | - | [56] |
| A. bangkokensis 44EHWT | thailandins A and B | this study MKQR01000000 | [7,8] |
| A. cianjurensis ID03-0810T | ? | - | [56] |
| A. cibodasensis ID03-0784T | ? | - | [56] |
| A. diospyrosa NRRL B-24047 | ? | - | [57] |
| A. enzanensis IFO 16517 | ? | GCA_000374445.1 | [55] |
| A. fastidiosa NRRL B-16697 | macrobicyclic peptide antibiotic | - | [58,59,60] |
| A. globicatena NRRL B-24048 | ? | - | [57] |
| A. guangxiensis GK-6T | ? | - | [61] |
| A. inagensis NRRL B-24050 | ? | GCA_000482865.1 | [57] |
| A. mzabensis PAL84 | ? | - | [62] |
| A. riparia IFO 14541 | compound with antimycoplasmic activity | - | [63] |
| A. soli YIM 75948T | ? | - | [64] |
| A. spheciospongiae EG49 | actinosporins A–D, products from co-cultivation | GCA_000564855.1 | [6,39,40,41,65,66] |
| A. terrae IFO 15668 | ? | - | [57] |
References
- Gomez-Escribano, J.P.; Bibb, M.J. Heterologous expression of natural product biosynthetic gene clusters in Streptomyces coelicolor: From genome mining to manipulation of biosynthetic pathways. J. Ind. Microbiol. Biotechnol. 2014, 41, 425–531. [Google Scholar] [CrossRef] [PubMed]
- Bode, H.B.; Bethe, B.; Höfs, R.; Zeeck, A. Big effects from small changes: possible ways to explore nature’s chemical diversity. Chembiochem 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Gao, C.; Hindra; Mulder, D.; Yin, C.; Elliot, M.A. Crp is a global regulator of antibiotic production in Streptomyces. MBio 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Gessner, A.; Heitzler, T.; Zhang, S.; Klaus, C.; Murillo, R.; Zhao, H.; Vanner, S.; Zechel, D.L.; Bechthold, A. Changing biosynthetic profiles by expressing bldA in Streptomyces strains. ChemBioChem 2015, 16, 2244–2252. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T. Actinokineospora: A new genus of the Actinomycetales. Actinomycetologica 1988, 2, 31–45. [Google Scholar] [CrossRef]
- Harjes, J.; Ryu, T.; Abdelmohsen, U.R.; Moitinho-Silva, L.; Horn, H.; Ravasi, T.; Hentschel, U. Draft genome sequence of the antitrypanosomally active sponge-associated bacterium Actinokineospora sp. strain EG49. Genome Announc. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Intra, B.; Matsumoto, A.; Inahashi, Y.; Omura, S.; Takahashi, Y.; Panbangred, W. Actinokineospora bangkokensis sp. nov., isolated from rhizospheric soil. Int. J. Syst. Evol. Microbiol. 2013, 63, 2655–2660. [Google Scholar] [CrossRef] [PubMed]
- Intra, B.; Greule, A.; Bechthold, A.; Euanorasetr, J.; Paululat, T.; Panbangred, W. Thailandins A and B, new polyene macrolactone compounds isolated from Actinokineospora bangkokensis strain 44EHWT, possessing antifungal activity against anthracnose fungi and pathogenic yeasts. J. Agric. Food Chem. 2016, 64, 5171–5179. [Google Scholar] [CrossRef] [PubMed]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Blin, K.; Duddela, S.; Krug, D.; Kim, H.U.; Bruccoleri, R.; Lee, S.Y.; Fischbach, M.A.; Müller, R.; Wohlleben, W.; et al. antiSMASH 3.0—A comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 2015, 43, W237–W243. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.; Gokhale, R.S.; Mohanty, D. Computational approach for prediction of domain organization and substrate specificity of modular polyketide synthases. J. Mol. Biol. 2003, 328, 335–363. [Google Scholar] [CrossRef]
- Bisang, C.; Long, P.F.; Corte´s, J.; Westcott, J.; Crosby, J.; Matharu, A.-L.; Cox, R.J.; Simpson, T.J.; Staunton, J.; Leadlay, P.F. A chain initiation factor common to both modular and aromatic polyketide synthases. Nature 1999, 401, 502–505. [Google Scholar] [PubMed]
- Erb, T.J.; Berg, I.A.; Brecht, V.; Müller, M.; Fuchs, G.; Alber, B.E. Synthesis of C5-dicarboxylic acids from C2-units involving crotonyl-CoA carboxylase/reductase: the ethylmalonyl-CoA pathway. Proc. Natl. Acad. Sci. USA 2007, 104, 10631–10636. [Google Scholar] [CrossRef] [PubMed]
- Erb, T.J.; Brecht, V.; Fuchs, G.; Müller, M.; Alber, B.E. Carboxylation mechanism and stereochemistry of crotonyl-CoA carboxylase/reductase, a carboxylating enoyl-thioester reductase. Proc. Natl. Acad. Sci. USA 2009, 106, 8871–8876. [Google Scholar] [CrossRef] [PubMed]
- Quade, N.; Huo, L.; Rachid, S.; Heinz, D.W.; Müller, R. Unusual carbon fixation gives rise to diverse polyketide extender units. Nat. Chem. Biol. 2012, 8, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Sandy, M.; Rui, Z.; Gallagher, J.; Zhang, W. Enzymatic synthesis of dilactone scaffold of antimycins. ACS Chem. Biol. 2012, 7, 1956–1961. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.C.; Moore, B.S. Beyond ethylmalonyl-CoA: The functional role of crotonyl-CoA-carboxylase/reductase homologs in expanding polyketide diversity. Nat. Prod. Rep. 2012, 29, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Keatinge-Clay, A. Crystal structure of the erythromycin polyketide synthase dehydratase. J. Mol. Biol. 2008, 384, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, P.; Lynch, S.; Flood, E.; Finnan, S.; Oliynyk, M. Amphotericin biosynthesis in Streptomyces nodosus: deductions from analysis of polyketide synthase and late genes. Chem. Biol. 2001, 8, 713–723. [Google Scholar] [CrossRef]
- Fjærvik, E.; Zotchev, S.B. Biosynthesis of the polyene macrolide antibiotic nystatin in Streptomyces noursei. Appl. Microbiol. Biotechnol. 2005, 67, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, P. Conserved amino acid residues correlating with ketoreductase stereospecificity in modular polyketide synthases. ChemBioChem 2003, 4, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Keatinge-Clay, A.T.; Stroud, R.M. The structure of a ketoreductase determines the organization of the β-carbon processing enzymes of modular polyketide synthases. Structure 2006, 14, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, S.A.; Whicher, J.R.; Papireddy, K.; Florova, G.; Smith, J.L.; Reynolds, K.A. Structural and stereochemical analysis of a modular polyketide synthase ketoreductase domain required for the generation of a cis-alkene. Chem. Biol. 2013, 20, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Pöplau, P.; Frank, S.; Morinaka, B.I.; Piel, J. An enzymatic domain for the formation of cyclic ethers in complex polyketides. Angew. Chem. Int. Ed. 2013, 52, 13215–13218. [Google Scholar] [CrossRef] [PubMed]
- Berkhan, G.; Hahn, F. A dehydratase domain in ambruticin biosynthesis displays additional activity as a pyran-forming cyclase. Angew. Chem. Int. Ed. 2014, 53, 14240–14244. [Google Scholar] [CrossRef] [PubMed]
- Luhavaya, H.; Dias, M.V.B.; Williams, S.R.; Hong, H.; de Oliveira, L.G.; Leadlay, P.F. Enzymology of pyran ring A formation in salinomycin biosynthesis. Angew. Chem. Int. Ed. 2015, 54, 13622–13625. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, D.M. Recent progress in the study of the interactions of amphotericin B with cholesterol and ergosterol in lipid environments. Eur. Biophys. J. 2014, 43, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Fanos, V.; Cataldi, L. Amphotericin B-induced nephrotoxicity: A review. J. Chemother. 2000, 12, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Tevyashova, A.N.; Olsufyeva, E.N.; Solovieva, S.E.; Printsevskaya, S.S.; Reznikova, M.I.; Trenin, A.S.; Galatenko, O.A.; Treshalin, I.D.; Pereverzeva, E.R.; Mirchink, E.P.; et al. Structure-antifungal activity relationships of polyene antibiotics of the amphotericin B group. Antimicrob. Agents Chemother. 2013, 57, 3815–3822. [Google Scholar] [CrossRef] [PubMed]
- Brautaset, T.; Sletta, H.; Nedal, A.; Borgos, S.E.F.; Degnes, K.F.; Bakke, I.; Volokhan, O.; Sekurova, O.N.; Treshalin, I.D.; Mirchink, E.P.; et al. Improved antifungal polyene macrolides via engineering of the nystatin biosynthetic genes in Streptomyces noursei. Chem. Biol. 2008, 15, 1198–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Huang, X.; Zhou, X.; Bai, L.; He, J.; Jeong, K.J.; Lee, S.Y.; Deng, Z. Organizational and mutational analysis of a complete FR-008/candicidin gene cluster encoding a structurally related polyene complex. Chem. Biol. 2003, 10, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Palacios, D.S.; Dailey, I.; Siebert, D.M.; Wilcock, B.C.; Burke, M.D. Synthesis-enabled functional group deletions reveal key underpinnings of amphotericin B ion channel and antifungal activities. Proc. Natl. Acad. Sci. USA 2011, 108, 6733–6738. [Google Scholar] [CrossRef] [PubMed]
- Gary-Bobo, C.M. Polyen-sterol interaction and selective toxicity. Biochimie 1989, 71, 37–47. [Google Scholar] [CrossRef]
- Cybulska, B.; Bolard, J.; Seksek, O.; Czerwinski, A.; Borowski, E. Identification of the structural elements of amphotericin B and other polyene macrolide antibiotics of the hepteane group influencing the ionic selectivity of the permeability pathways formed in the red cell membrane. Biochim. Biophys. Acta 1995, 1240, 167–178. [Google Scholar] [CrossRef]
- Pandey, R.C.; Narasimhachari, N.; Rinehart, K.L.; Millington, D.S. Polyene antibiotics. IV. Structure of chainin. J. Am. Chem. Soc. 1972, 94, 4306–4310. [Google Scholar] [CrossRef] [PubMed]
- Ceder, O.; Ryhage, R. The structure of filipin. Acta Chem. Scand. 1964, 18, 558–560. [Google Scholar] [CrossRef]
- Shih, H.-D.; Liu, Y.-C.; Hsu, F.-L.; Mulabagal, V.; Dodda, R.; Huang, J.-W. Fungichromin: A substance from Streptomyces padanus with inhibitory effects on Rhizoctonia solani. J. Agric. Food Chem. 2003, 51, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Wei, S.-J.; Zhang, Z.-P.; Zhan, T.-H.; Tu, G.-Q. Antifungalmycin, an antifungal macrolide from Streptomyces padanus 702. Nat. Products Bioprospect. 2012, 2, 41–45. [Google Scholar] [CrossRef]
- Abdelmohsen, U.; Cheng, C.; Viegelmann, C.; Zhang, T.; Grkovic, T.; Ahmed, S.; Quinn, R.; Hentschel, U.; Edrada-Ebel, R. Dereplication strategies for targeted isolation of new antitrypanosomal actinosporins A and B from a marine sponge associated-Actinokineospora sp. EG49. Mar. Drugs 2014, 12, 1220–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grkovic, T.; Abdelmohsen, U.R.; Othman, E.M.; Stopper, H.; Edrada-Ebel, R.; Hentschel, U.; Quinn, R.J. Two new antioxidant actinosporin analogues from the calcium alginate beads culture of sponge-associated Actinokineospora sp. strain EG49. Bioorg. Med. Chem. Lett. 2014, 24, 5089–5092. [Google Scholar] [CrossRef] [PubMed]
- Dashti, Y.; Grkovic, T.; Abdelmohsen, U.; Hentschel, U.; Quinn, R. Production of induced secondary metabolites by a co-culture of sponge-associated actinomycetes, Actinokineospora sp. EG49 and Nocardiopsis sp. RV163. Mar. Drugs 2014, 12, 3046–3059. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-K.; Jin, J.-L. Crucial factor for increasing the conjugation frequency in Streptomyces netropsis SD-07 and other strains. FEMS Microbiol. Lett. 2014, 357, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, J.F.; Caffrey, P.; Gil, J.A.; Zotchev, S.B. Polyene antibiotic biosynthesis gene clusters. Appl. Microbiol. Biotechnol. 2003, 61, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Toyoda, A.; Sekiyama, Y.; Takagi, H.; Nogawa, T.; Uramoto, M.; Suzuki, R.; Koshino, H.; Kumano, T.; Panthee, S.; et al. Reveromycin A biosynthesis uses RevG and RevJ for stereospecific spiroacetal formation. Nat. Chem. Biol. 2011, 7, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Y.; Lu, C.; Zhang, J.; Zhu, J.; Wang, H.; Shen, Y. Activating a cryptic ansamycin biosynthetic gene cluster to produce three new naphthalenic octaketide ansamycins with n-pentyl and n-butyl side chains. Org. Lett. 2015, 17, 3706–3709. [Google Scholar] [CrossRef] [PubMed]
- Seipke, R.F.; Patrick, E.; Hutchings, M.I. Regulation of antimycin biosynthesis by the orphan ECF RNA polymerase sigma factor σ AntA. PeerJ 2014, 2, e253. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.T.; Nietsche, J.A.; Hertz, M.R.; Williams, D.R.; Siegel, M.M.; Morton, G.O.; James, J.C.; Borders, D.B. LL-F28249 antibiotic complex: A new family of antiparasitic macrocyclic lactones. Isolation, characterization and structures of LL-F28249 α, β, δ, λ. J. Antibiot. (Tokyo) 1988, 41, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, Y.; Huang, T.; Tao, M.; Deng, Z.; Lin, S. Identification and characterization of the biosynthetic gene cluster of polyoxypeptin A, a potent apoptosis inducer. BMC Microbiol. 2014, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Madden, T. The BLAST Sequence Analysis Tool. In The NCBI Handbook, 2nd ed.; National Center for Biotechnology Information: Bethesda, MD, USA, 2003; Chapter 16. [Google Scholar]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. Circlize implements and enhances circular visualization in R. Bioinformatics 2014, 30, 2811–2812. [Google Scholar] [CrossRef] [PubMed]
- Petzke, L.; Bechthold, A. Transgenese in Streptomyceten: Transposons, Rekombinasen und Meganukleasen. Ph.D. Thesis, Albert-Ludwigs-University of Freiburg, Freiburg im Breisgau, Germany, 2010. [Google Scholar]
- MacNeil, D.J.; Gewain, K.M.; Ruby, C.L.; Dezeny, G.; Gibbons, P.H.; MacNeil, T. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene 1992, 111, 61–68. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Otoguro, M.; Hayakawa, M.; Yamazaki, T.; Tamura, T.; Hatano, K.; Iimura, Y. Numerical phenetic and phylogenetic analyses of Actinokineospora isolates, with a description of Actinokineospora auranticolor sp. nov. and Actinokineospora enzanensis sp. nov. Actinomycetologica 2001, 15, 30–39. [Google Scholar] [CrossRef]
- Lisdiyanti, P.; Otoguro, M.; Ratnakomala, S.; Lestari, Y.; Hastuti, R.D.; Triana, E.; Katsuhiko, A.; Widyastuti, Y. Actinokineospora baliensis sp. nov., Actinokineospora cibodasensis sp. nov. and Actinokineospora cianjurensis sp. nov., isolated from soil and plant litter. Int. J. Syst. Evol. Microbiol. 2010, 60, 2331–2335. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Hayakawa, M.; Nonomura, H.; Yokota, A.; Hatano, K. Four new species of the genus Actinokineospora: Actinokineospora inagensis sp. nov., Actinokineospora globicatena sp. nov., Actinokineospora terrae sp. nov., and Actinokineospora diospyrosa sp. nov. Int. J. Syst. Bacteriol. 1995, 45, 371–378. [Google Scholar] [CrossRef]
- Celmer, W.D.; Cullen, W.P.; Moppett, C.E.; Routien, J.B.; Shibakawa, R.; Tone, J. Antibiotics Produced by Species of Pseudonocardia. U.S. Patent 4031206 A, 21 June 1977. [Google Scholar]
- Henssen, A.; Kothe, H.W.; Kroppenstedt, R.M. Transfer of Pseudonocardia azurea and Pseudonocardia fastidiosa to the genus Amycolatopsis, with emended species description. Int. J. Syst. Bacteriol. 1987, 37, 292–295. [Google Scholar] [CrossRef]
- Labeda, D.P.; Price, N.P.; Tan, G.Y.A.; Goodfellow, M.; Klenk, H.-P. Emended description of the genus Actinokineospora (Hasegawa 1988) and transfer of Amycolatopsis fastidiosa (Henssen et al. 1987) as Actinokineospora fastidiosa comb. nov. Int. J. Syst. Evol. Microbiol. 2010, 60, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, B. Actinokineospora guangxiensis sp. nov., isolated from soil. Int. J. Syst. Evol. Microbiol. 2015, 65, 4650–4654. [Google Scholar] [CrossRef] [PubMed]
- Aouiche, A.; Bouras, N.; Mokrane, S.; Zitouni, A.; Schumann, P.; Spröer, C.; Sabaou, N.; Klenk, H.-P. Actinokineospora mzabensis sp. nov., a novel actinomycete isolated from Saharan soil. Antonie Van Leeuwenhoek 2015, 107, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T. Studies on motile arthrospore-bearing rare actinomycetes. Actinomycetologica 1991, 5, 64–71. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, Y.; Zhang, J.; Ming, H.; Nie, G.-X.; Yang, L.-L.; Tang, S.-K.; Li, W.-J. Actinokineospora soli sp. nov., a thermotolerant actinomycete isolated from soil, and emended description of the genus Actinokineospora. Int. J. Syst. Evol. Microbiol. 2012, 62, 1845–1849. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Pimentel-Elardo, S.M.; Hanora, A.; Radwan, M.; Abou-El-Ela, S.H.; Ahmed, S.; Hentschel, U. Isolation, phylogenetic analysis and anti-infective activity screening of marine sponge-associated actinomycetes. Mar. Drugs 2010, 8, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Kämpfer, P.; Glaeser, S.P.; Busse, H.-J.; Abdelmohsen, U.R.; Ahmed, S.; Hentschel, U. Actinokineospora spheciospongiae sp. nov., isolated from the marine sponge Spheciospongia vagabunda. Int. J. Syst. Evol. Microbiol. 2015, 65, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Strain Actinokineospora bangkokensis 44EHWT is available from the author W.P.
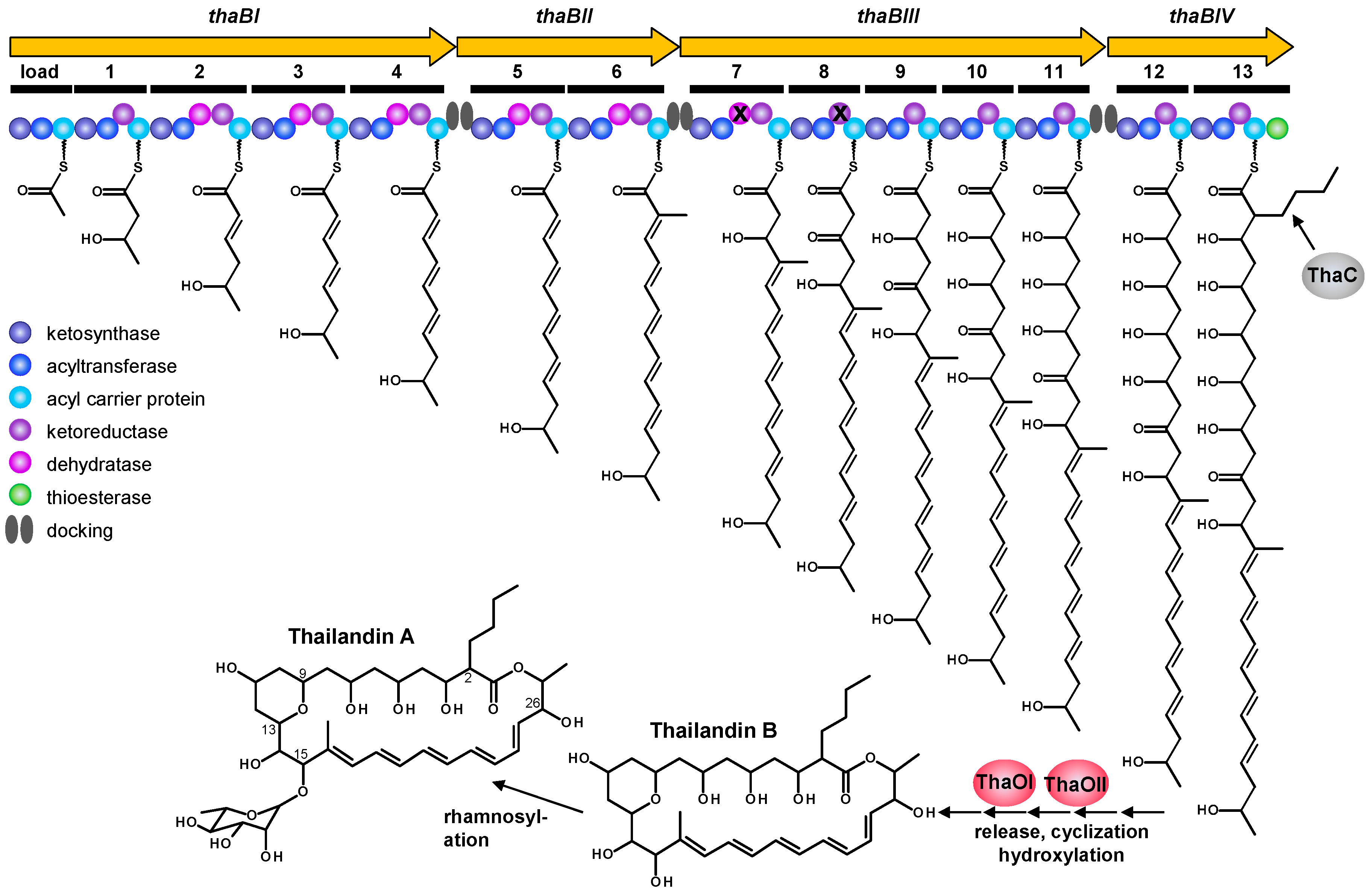
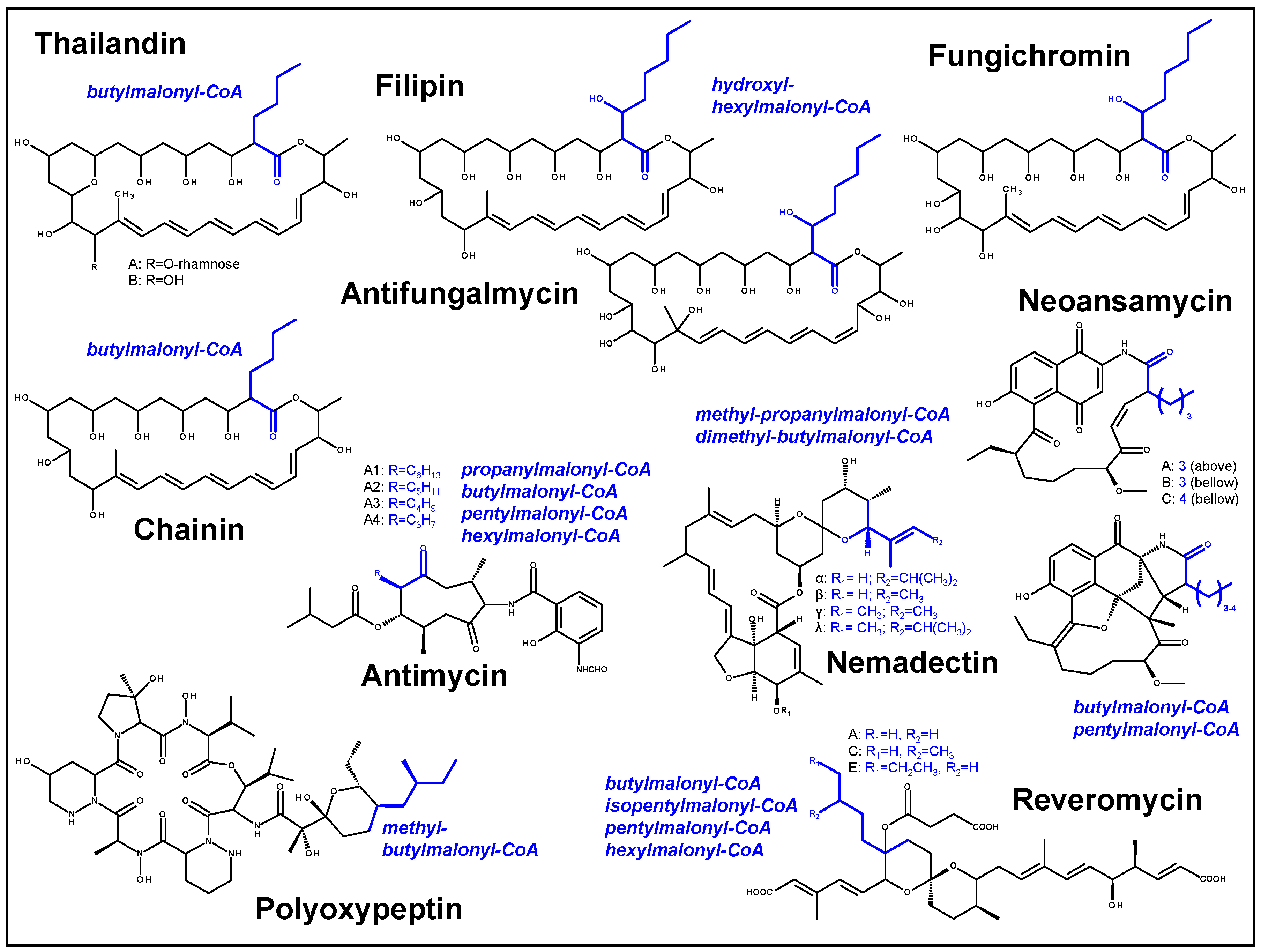
| Feature | Property |
|---|---|
| total length | 7,453,713 bp |
| GC content | 74.1% |
| number of scaffold | 32 |
| number of contigs | 79 |
| CDS (total) | 6287 |
| genes (coding) | 6191 |
| tRNA | 50 |
| secondary metabolite biosynthetic gene clusters | 35 |
| Gene | Protein-ID (PRJNA345323:) | Protein [aa] | Putative Product | Closest Similarity in the Databases (Identity %) |
|---|---|---|---|---|
| thaRI | BJP25_14755 | 953 | LuxR transcriptional regulator | Streptomyces himastatinicus (47%) |
| thaRII | BJP25_14760 | 923 | LuxR transcriptional regulator | Amycolatopsis azurea (38%) |
| thaRIII | BJP25_14765 | 921 | LuxR transcriptional regulator | Streptomyces sp. TAA204 (38%) |
| thaRIV | BJP25_14770 | 228 | LuxR transcriptional regulator | Allokutzneria albata (41%) |
| thaOI | BJP25_14775 | 402 | P450 monooxygenase | Streptomyces sp. LamerLS-31b (69%) |
| thaF | BJP25_14780 | 66 | ferredoxin | Streptomyces niger (73%) |
| thaBI | BJP25_14785 | 7524 | polyketide synthase type I | Streptomyces sp. MBT76 (60%) |
| thaBII | BJP25_14790 | 3501 | polyketide synthase type I | Streptomyces avermitilis (59%) |
| thaBIII | BJP25_14795 | 7685 | polyketide synthase type I | Streptomyces sp. NRRL B-24891 (54%) |
| thaBIV | BJP25_14800 | 3310 | polyketide synthase type I | Streptomyces avermitilis (58%) |
| thaC | BJP25_14805 | 418 | crotonyl-CoA carboxylase/reductase | Streptomyces durhamensis (71%) |
| thaOII | BJP25_14810 | 408 | P450 monooxygenase | Streptomyces avermitilis (65%) |
| orf1 | BJP25_14815 | 295 | phosphoesterase PA-phosphatase | Streptomyces regensis (70%) |
| orf2 | BJP25_14820 | 297 | hypothetical protein | Blastococcus sp. URHD0036 (70%) |
| orf3 | BJP25_14825 | 178 | hypothetical protein | Blastococcus sp. URHD0036 (60%) |
| orf4 | BJP25_14830 | 489 | chromosome segregation ATPase | Kibdelosporangium aridum (71%) |
| orf5 | BJP25_14835 | 172 | hypothetical protein | Lentzea sp. DHS C013 (65%) |
| orf6 | BJP25_14840 | 315 | hypothetical protein | Mycobacterium sp. Root135 (72%) |
| orf7 | BJP25_14845 | 297 | oxidoreductase | Nocardia niigatensis (63%) |
| orf8 | BJP25_14850 | 312 | LuxR transcriptional regulator | Saccharomonospora sp. CNQ490 (46%) |
| orf9 | BJP25_14855 | 300 | hypothetical protein | Actinokineospora spheciospongiae (69%) |
| orf10 | BJP25_14860 | 529 | serine protease | Actinokineospora spheciospongiae (73%) |
| orf11 | BJP25_14865 | 946 | LuxR transcriptional regulator | Actinokineospora inagensis (69%) |
| orf12 | BJP25_14870 | 294 | LysR transcriptional regulator | Alloactinosynnema sp. L-07 (70%) |
| thaT | BJP25_14875 | 405 | MFS transporter | Blastococcus saxobsidens (68%) |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greule, A.; Intra, B.; Flemming, S.; Rommel, M.G.E.; Panbangred, W.; Bechthold, A. The Draft Genome Sequence of Actinokineospora bangkokensis 44EHWT Reveals the Biosynthetic Pathway of the Antifungal Thailandin Compounds with Unusual Butylmalonyl-CoA Extender Units. Molecules 2016, 21, 1607. https://doi.org/10.3390/molecules21111607
Greule A, Intra B, Flemming S, Rommel MGE, Panbangred W, Bechthold A. The Draft Genome Sequence of Actinokineospora bangkokensis 44EHWT Reveals the Biosynthetic Pathway of the Antifungal Thailandin Compounds with Unusual Butylmalonyl-CoA Extender Units. Molecules. 2016; 21(11):1607. https://doi.org/10.3390/molecules21111607
Chicago/Turabian StyleGreule, Anja, Bungonsiri Intra, Stephan Flemming, Marcel G. E. Rommel, Watanalai Panbangred, and Andreas Bechthold. 2016. "The Draft Genome Sequence of Actinokineospora bangkokensis 44EHWT Reveals the Biosynthetic Pathway of the Antifungal Thailandin Compounds with Unusual Butylmalonyl-CoA Extender Units" Molecules 21, no. 11: 1607. https://doi.org/10.3390/molecules21111607





