Circulating IL-27 Is Elevated in Rheumatoid Arthritis Patients
Abstract
:1. Introduction
2. Results
2.1. RA Patients and Control Subjects
2.2. Serum Levels of IL-27
2.3. Serum Concentration of IL-27 and RA Disease Activity
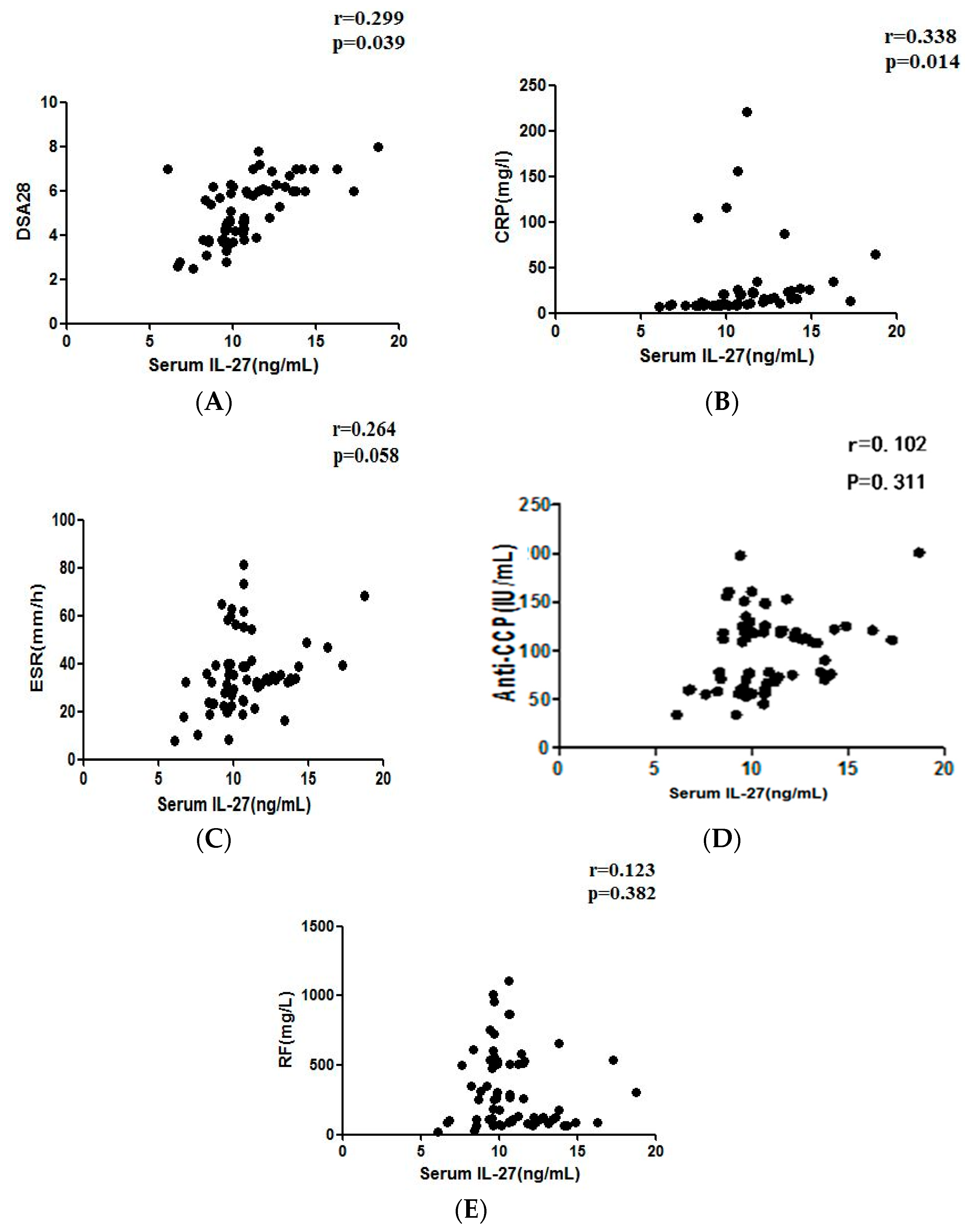
2.4. Correlation between Serum IL-27 and RF, Anti-CCP, CRP or ESR Levels in RA Patients
2.5. Effect of Immunosuppressant Treatment on the Production of IL-27
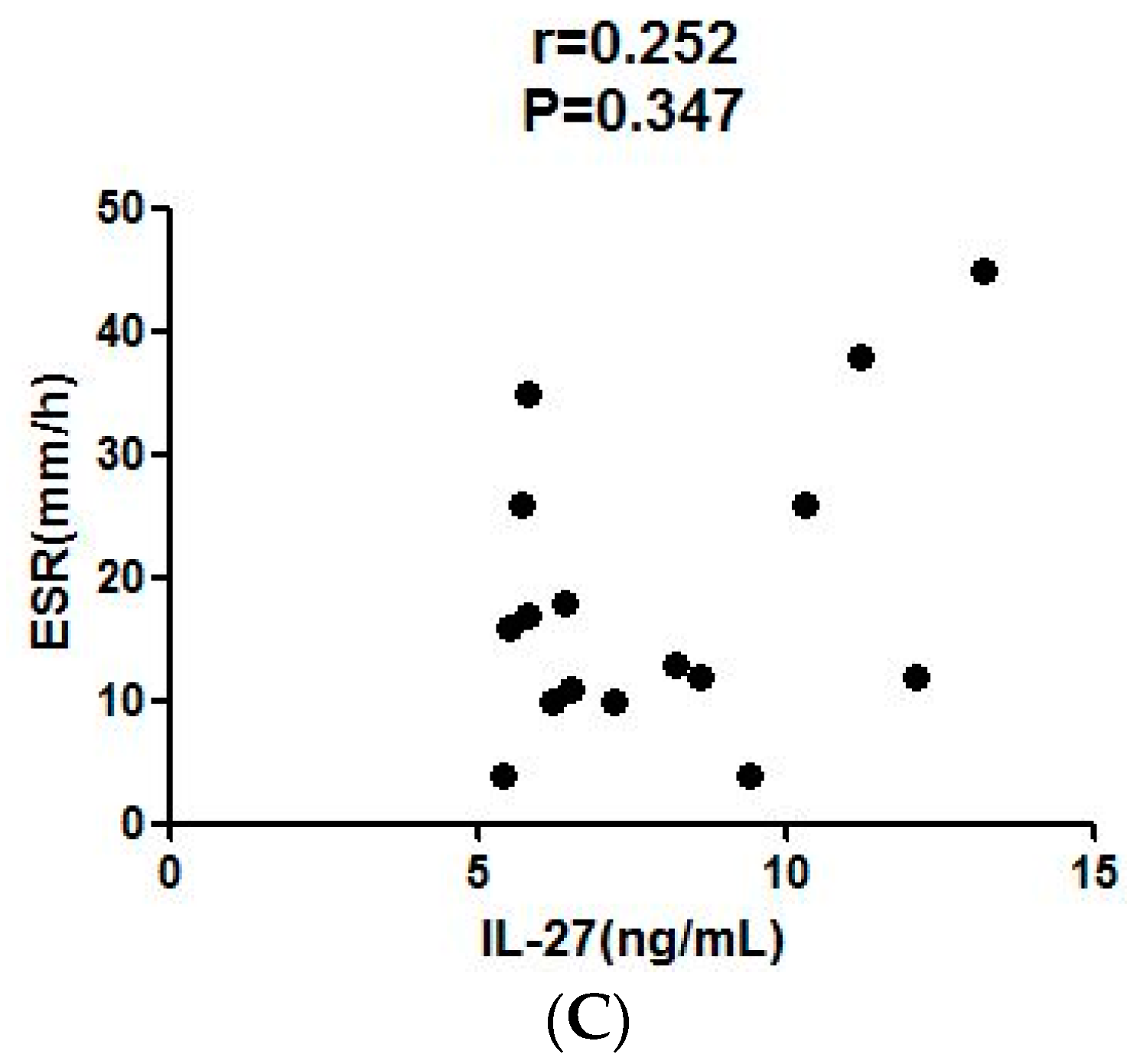
2.6. Correlation of Serum IL-27 to Parameters after LEF Treatment
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Measurement of Auto-Antibodies
4.3. Assay of IL-27
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Feldmann, M.; Brennan, F.M.; Maini, R.N. Role of cytokines in rheumatoid arthritis. Annu. Rev. Immunol. 1996, 14, 397–440. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Yong, J.; Dang, H.; Kaufman, D.L. Oral gaba treatment downregulates inflammatory responses in a mouse model of rheumatoid arthritis. Autoimmunity 2011, 44, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Benham, H.; Cope, A.P.; Thomas, R. T-cell receptor signaling and the pathogenesis of autoimmune arthritis: Insights from mouse and man. Immunol. Cell. Biol. 2012, 90, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.H.; Panayi, G.S. Cytokine pathways and joint inflammation in rheumatoid arthritis. Engl. J. Med. 2001, 344, 907–916. [Google Scholar]
- Linsley, P.S.; Nadler, S.G. The clinical utility of inhibiting cd28-mediated costimulation. Immunol. Rev. 2009, 229, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Sprent, J.; Surh, C.D. Normal t cell homeostasis: The conversion of naive cells into memory-phenotype cells. Nat. Immunol. 2011, 12, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Devergne, O.; Hummel, M.; Koeppen, H.; Le Beau, M.M.; Nathanson, E.C.; Kieff, E.; Birkenbach, M. A novel interleukin-12 p40-related protein induced by latent epstein-barr virus infection in b lymphocytes. J. Virol. 1996, 70, 1143–1153. [Google Scholar] [PubMed]
- Pflanz, S.; Timans, J.C.; Cheung, J.; Rosales, R.; Kanzler, H.; Gilbert, J.; Hibbert, L.; Churakova, T.; Travis, M.; Vaisberg, E.; et al. Il-27, a heterodimeric cytokine composed of ebi3 and p28 protein, induces proliferation of naive cd4+ t cells. Immunity 2002, 16, 779–790. [Google Scholar] [CrossRef]
- Sun, J.; Dodd, H.; Moser, E.K.; Sharma, R.; Braciale, T.J. Cd4+ t cell help and innate-derived il-27 induce blimp-1-dependent il-10 production by antiviral ctls. Nat. Immunol. 2011, 12, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.; Ghilardi, N.; Li, J.; de Sauvage, F.J. Il-27 regulates il-12 responsiveness of naive cd4+ t cells through stat1-dependent and -independent mechanisms. Proc. Natl. Acad. Sci. USA 2003, 100, 15047–15052. [Google Scholar] [CrossRef] [PubMed]
- Bosmann, M.; Ward, P.A. Modulation of inflammation by interleukin-27. J. Leukoc. Biol. 2013, 94, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Sprecher, C.A.; Grant, F.J.; Baumgartner, J.W.; Presnell, S.R.; Schrader, S.K.; Yamagiwa, T.; Whitmore, T.E.; O'Hara, P.J.; Foster, D.F. Cloning and characterization of a novel class i cytokine receptor. Biochem. Biophys. Res. Commun. 1998, 246, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Hamano, S.; Senaldi, G.; Covey, T.; Faggioni, R.; Mu, S.; Xia, M.; Wakeham, A.C.; Nishina, H.; Potter, J.; et al. Wsx-1 is required for the initiation of th1 responses and resistance to l. Major infection. Immunity 2001, 15, 569–578. [Google Scholar] [CrossRef]
- Hunter, C.A.; Kastelein, R. Interleukin-27: Balancing protective and pathological immunity. Immunity 2012, 37, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Takeda, A.; Hamano, S.; Miyazaki, Y.; Kinjyo, I.; Ishibashi, T.; Yoshimura, A.; Yoshida, H. Two-sided roles of il-27: Induction of th1 differentiation on naive cd4+ t cells versus suppression of proinflammatory cytokine production including il-23-induced il-17 on activated cd4+ t cells partially through stat3-dependent mechanism. J. Immunol. 2006, 177, 5377–5385. [Google Scholar] [CrossRef] [PubMed]
- El-behi, M.; Ciric, B.; Yu, S.; Zhang, G.X.; Fitzgerald, D.C.; Rostami, A. Differential effect of il-27 on developing versus committed th17 cells. J. Immunol. 2009, 183, 4957–4967. [Google Scholar] [CrossRef] [PubMed]
- Larousserie, F.; Charlot, P.; Bardel, E.; Froger, J.; Kastelein, R.A.; Devergne, O. Differential effects of il-27 on human b cell subsets. J. Immunol. 2006, 176, 5890–5897. [Google Scholar] [CrossRef] [PubMed]
- Kastelein, R.A.; Hunter, C.A.; Cua, D.J. Discovery and biology of il-23 and il-27: Related but functionally distinct regulators of inflammation. Annu. Rev. Immunol. 2007, 25, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Eri, R.; Zunt, S.L.; Summerlin, D.J.; Brand, D.D.; Blum, J.S. Suppression of immune responses in collagen-induced arthritis by a rationally designed cd80-binding peptide agent. Arthritis Rheum. 2007, 56, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Arakawa, F.; Higuchi, F.; Ishibashi, Y.; Goto, M.; Sugita, Y.; Nomura, Y.; Niino, D.; Shimizu, K.; Aoki, R.; et al. Gene expression analysis of rheumatoid arthritis synovial lining regions by cdna microarray combined with laser microdissection: Up-regulation of inflammation-associated stat1, irf1, cxcl9, cxcl10, and ccl5. Scand. J. Rheumatol. 2012, 41, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Mohan, C. The akt axis as a therapeutic target in autoimmune diseases. Endocr. Metab. Immune Disord. Drug Targets 2009, 9, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Warrington, K.J.; Takemura, S.; Goronzy, J.J.; Weyand, C.M. Cd4+,cd28- t cells in rheumatoid arthritis patients combine features of the innate and adaptive immune systems. Arthritis Rheum. 2001, 44, 13–20. [Google Scholar] [CrossRef]
- Boilard, E.; Blanco, P.; Nigrovic, P.A. Platelets: Active players in the pathogenesis of arthritis and sle. Nat. Rev. Rheumatol. 2012, 8, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Boilard, E.; Nigrovic, P.A.; Larabee, K.; Watts, G.F.; Coblyn, J.S.; Weinblatt, M.E.; Massarotti, E.M.; Remold-O’Donnell, E.; Farndale, R.W.; Ware, J.; et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 2010, 327, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Garraud, O.; Hamzeh-Cognasse, H.; Pozzetto, B.; Cavaillon, J.M.; Cognasse, F. Bench-to-bedside review: Platelets and active immune functions—New clues for immunopathology? Crit. Care 2013, 17, 236. [Google Scholar] [CrossRef] [PubMed]
- Michou, L.; Cornelis, F.; Baron, M.; Bombardieri, S.; Balsa, A.; Westhovens, R.; Barrera, P.; Alves, H.; Radstake, T.R.; Migliorini, P.; et al. Association study of the platelet collagen receptor glycoprotein vi gene with rheumatoid arthritis. Clin. Exp. Rheumatol. 2013, 31, 770–772. [Google Scholar] [PubMed]
- Gasparyan, A.Y.; Stavropoulos-Kalinoglou, A.; Mikhailidis, D.P.; Douglas, K.M.; Kitas, G.D. Platelet function in rheumatoid arthritis: Arthritic and cardiovascular implications. Rheumatol. Int. 2011, 31, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Hamzeh-Cognasse, H.; Damien, P.; Nguyen, K.A.; Zeni, F.; Pozzetto, B.; Cognasse, F.; Garraud, O. Contribution of activated platelets to plasma il-27 levels. Crit. Care 2013, 17, 411. [Google Scholar] [CrossRef] [PubMed]
- Yazici, S.; Yazici, M.; Erer, B.; Erer, B.; Calik, Y.; Ozhan, H.; Ataoglu, S. The platelet indices in patients with rheumatoid arthritis: Mean platelet volume reflects disease activity. Platelets 2010, 21, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Gasparyan, A.Y.; Sandoo, A.; Stavropoulos-Kalinoglou, A.; Kitas, G.D. Mean platelet volume in patients with rheumatoid arthritis: The effect of anti-tnf-alpha therapy. Rheumatol. Int. 2010, 30, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, J.; Wang, S. The potential therapeutic role of myeloid-derived suppressor cells in autoimmune arthritis. Semin. Arthritis. Rheum. 2016, 45, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Chen, D.P.; Tam, L.S.; Li, E.K.; Yin, Y.B.; Lam, C.W. Effects of inflammatory cytokine il-27 on the activation of fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis. Res. Ther. 2010, 12, R129. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Xia, L.; Xiao, W.; Lu, J. Increased levels of interleukin-27 in patients with rheumatoid arthritis. Arthritis. Rheum. 2011, 63, 860–861. [Google Scholar] [CrossRef] [PubMed]
- Tanida, S.; Yoshitomi, H.; Ishikawa, M.; Kasahara, T.; Murata, K.; Shibuya, H.; Ito, H.; Nakamura, T. Il-27-producing cd14 (+) cells infiltrate inflamed joints of rheumatoid arthritis and regulate inflammation and chemotactic migration. Cytokine 2011, 55, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strand, V.; Cohen, S.; Schiff, M.; Weaver, A.; Fleischmann, R.; Cannon, G.; Fox, R.; Moreland, L.; Olsen, N.; Furst, D.; et al. Treatment of active rheumatoid arthritis with leflunomide compared with placebo and methotrexate. Leflunomide rheumatoid arthritis investigators group. Arch. Intern. Med. 1999, 159, 2542–2550. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.I.; Herrmann, M.L.; Frangou, C.G.; Wahl, G.M.; Morris, R.E.; Strand, V.; Kirschbaum, B.J. Mechanism of action for leflunomide in rheumatoid arthritis. Clin. Immunol. 1999, 93, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Sulli, A.; Ghiorzo, P.; Pizzorni, C.; Craviotto, C.; Villaggio, B. Anti-inflammatory effects of leflunomide on cultured synovial macrophages from patients with rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Litinsky, I.; Paran, D.; Levartovsky, D.; Wigler, I.; Kaufman, I.; Yaron, I.; Yaron, M.; Caspi, D.; Elkayam, O. The effects of leflunomide on clinical parameters and serum levels of il-6, il-10, mmp-1 and mmp-3 in patients with resistant rheumatoid arthritis. Cytokine 2006, 33, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Rajaiah, R.; Puttabyatappa, M.; Polumuri, S.K.; Moudgil, K.D. Interleukin-27 and interferon-gamma are involved in regulation of autoimmune arthritis. J. Biol. Chem. 2011, 286, 2817–2825. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.W.; Bombardieri, M.; Greenhill, C.J.; McLeod, L.; Nerviani, A.; Rocher-Ros, V.; Cardus, A.; Williams, A.S.; Pitzalis, C.; Jenkins, B.J.; et al. Interleukin-27 inhibits ectopic lymphoid-like structure development in early inflammatory arthritis. J. Exp. Med. 2015, 212, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.


| Features | Pre-Treatment | Post-Treatment | p * | Correlation | |
|---|---|---|---|---|---|
| r | p | ||||
| T28 (IQ range) | 15.5 (12.4–17.8) | 6 (3.7–8.7) | 0.000 | 0.886 | 0.000 |
| SW28 | 8.5 (7.1–10.7) | 1.5 (1.1–2.8) | 0.092 | 0.614 | 0.011 |
| WBC (×109/L) | 5.8 (4.5–6.5) | 6.2 (5.4–6.9) | 0.000 | 0.610 | 0.012 |
| Leukocyte % | 55 (48.9–57.6) | 49 (43.4–50.4) | 0.001 | 0.845 | 0.000 |
| Neutrophile % | 40 (38.5–43.1) | 45 (44–46) | 0.007 | 0.542 | 0.03 |
| PLT (×109/L) | 263 (256–293) | 271 (248–288) | 0.176 | 0.227 | 0.397 |
| Hb (g/L) | 119 (113.8–125.2) | 117 (111.2–122.4) | 0.000 | 0.828 | 0.000 |
| RF, IU/mL | 134.2 (61.3–331.8) | 49 (37–77) | 0.021 | 0.704 | 0.002 |
| Anti-CCP, U/mL | 85.5 (88.7–102.9) | 11.5 (9.7–15.7) | 0.157 | 0.126 | 0.643 |
| ESR, mm/h | 31.5 (27.5–37.3) | 14.5 (12.0–25.0) | 0.015 | 0.252 | 0.347 |
| CRP, mg/L | 22 (16.8–45.3) | 13.5 (12.2–22.4) | 0.004 | 0.415 | 0.11 |
| HAQ | 7 (6.2–7.2) | 2.8 ± 1.1 | 0.000 | 0.893 | 0.000 |
| DAS28 | 5.8 ± 1.2 | 4 (3.3–4.9) | 0.000 | 0.934 | 0.000 |
| Features | Healthy Controls (n = 36) | Rheumatoid Arthritis Group (n = 67) | Active Patients (n = 23) |
|---|---|---|---|
| Age, years | 57.6 (45.5–66.1) | 55.2 (48.1–66.7) | 41 (40.3–44.4) |
| Male/female | 15/21 | 28/39 | 6/17 |
| Disease duration, years | No | 9.6 ± 3.5 | 8 ± 1.5 |
| RF, IU/mL | 12 (1–21) | 311.0 (83.1–1111.0) * | 88 (47.9–24.2) |
| Anti-CCP, U/mL | 7 (0–19) | 111.6 (68.5–186.5) * | 79 (89.5–96.2) |
| ESR, mm/h | 7 (5–15) | 28.1 (15.0–34.2) * | 31 (28.3–35.0) |
| CRP, mg/L | 1.11 (0.4–2.1) | 14.6 (3.6–48.5) * | 17 (15.8–36.1) |
| DAS28 | No | 5.5 ± 1.1 * | 5.8 ± 1.3 * |
| IL-27 (ng/mL) | 6.2 (4.2–8.9) | 10.7 (6.2–11.1) * | 9.4 (8.5–12.2) |
| T28 | 0 | 8 (6.6–13.2) | 14 (12.3–16.5) ● |
| Sw28 | 0 | 5.2 (4.2–9.8) | 9.7 (8.3–10.9) ● |
| WBC (×109) | 5.8 (4.8–7.2) | 5.5 (4.9–6.3) * | 5.2 (4.7–5.9) |
| Leukocyte % | 46 (41.9–53.9) | 47.3 (40.1–48.9) * | 48 (45.9–52.3) |
| Neutrophile % | 58 (50.9–62.3) | 48 (46.9–60.1) * | 45 (43.6–50.6) |
| PLT (×109) | 302 (290.3–311.3) | 285 (265.3–288.2) * | 278 (270.1–292.5) |
| Hb (g/L) | 127.3 (120.4–131.5) | 118 (117.6–123.3) * | 115 (111.9–119.7) |
| HAQ | 0 | 6 (2.7–6.5) | 7 (4.7–5.6) |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, X.; Wang, H.; Cao, J.; Li, Y.; Dai, Y.; Xiang, Y.; Zhang, L. Circulating IL-27 Is Elevated in Rheumatoid Arthritis Patients. Molecules 2016, 21, 1565. https://doi.org/10.3390/molecules21111565
Lai X, Wang H, Cao J, Li Y, Dai Y, Xiang Y, Zhang L. Circulating IL-27 Is Elevated in Rheumatoid Arthritis Patients. Molecules. 2016; 21(11):1565. https://doi.org/10.3390/molecules21111565
Chicago/Turabian StyleLai, Xiaofei, Hongxu Wang, Ju Cao, Ying Li, Yubing Dai, Yu Xiang, and Liping Zhang. 2016. "Circulating IL-27 Is Elevated in Rheumatoid Arthritis Patients" Molecules 21, no. 11: 1565. https://doi.org/10.3390/molecules21111565





