1-Deoxynojirimycin: Occurrence, Extraction, Chemistry, Oral Pharmacokinetics, Biological Activities and In Silico Target Fishing
Abstract
:1. Introduction
2. 1-Deoxynojirimycin
2.1. Occurrence
2.2. Extraction
2.3. Quantitation of DNJ
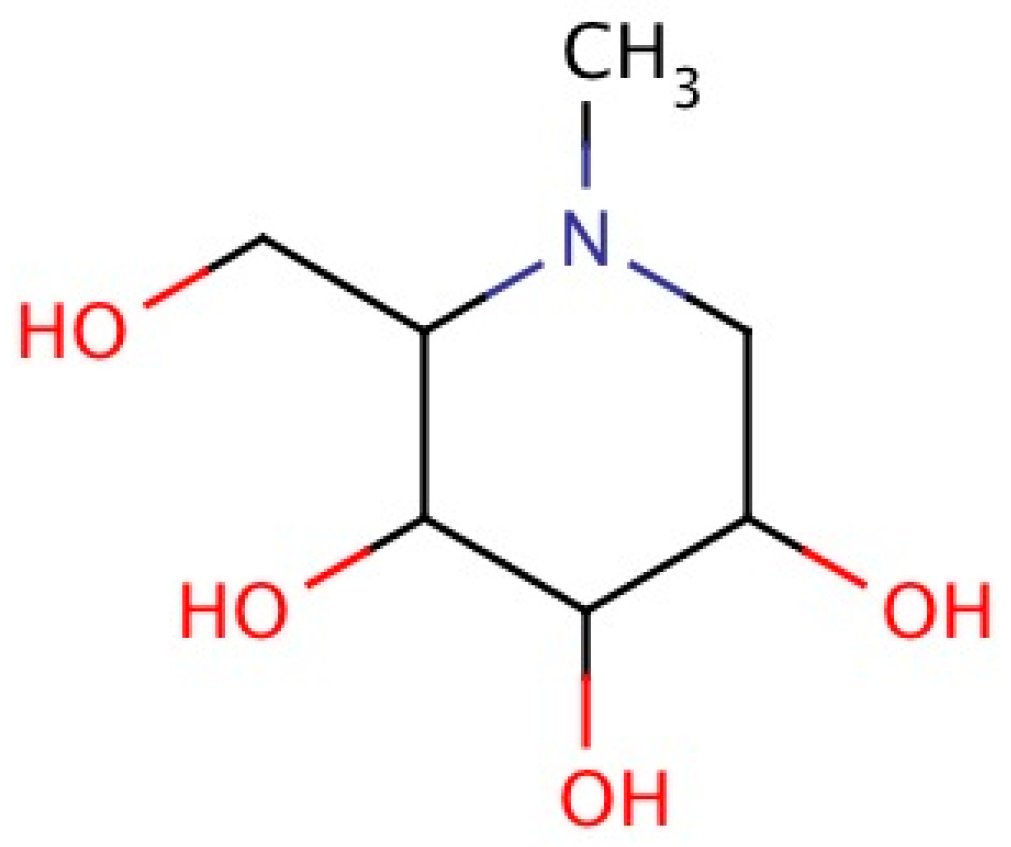
2.4. Chemistry
2.5. Oral Pharmacokinetic of 1-Deoxynojirimycin
2.6. Biological Activities
2.7. Antihyperglycemic Activity
2.8. Anti-Obesity Activity
2.9. Anti-Viral Activity
2.10. In Silico Target Fishing
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pearson, M.S.M.; Mathe-Allainmat, M.; Fargeas, V.; Lebreton, J. Recent Advances in the Total Synthesis of Piperidine Azasugars. Annalen Der Chemie Und Pharmacie 2005, 36, 2159–2191. [Google Scholar]
- Zechel, D.L.; Withers, S.G. Glycosidase mechanisms: Anatomy of a finely tuned catalyst. Acc. Chem. Res. 2000, 33, 11–18. [Google Scholar] [PubMed]
- Inouye, S.; Tsuruoka, T.; Ito, T.; Niida, T. Structure and synthesis of nojirimycin. Tetrahedron 1968, 24, 2125–2144. [Google Scholar] [CrossRef]
- Yagi, M.; Kouno, T.; Aoyagi, Y.; Murai, H. The structure of moranoline, a piperidine alkaloid from Morus species. J. Agric. Chem. Soc. Jpn. 1976, 50, 571–572. [Google Scholar]
- Butters, T.D.; Dwek, R.A.; Platt, F.M. Therapeutic applications of imino sugars in lysosomal storage disorders. Curr. Top. Med. Chem. 2003, 3, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Vichasilp, C.; Nakagawa, K.; Sookwong, P.; Higuchi, O.; Luemunkong, S.; Miyazawa, T. Development of high 1-deoxynojirimycin (DNJ) content mulberry tea and use of response surface methodology to optimize tea-making conditions for highest DNJ extraction. LWT Food Sci. Technol. 2012, 45, 226–232. [Google Scholar] [CrossRef]
- Bajpai, S.; Rao, A.V.B. Quantitative determination of 1-Deoxynojirimycin in different Mulberry Varieties of India. J. Pharm. Phytochem. 2014, 3, 17–22. [Google Scholar]
- Onose, S.; Ikeda, R.; Nakagawa, K.; Kimura, T.; Yamagishi, K.; Higuchi, O.; Miyazawa, T. Production of the α-glycosidase inhibitor 1-deoxynojirimycin from Bacillus species. Food Chem. 2013, 138, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Ezure, Y.; Maruo, S.; Miyazaki, K.; Kawamata, M. Moranoline (1-deoxynojirimycin) fermentation and its improvement. Agric. Biol. Chem. 1985, 49, 1119–1125. [Google Scholar]
- Sharpless, K.E.; Margolis, S.; Thomas, J.B. Determination of vitamins in food-matrix Standard Reference Materials. J. Chromatogr. A 2000, 881, 171–181. [Google Scholar] [CrossRef]
- Kimura, T.; Nakagawa, K.; Saito, Y.; Yamagishi, K.; Suzuki, M.; Yamaki, K.; Shinmoto, H.; Miyazawa, T. Determination of 1-deoxynojirimycin in mulberry leaves using hydrophilic interaction chromatography with evaporative light scattering detection. J. Agric. Food Chem. 2004, 52, 1415–1418. [Google Scholar] [CrossRef] [PubMed]
- Yoshihashi, T.; Do, H.T.T.; Tungtrakul, P.; Boonbumrung, S.; Yamaki, K. Simple, Selective, and Rapid Quantification of 1-Deoxynojirimycin in Mulberry Leaf Products by High-Performance Anion-Exchange Chromatography with Pulsed Amperometric Detection. J. Food Sci. 2010, 75, C246–C250. [Google Scholar] [CrossRef] [PubMed]
- Einarsson, S.; Josefsson, B.; Lagerkvist, S. Determination of amino acids with 9-fluorenylmethyl chloroformate and reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1983, 282, 609–618. [Google Scholar] [CrossRef]
- Lewis, J.; Morley, J.; Venn, R. Analysis of human β-endorphin 28–31 (melanotropin potentiating factor) and analogues by high-performance liquid chromatography of their 9-fluorenylmethoxy-carbonyl derivatives. J. Chromatogr. B Biomed. Sci. Appl. 1993, 615, 37–45. [Google Scholar] [CrossRef]
- Stead, D.; Richards, R. Sensitive fluorimetric determination of gentamicin sulfate in biological matrices using solid-phase extraction, pre-column derivatization with 9-fluorenylmethyl chloroformate and reversed-phase high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1996, 675, 295–302. [Google Scholar] [CrossRef]
- Shangguan, D.; Zhao, Y.; Han, H.; Zhao, R.; Liu, G. Derivatization and fluorescence detection of amino acids and peptides with 9-fluorenylmethyl chloroformate on the surface of a solid adsorbent. Anal. Chem. 2001, 73, 2054–2057. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Kubota, H.; Kimura, T.; Yamashita, S.; Tsuzuki, T.; Oikawa, S.; Miyazawa, T. Occurrence of orally administered mulberry 1-deoxynojirimycin in rat plasma. J. Agric. Food Chem. 2007, 55, 8928–8933. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kwon, H.J.; Jung, J.Y.; Kwon, H.Y.; Baek, J.G.; Kim, Y.-S.; Kwon, O. Comparison of absorption of 1-deoxynojirimycin from mulberry water extract in rats. J. Agric. Food Chem. 2010, 58, 6666–6671. [Google Scholar] [CrossRef] [PubMed]
- Ahr, H.; Boberg, M.; Krause, H.; Maul, W.; Müller, F.; Ploschke, H.; Weber, H.; Wünsche, C. Pharmacokinetics of acarbose. Part I: Absorption, concentration in plasma, metabolism and excretion after single administration of [14C] acarbose to rats, dogs and man. Arzneimittel-Forschung 1989, 39, 1254–1260. [Google Scholar] [PubMed]
- Asano, N.; Yamashita, T.; Yasuda, K.; Ikeda, K.; Kizu, H.; Kameda, Y.; Kato, A.; Nash, R.J.; Lee, H.S.; Ryu, K.S. Polyhydroxylated alkaloids isolated from mulberry trees (Morus alba L.) and silkworms (Bombyx mori L.). J. Agric. Food Chem. 2001, 49, 4208–4213. [Google Scholar] [CrossRef] [PubMed]
- Gui, Z.; Zhuang, D.; Chen, J.; Chen, W. Effect of silkworm powder (SP) lowering blood-glucose levels in mice and its mechanism. Acta Sericol. Sin. 2000, 27, 114–118. [Google Scholar]
- Bembi, B.; Deegan, P. Gaucher disease: Improving management. Acta Paediatr. 2008, 97, 81–82. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, C.; Kamiyama, O.; Ikeda, K.; Sanae, F.; Kato, A.; Adachi, I.; Imahori, T.; Takahata, H.; Okamoto, T.; Asano, N. In vitro inhibition of glycogen-degrading enzymes and glycosidases by six-membered sugar mimics and their evaluation in cell cultures. Bioorg. Med. Chem. 2008, 16, 7330–7336. [Google Scholar] [CrossRef] [PubMed]
- Newbrun, E.; Hoover, C.; Walker, G.J. Inhibition by acarbose, nojirimycin and 1-deoxynojirimycin of glucosyltransferase produced by oral streptococci. Arch. Oral Biol. 1983, 28, 531–536. [Google Scholar] [CrossRef]
- Yatsunami, K.; Ichida, M.; Onodera, S. The relationship between 1-deoxynojirimycin content and α-glucosidase inhibitory activity in leaves of 276 mulberry cultivars (Morus spp.) in Kyoto, Japan. J. Nat. Med. 2008, 62, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, L.; Ma, D.; Qu, X.; Guo, H.; Xu, X.; Mason, P.M.; Bourne, N.; Moriarty, R.; Gu, B. Novel imino sugar derivatives demonstrate potent antiviral activity against flaviviruses. Antimicrob. Agents Chemother. 2009, 53, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kato, J.; Kohara, M.; Galinski, M.S. Antiviral effects of glycosylation and glucose trimming inhibitors on human parainfluenza virus type 3. Antivir. Res. 2006, 72, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Durantel, D.; Branza-Nichita, N.; Carrouée-Durantel, S.; Butters, T.D.; Dwek, R.A.; Zitzmann, N. Study of the mechanism of antiviral action of iminosugar derivatives against bovine viral diarrhea virus. J. Virol. 2001, 75, 8987–8998. [Google Scholar] [CrossRef] [PubMed]
- Lazar, C.; Durantel, D.; Macovei, A.; Zitzmann, N.; Zoulim, F.; Dwek, R.A.; Branza-Nichita, N. Treatment of hepatitis B virus-infected cells with α-glucosidase inhibitors results in production of virions with altered molecular composition and infectivity. Antivir. Res. 2007, 76, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Papandréou, M.-J.; Barbouche, R.; Guieu, R.; Kieny, M.P.; Fenouillet, E. The α-glucosidase inhibitor 1-deoxynojirimycin blocks human immunodeficiency virus envelope glycoprotein-mediated membrane fusion at the CXCR4 binding step. Mol. Pharmacol. 2002, 61, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Do, H.J.; Chung, J.H.; Hwang, J.W.; Kim, O.Y.; Lee, J.-Y.; Shin, M.-J. 1-Deoxynojirimycin isolated from Bacillus subtilis improves hepatic lipid metabolism and mitochondrial function in high-fat–fed mice. Food Chem. Toxicol. 2015, 75, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Kimura, T.; Nakagawa, K.; Asai, A.; Hasumi, K.; Oikawa, S.; Miyazawa, T. Effects of mulberry leaf extract rich in 1-deoxynojirimycin on blood lipid profiles in humans. J. Clin. Biochem. Nutr. 2010, 47, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-Q.; Zhu, L.; Ma, M.-L.; Chen, X.-C.; Gao, Y.; Yu, T.-Y.; Yang, G.-S.; Pang, W.-J. Mulberry 1-Deoxynojirimycin Inhibits Adipogenesis by Repression of the ERK/PPARγ Signaling Pathway in Porcine Intramuscular Adipocytes. J. Agric. Food Chem. 2015, 63, 6212–6220. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.-H.; Oh, S.-H.; Ahn, Y.-R.; Kim, K.-W.; Kim, J.-H.; Seo, S.-W. Antiobesity effects and improvement of insulin sensitivity by 1-deoxynojirimycin in animal models. J. Agric. Food Chem. 2008, 56, 2613–2619. [Google Scholar] [CrossRef] [PubMed]
- Monte, S.V.; Schentag, J.J.; Adelman, M.H.; Paladino, J.A. Glucose supply and insulin demand dynamics of antidiabetic agents. J. Diabetes sci. Technol. 2010, 4, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Koutsoukas, A.; Simms, B.; Kirchmair, J.; Bond, P.J.; Whitmore, A.V.; Zimmer, S.; Young, M.P.; Jenkins, J.L.; Glick, M.; Glen, R.C. From in silico target prediction to multi-target drug design: Current databases, methods and applications. J. Proteom. 2011, 74, 2554–2574. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, J.L.; Bender, A.; Davies, J.W. In silico target fishing: Predicting biological targets from chemical structure. Drug Discov. Today Technol. 2007, 3, 413–421. [Google Scholar] [CrossRef]
- Chen, F.; Nakashima, N.; Kimura, I.; Kimura, M. [Hypoglycemic activity and mechanisms of extracts from mulberry leaves (Folium mori) and cortex mori radicis in streptozotocin-induced diabetic mice]. Yakugaku Zasshi J. Pharm. Soc. Jpn. 1995, 115, 476–482. [Google Scholar]
- Hocking, D. Trees For Drylands; International Science Publisher: New York, NY, USA, 1993. [Google Scholar]
- Arabshahi-Delouee, S.; Urooj, A. Antioxidant properties of various solvent extracts of mulberry (Morus indica L.) leaves. Food Chem. 2007, 102, 1233–1240. [Google Scholar] [CrossRef]
- Ercisli, S. A short review of the fruit germplasm resources of Turkey. Genet. Resour. Crop Evol. 2004, 51, 419–435. [Google Scholar] [CrossRef]
- Halliwell, B. Dietary polyphenols: Good, bad, or indifferent for your health? Cardiovasc. Res. 2007, 73, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Mazur, A.; Scalbert, A. Polyphenols and prevention of cardiovascular diseases. Curr. Opin. Lipidol. 2005, 16, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Ercisli, S.; Orhan, E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 2007, 103, 1380–1384. [Google Scholar] [CrossRef]
- Butt, M.S.; Nazir, A.; Sultan, M.T.; Schroën, K. Morus alba L. nature's functional tonic. Trends Food Sci. Technol. 2008, 19, 505–512. [Google Scholar] [CrossRef]
- Kooij, R.; Branderhorst, H.M.; Bonte, S.; Wieclawska, S.; Martin, N.I.; Pieters, R.J. Glycosidase inhibition by novel guanidinium and urea iminosugar derivatives. MedChemComm 2013, 4, 387–393. [Google Scholar] [CrossRef]
- Tsuduki, T.; Kikuchi, I.; Kimura, T.; Nakagawa, K.; Miyazawa, T. Intake of mulberry 1-deoxynojirimycin prevents diet-induced obesity through increases in adiponectin in mice. Food Chem. 2013, 139, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Kimuar, M.; Chen, F.; Nakashima, N. Antihyperglycemic effects of N-containing sugars derived from mulberry leaves is streptozocin-in-duced diabetic mice. Wakan Iyakugaku Zasshi 1995, 12, 214–219. [Google Scholar]
- Lee, S.H.; Choi, S.Y.; Kim, H.; Hwang, J.S.; Lee, B.G.; Gao, J.J.; Kim, S.Y. Mulberroside F isolated from the leaves of Morus alba inhibits melanin biosynthesis. Biol. Pharm. Bull. 2002, 25, 1045–1048. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.-H.; Hou, Y.-C.; Chao, P.-D.L. Pharmacokinetic and pharmacodynamic interactions of morin and cyclosporin. Toxicol. Appl. Pharmacol. 2005, 205, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Niidome, T.; Takahashi, K.; Goto, Y.; Goh, S.; Tanaka, N.; Kamei, K.; Ichida, M.; Hara, S.; Akaike, A.; Kihara, T. Mulberry leaf extract prevents amyloid β-peptide fibril formation and neurotoxicity. Neuroreport 2007, 18, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-G.; Wang, C.-Y.; Jin, C.; Jia, J.-Q.; Guo, X.; Zhang, G.-Z.; Gui, Z.-Z. Improved 1-Deoxynojirimycin (DNJ) production in mulberry leaves fermented by microorganism. Braz. J. Microbiol. 2014, 45, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, X.; Li, C.; Zheng, Y.; Peng, G. 1-Deoxynojirimycin Alleviates Insulin Resistance via Activation of Insulin Signaling PI3K/AKT Pathway in Skeletal Muscle of db/db Mice. Molecules 2015, 20, 21700–21714. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Nakagawa, K.; Higuchi, O.; Kimura, T.; Kojima, Y.; Kariya, J.; Miyazawa, T.; Oikawa, S. Effect of mulberry leaf extract with enriched 1-deoxynojirimycin content on postprandial glycemic control in subjects with impaired glucose metabolism. J. Diabetes Investig. 2011, 2, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Frommer, W.; Junge, B.; Müller, L.; Wingender, W.; Truscheit, E.; Schäfer, D. α-Glucosidase inhibitors. Naturwissenschaften 1977, 64, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Kanieda, Y.; Asano, N.; Teranishi, M.; Matsui, K. New cyclitols, degradation of validamycin a by Flavobacterium saccharophilum. J. Antibiot. 1980, 33, 1573–1574. [Google Scholar] [CrossRef]
- Kimura, T.; Nakagawa, K.; Kubota, H.; Kojima, Y.; Goto, Y.; Yamagishi, K.; Oita, S.; Oikawa, S.; Miyazawa, T. Food-grade mulberry powder enriched with 1-deoxynojirimycin suppresses the elevation of postprandial blood glucose in humans. J. Agric. Food Chem. 2007, 55, 5869–5874. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Wang, H.-J.; Bucheli, P.; Zhang, P.-F.; Wei, D.-Z.; Lu, Y.-H. Phytochemical profiles of different mulberry (Morus sp.) species from China. J. Agric. Food Chem. 2009, 57, 9133–9140. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Li, Y.; Du, Y.; Su, B.; Lu, D. Analysis of 1-deoxynojirimycin component correlation between medicinal parasitic loranthus from loranthaceae and their mulberry host trees. J. Med. Plants Res. 2011, 5, 4326–4331. [Google Scholar]
- Hu, X.-Q.; Jiang, L.; Zhang, J.-G.; Deng, W.; Wang, H.-L.; Wei, Z.-J. Quantitative determination of 1-deoxynojirimycin in mulberry leaves from 132 varieties. Ind. Crop. Prod. 2013, 49, 782–784. [Google Scholar] [CrossRef]
- Jeong, J.H.; Lee, N.K.; Cho, S.H.; Jeong, Y.-S. Enhancement of 1-deoxynojirimycin content and α-glucosidase inhibitory activity in mulberry leaf using various fermenting microorganisms isolated from Korean traditional fermented food. Biotechnol. Bioprocess Eng. 2014, 19, 1114–1118. [Google Scholar] [CrossRef]
- Wang, T.; Li, C.-Q.; Zhang, H.; Li, J.-W. Response surface optimized extraction of 1-deoxynojirimycin from mulberry leaves (Morus alba L.) and preparative separation with resins. Molecules 2014, 19, 7040–7056. [Google Scholar] [CrossRef] [PubMed]
- Vichasilp, C.; Nakagawa, K.; Sookwong, P.; Suzuki, Y.; Kimura, F.; Higuchi, O.; Miyazawa, T. Optimization of 1-deoxynojirimycin extraction from mulberry leaves by using response surface methodology. Biosci. Biotechnol. Biochem. 2009, 73, 2684–2689. [Google Scholar] [CrossRef] [PubMed]
- Tolstikov, V.V.; Fiehn, O. Analysis of highly polar compounds of plant origin: Combination of hydrophilic interaction chromatography and electrospray ion trap mass spectrometry. Anal. Biochem. 2002, 301, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Kim, S.-U.; Lee, H.S.; Kim, I.; Ahn, M.Y.; Ryu, K.S. Determination of 1-deoxynojirimycin in Morus alba L. leaves by derivatization with 9-fluorenylmethyl chloroformate followed by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2003, 1002, 93–99. [Google Scholar] [CrossRef]
- Nuengchamnong, N.; Ingkaninan, K.; Kaewruang, W.; Wongareonwanakij, S.; Hongthongdaeng, B. Quantitative determination of 1-deoxynojirimycin in mulberry leaves using liquid chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 2007, 44, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Keiner, R.; Dräger, B. Calystegine distribution in potato (Solanum tuberosum) tubers and plants. Plant Sci. 2000, 150, 171–179. [Google Scholar] [CrossRef]
- Nakagawa, K.; Ogawa, K.; Higuchi, O.; Kimura, T.; Miyazawa, T.; Hori, M. Determination of iminosugars in mulberry leaves and silkworms using hydrophilic interaction chromatography–tandem mass spectrometry. Anal. Biochem. 2010, 404, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Herraez-Hernandez, R.; Campins-Falco, P. Derivatization of tertiary amphetamines with 9-fluorenylmethyl chloroformate for liquid chromatography: Determination of N-methylephedrine. Analyst 2000, 125, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zhe, S.; Chu, Q.; Lin, T.; Xin, L.; Feng, X.; Li, Z.; Yang, S.; Xianghui, L.; Jingxian, Z. Therapeutic target database update 2012: A resource for facilitating target-oriented drug discovery. Nucleic Acids Res. 2012, 40, D1128–D1136. [Google Scholar]
- Wang, L.; Peng, J.; Wang, X.; Zhu, X.; Cheng, B.; Gao, J.; Jiang, M.; Bai, G.; Hou, Y. Carboxymethylcellulose sodium improves the pharmacodynamics of 1-deoxynojirimycin by changing its absorption characteristics and pharmacokinetics in rats. Pharm. Int. J. Pharm. Sci. 2012, 67, 168–173. [Google Scholar]
- Nirogi, R.V.; Kandikere, V.N.; Shukla, M.; Mudigonda, K.; Maurya, S.; Boosi, R.; Yerramilli, A. Liquid chromatographic tandem mass spectrometry method for the quantification of miglitol in human plasma. Arzneimittelforschung 2006, 56, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Faber, E.D.; Oosting, R.; Neefjes, J.J.; Ploegh, H.L.; Meijer, D.K. Distribution and elimination of the glycosidase inhibitors 1-deoxymannojirimycin and N-methyl-1-deoxynojirimycin in the rat in vivo. Pharm. Res. 1992, 9, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Dyer, J.; Merediz, E.F.-C.; Salmon, K.; Proudman, C.; Edwards, G.; Shirazi-Beechey, S. Molecular characterisation of carbohydrate digestion and absorption in equine small intestine. Equine Vet. J. 2002, 34, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.M. Starch digestion and absorption in nonruminants. J. Nutr. 1992, 122, 172. [Google Scholar] [PubMed]
- Koh, L.W.; Wong, L.L.; Loo, Y.Y.; Kasapis, S.; Huang, D. Evaluation of different teas against starch digestibility by mammalian glycosidases. J. Agric. Food Chem. 2009, 58, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Drozdowski, L.; Thomson, A.B. Citation of This Article. World J. Gastroenterol. 2006, 12, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, H. The mechanism of α-glucosidase inhibition in the management of diabetes. Clin. Investig. Med. 1995, 18, 303–311. [Google Scholar]
- Krentz, A.J. Comparative safety of newer oral antidiabetic drugs. Expert Opin. Drug Saf. 2006, 5, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Sels, J.-P.J.; Huijberts, M.S.; Wolffenbuttel, B.H. Miglitol, a new α-glucosidase inhibitor. Expert Opin. Pharmacother. 1999, 1, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Martin, O. Iminosugars: Current and future therapeutic applications. Ann. Pharm. Francaises 2007, 65, 5–13. [Google Scholar] [CrossRef]
- Li, Y.-G.; Ji, D.-F.; Zhong, S.; Lin, T.-B.; Lv, Z.-Q.; Hu, G.-Y.; Wang, X. 1-deoxynojirimycin inhibits glucose absorption and accelerates glucose metabolism in streptozotocin-induced diabetic mice. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Shin, M.J.; Hwang, K.Y.; Kwon, O.; Chung, J.H. 1-Deoxynojirimycin isolated from a Bacillus subtilis stimulates adiponectin and GLUT4 expressions in 3T3-L1 adipocytes. J. Microbiol. Biotechnol. 2013, 23, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, T.; Mukoyama, S.; Soda, K. Chemo-enzymatic d-enantiomerization of dl-lactate. Biotechnol. Bioeng. 2001, 73, 80–82. [Google Scholar] [CrossRef]
- Farmer, S.; Auwerx, J. Adipose tissue: New therapeutic targets from molecular and genetic studies–IASO Stock Conference 2003 report. Obes. Rev. 2004, 5, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Kersten, S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2001, 2, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Park, N.-Y.; Lim, Y. Anti-adipogenic effect of mulberry leaf ethanol extract in 3T3-L1 adipocytes. Nutr. Res. Pract. 2014, 8, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Dedera, D.; Vander Heyden, N.; Ratner, L. Attenuation of HIV-1 infectivity by an inhibitor of oligosaccharide processing. AIDS Res. Hum. Retrovir. 1990, 6, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Fenouillet, E.; Gluckman, J.-C. Effect of a glucosidase inhibitor on the bioactivity and immunoreactivity of human immunodeficiency virus type 1 envelope glycoprotein. J. Gen. Virol. 1991, 72, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.M.; Jacob, G.S. Anti-HIV drug mechanism. Nature 1991, 352, 198. [Google Scholar] [CrossRef] [PubMed]
- Ratner, L. Glucosidase inhibitors for treatment of HIV-1 infection. AIDS Res. Hum. Retrovir. 1992, 8, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Fischer, P.; Collin, M.; Karlsson, G.; James, W.; Butters, T.; Davis, S.; Gordon, S.; Dwek, R.; Platt, F. The α-glucosidase inhibitor N-butyldeoxynojirimycin inhibits human immunodeficiency virus entry at the level of post-CD4 binding. J. Virol. 1995, 69, 5791–5797. [Google Scholar] [PubMed]
- Jacob, J.R.; Mansfield, K.; You, J.E.; Tennant, B.C.; Kim, Y.H. Natural iminosugar derivatives of 1-deoxynojirimycin inhibit glycosylation of hepatitis viral envelope proteins. J. Microbiol. 2007, 45, 431–440. [Google Scholar] [PubMed]
- Kang, K.-D.; Park, J.-S.; Cho, Y.-S.; Park, Y.-S.; Lee, J.-Y.; Hwang, K.-Y.; Yuk, W.-J.; Kamita, S.G.; Suzuki, K.; Seong, S.-I. Effect of 1-deoxynojirimycin on the replication of baculoviruses, Bombyx mori Nucleopolyhedrovirus and Autographa californica multiple nucleopolyhedrovirus. Int. J. Ind. Entomol. 2011, 23, 123–128. [Google Scholar] [CrossRef]




| No. | Biological Activity | Reference |
|---|---|---|
| 1 | Antihyperglycemic | [20,21,22,23,24,25] |
| 2 | Anti-viral | [26,27,28,29,30] |
| 3 | Anti-obesity | [31,32,33,34,35] |
| No. | Pa | Activity | No. | Pa | Activity |
|---|---|---|---|---|---|
| 1 | 0.953 | Phosphatidylcholine-sterol O-acyltransferase inhibitor | 23 | 0.829 | CDP-glycerol glycerophosphotransferase inhibitor |
| 2 | 0.937 | Glucan 1,3-β-glucosidase inhibitor | 24 | 0.802 | β-glucosidase inhibitor |
| 3 | 0.924 | l-iduronidase inhibitor | 25 | 0.801 | Fucosterol-epoxide lyase inhibitor |
| 4 | 0.916 | Mannosyl-oligosaccharide 1,2-α-mannosidase inhibitor | 26 | 0.792 | Ceramide glucosyltransferase inhibitor |
| 5 | 0.905 | Glycosylceramidase inhibitor | 27 | 0.798 | Glucan endo-1,6-β-glucosidase inhibitor |
| 6 | 0.903 | Oligo-1,6-glucosidase inhibitor | 28 | 0.783 | Mucinaminylserine mucinaminidase inhibitor |
| 7 | 0.886 | Sucrose α-glucosidase inhibitor | 29 | 0.776 | Fructan β-fructosidase inhibitor |
| 8 | 0.880 | Mannosidase inhibitor | 30 | 0.773 | Manganese peroxidase inhibitor |
| 9 | 0.879 | Glucan 1,3-α-glucosidase inhibitor | 31 | 0.784 | Alkenylglycerophosphocholine hydrolase inhibitor |
| 10 | 0.880 | β-mannosidase inhibitor | 32 | 0.788 | Testosterone 17β-dehydrogenase (NADP+) inhibitor |
| 11 | 0.869 | Sugar-phosphatase inhibitor | 33 | 0.750 | β-glucosidase inhibitor |
| 12 | 0.856 | α-mannosidase inhibitor | 34 | 0.753 | Glucan 1,4-α-maltotriohydrolase inhibitor |
| 13 | 0.850 | Mannosyl-oligosaccharide glucosidase inhibitor | 35 | 0.755 | Ribulose-phosphate 3-epimerase inhibitor |
| 14 | 0.853 | Nicotinic α6β3β4α5 receptor antagonist | 36 | 0.741 | α-glucosidase inhibitor |
| 15 | 0.842 | Amylo-α-1,6-glucosidase inhibitor | 37 | 0.756 | Benzoate-CoA ligase inhibitor |
| 16 | 0.838 | UDP-N-acetylglucosamine 4-epimerase inhibitor | 38 | 0.730 | Glucan 1,4-α-glucosidase inhibitor |
| 17 | 0.833 | α-l-fucosidase inhibitor | 39 | 0.727 | α,α-trehalose phosphorylase inhibitor |
| 18 | 0.828 | Exoribonuclease II inhibitor | 40 | 0.721 | Endo-1,3(4)-β-glucanase inhibitor |
| 19 | 0.827 | Nicotinic α2β2 receptor antagonist | 41 | 0.708 | Nucleoside oxidase (H2O2-forming) inhibitor |
| 20 | 0.829 | Glutamate-5-semialdehyde dehydrogenase inhibitor | 42 | 0.717 | Acylcarnitine hydrolase inhibitor |
| 21 | 0.812 | Interleukin 4 antagonist | 43 | 0.704 | Mannotetraose 2-α-N-acetylglucosaminyl transferase inhibitor |
| 22 | 0.808 | β-galactosidase inhibitor | 44 | 0.723 | Membrane integrity agonist |
| Node Color | Abbreviations | Protein Targets | UniProt ID | Score |
|---|---|---|---|---|
 | GLA | Galactosidase, α (429 aa) | P10253 | 0.921 |
 | GANAB | Glucosidase, α; neutral AB; Cleaves sequentially the 2 innermost α-1,3-linked glucose residues from the Glc(2)Man(9)GlcNAc(2) oligosaccharide precursor of immature glycoproteins (966 aa) | Q14697 | 0.854 |
 | CALR | Calreticulin; Molecular calcium binding chaperone promoting folding, oligomeric assembly and quality control in the ER via the calreticulin/calnexin cycle. This lectin interacts transiently with almost all of the monoglucosylated glycoproteins that are synthesized in the ER. Interacts with the DNA-binding domain of NR3C1 and mediates its nuclear export (417 aa) | P27797 | 0.843 |
 | MGAM | Maltase-glucoamylase (α-glucosidase); May serve as an alternate pathway for starch digestion when luminal α-amylase activity is reduced because of immaturity or malnutrition. May play a unique role in the digestion of malted dietary oligosaccharides used in food manufacturing (1857 aa) | O43451 | 0.833 |
 | GAA | Glucosidase, α; acid; Essential for the degradation of glygogen to glucose in lysosomes (952 aa) | P06280 | 0.833 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, K.; Zheng, C.; Wang, T.; Zhao, H.; Wang, J.; Wang, Z.; Zhai, X.; Jia, Z.; Chen, J.; Zhou, Y.; et al. 1-Deoxynojirimycin: Occurrence, Extraction, Chemistry, Oral Pharmacokinetics, Biological Activities and In Silico Target Fishing. Molecules 2016, 21, 1600. https://doi.org/10.3390/molecules21111600
Gao K, Zheng C, Wang T, Zhao H, Wang J, Wang Z, Zhai X, Jia Z, Chen J, Zhou Y, et al. 1-Deoxynojirimycin: Occurrence, Extraction, Chemistry, Oral Pharmacokinetics, Biological Activities and In Silico Target Fishing. Molecules. 2016; 21(11):1600. https://doi.org/10.3390/molecules21111600
Chicago/Turabian StyleGao, Kuo, Chenglong Zheng, Tong Wang, Huihui Zhao, Juan Wang, Zhiyong Wang, Xing Zhai, Zijun Jia, Jianxin Chen, Yingwu Zhou, and et al. 2016. "1-Deoxynojirimycin: Occurrence, Extraction, Chemistry, Oral Pharmacokinetics, Biological Activities and In Silico Target Fishing" Molecules 21, no. 11: 1600. https://doi.org/10.3390/molecules21111600





