The Protective Effects of Icariin against the Homocysteine-Induced Neurotoxicity in the Primary Embryonic Cultures of Rat Cortical Neurons
Abstract
:1. Introduction
2. Results
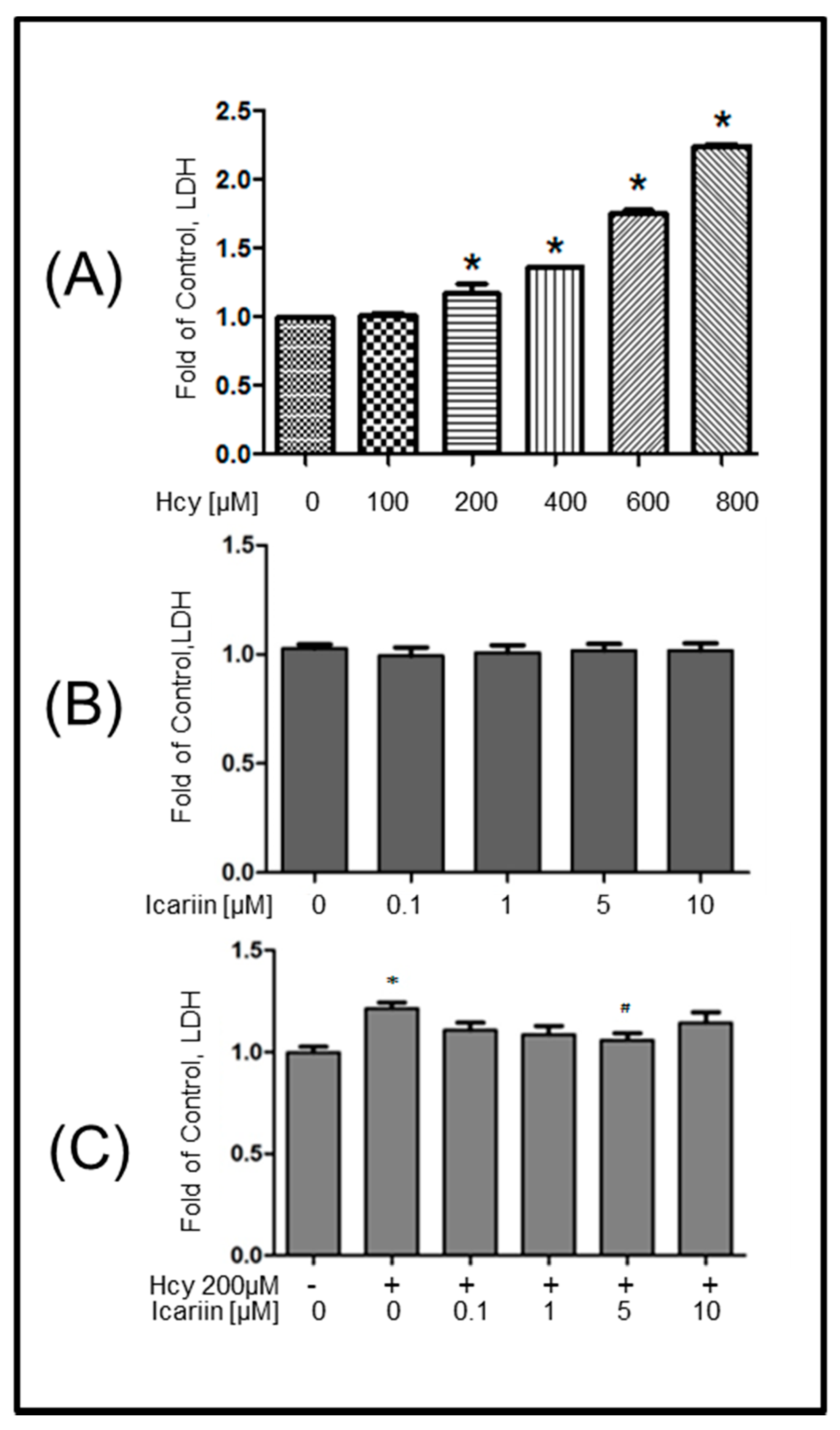
2.1. Icariin Protected Primary Embryonic Rat Cortical Neurons from Hcy-Induced Cytotoxicity
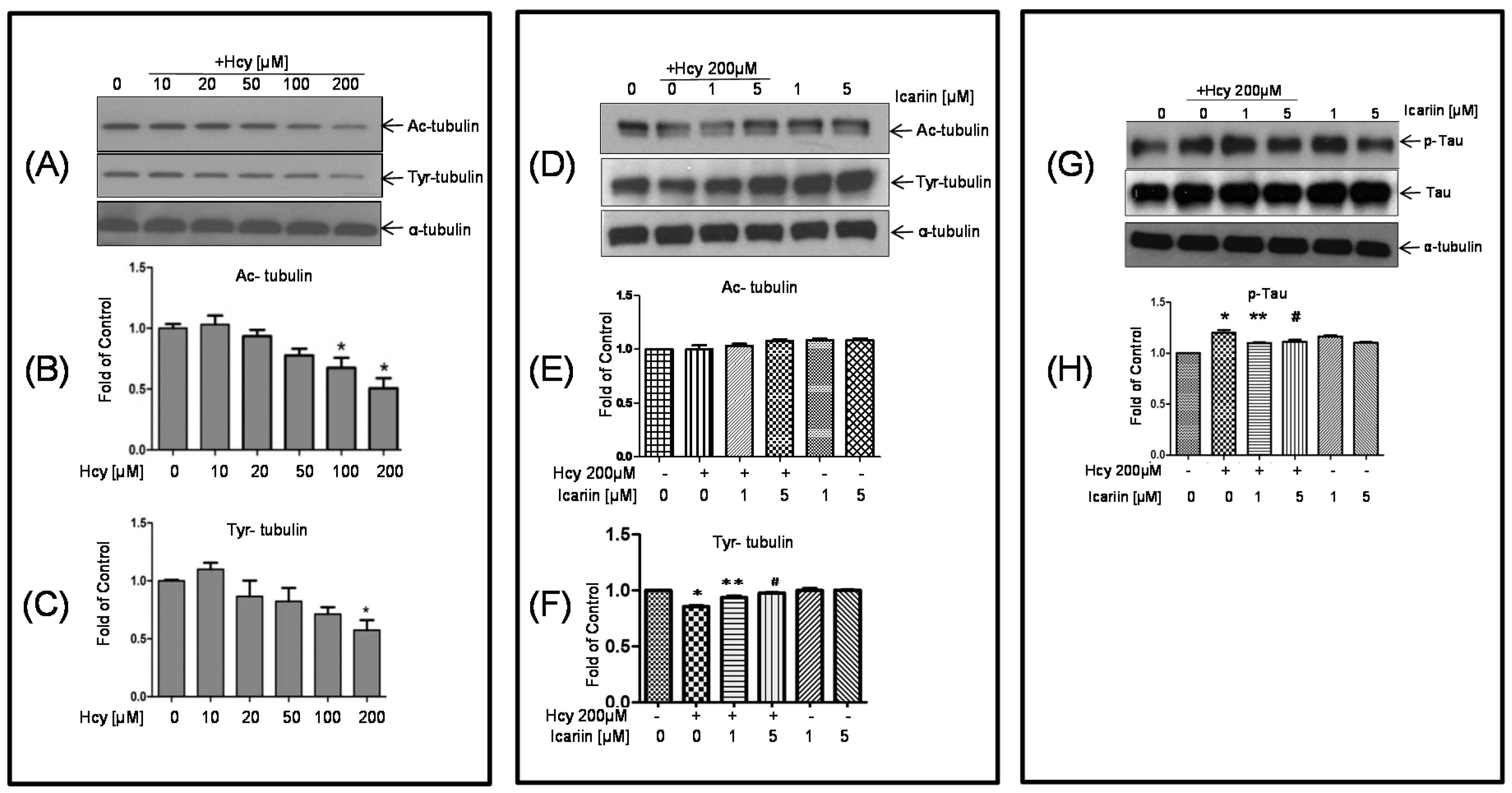
2.2. Icariin Restored the Hcy-Induced Acetylation and Phosphorylation of Tubulin in the Cultured Neuronal Cells
2.3. Icariin Decreased the Level of p-Tau Which Was Deregulated by Hcy Treatment
2.4. Immunohistochemical (IHC) Staining of Tubulin and Tau Proteins in Cortical Neuronal Cultures under the Treatments of Hcy and Icariin
2.5. Icariin Counteracts the Effects of Hcy on ERK, and AKT Signaling Molecules
2.6. Using RT2 Profiler PCR Array to Investigate the Expression Profiles in Neuronal Cells under Icariin/Hcy Treatments
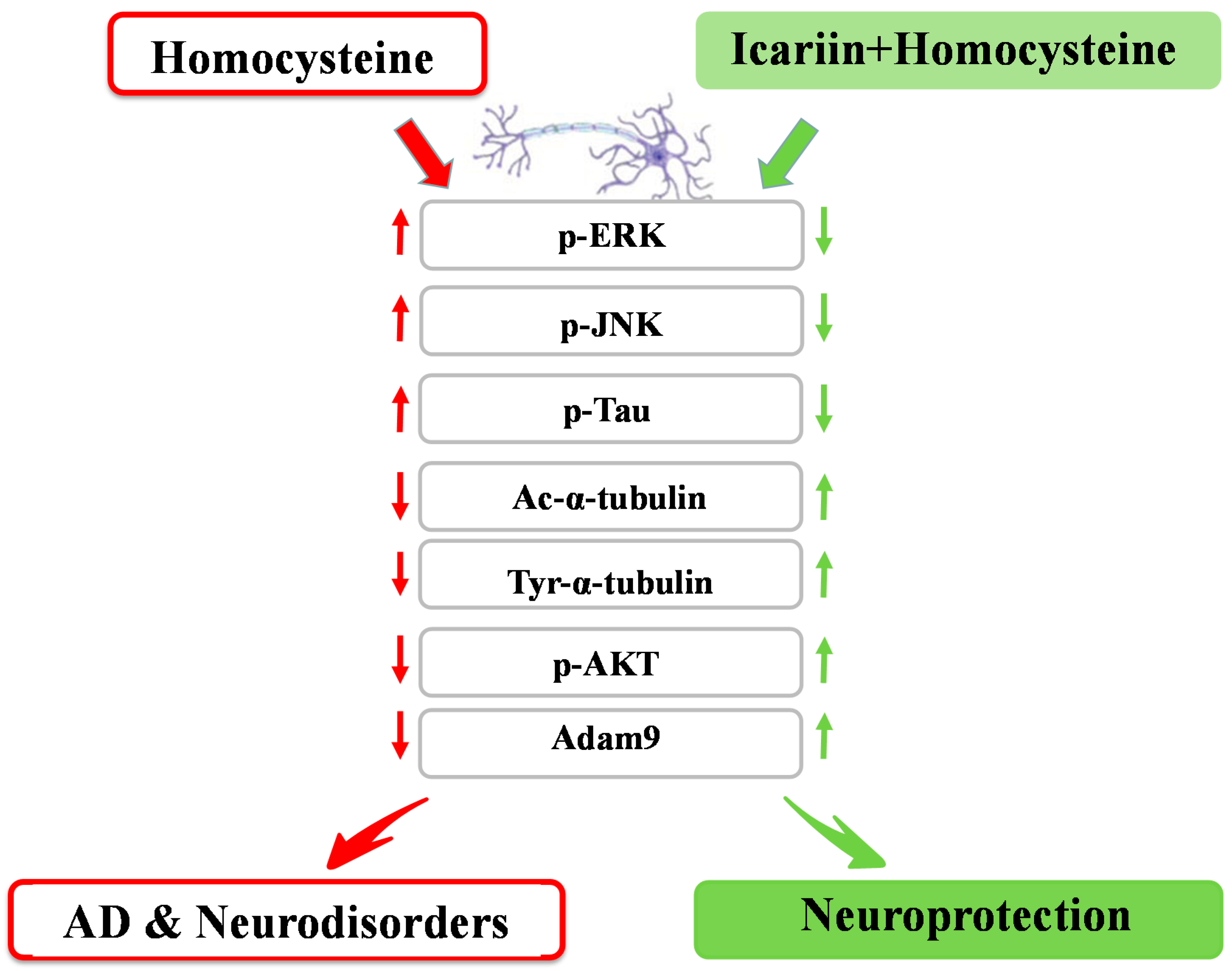
3. Discussion
4. Methods and Materials
4.1. Reagents
4.2. Preparation of Primary Embryonic Culture of Rat Cortical Neurons and Hcy/Icariin Treatments
4.3. LDH Cytotoxicity Assay
4.4. Protein Preparation for Western Blot Analysis
4.5. Immunohistochemical (IHC) Staining
4.6. RT2 Profiler PCR Arrays Analysis
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
| Gene Name | Gene Full Name | Primer Sequence |
|---|---|---|
| Adam9 | ADAM metallopeptidase domain 9 | F: 5’-aattaactgcattagtgtaa-3’ |
| R: 5’-cctgcttctc tgtgtactaa-3’ | ||
| Apbb2 | Amyloid β (A4) precursor protein-binding, family B, member 2 | F: 5’-aatgtgaagcgaggggtctt-3’ |
| R: 5’-gggcttctttagtgtagcta-3’ | ||
| BDNF | Brain-derived neurotrophic factor | F: 5’-cgccactccgaccccgcccg-3’ |
| R: 5’-cctgcagccttccttcgtgt -3’ | ||
| Cd8b | CD8b molecule | F: 5’-gactccttcatccctgctgg-3’ |
| R: 5’-tcctttggttgaagtccggg-3’ | ||
| Ldha | Lactate dehydrogenase A | F: 5’-gggccattggcctctccgtg-3’ |
| R: 5’-ggatgcacccgcctaaggtt-3’ | ||
| Rplp1 | Ribosomal protein, large, P1 | F: 5’-gcaccgtgccggcagtccac-3’ |
| R: 5’-gagggcggagtagatgcagg-3’ | ||
| β-actin | Actin, beta | F: 5’-cagaaggagattactgccct-3’ |
| R: 5‘-acatctgctggaaggtggac-3’ |
References
- Selhub, J. Homocysteine metabolism. Annu. Rev. Nutr. 1999, 19, 217–246. [Google Scholar] [CrossRef] [PubMed]
- Jacques, P.F.; Rosenberg, I.H.; Rogers, G.; Selhub, J.; Bowman, B.A.; Gunter, E.W.; Wright, J.D.; Johnson, C.L. Serum total homocysteine concentrations in adolescent and adult Americans: Results from the third National Health and Nutrition Examination Survey. Am. J. Clin. Nutr. 1999, 69, 482–489. [Google Scholar] [PubMed]
- Selhub, J.; Jacques, P.F.; Wilson, P.W.; Rush, D.; Rosenberg, I.H. Vitamin status and intake as primary determinants of Hyperhomocysteinemia in an elderly population. JAMA 1993, 270, 2693–2698. [Google Scholar] [CrossRef] [PubMed]
- Hankey, G.J.; Eikelboom, J.W. Homocysteine and stroke. Lancet 2005, 365, 194–196. [Google Scholar] [CrossRef]
- Bots, M.L.; Launer, L.J.; Lindemans, J.; Hofman, A.; Grobbee, D.E. Homocysteine, atherosclerosis and prevalent cardiovascular disease in the elderly: The Rotterdam Study. J. Intern. Med. 1997, 242, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Bots, M.L.; Launer, L.J.; Lindemans, J.; Hoes, A.W.; Hofman, A.; Witteman, J.C.; Koudstaal, P.J.; Grobbee, D.E. Homocysteine and short-term risk of myocardial infarction and stroke in the elderly: The Rotterdam Study. Arch. Intern. Med. 1999, 159, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Ansari, R.; Mahta, A.; Mallack, E.; Luo, J.J. Hyperhomocysteinemia and neurologic disorders: A review. J. Clin. Neurol. 2014, 10, 281–228. [Google Scholar] [CrossRef] [PubMed]
- McCaddon, A.; Davies, G.; Hudson, P.; Tandy, S.; Cattell, H. Total serum homocyeine in senile dementia of Alzheimer type. Int. J. Geriatr. Psychiatr. 1998, 13, 235–239. [Google Scholar] [CrossRef]
- Ravaglia, G.; Forti, P.; Maioli, F.; Martelli, M.; Servadei, L.; Brunetti, N.; Porcellini, E.; Licastro, F. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am. J. Clin. Nutr. 2005, 82, 636–643. [Google Scholar] [PubMed]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Hassin-Baer, S.; Cohen, O.; Vakil, E.; Sela, B.A.; Nitsan, Z.; Schwartz, R.; Chapman, J.; Tanne, D. Plasma homocysteine levels and Parkinson disease: Disease progression, carotid intima-media thickness and neuropsychiatric complications. Clin. Neuropharmacol. 2006, 29, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.P.; McCaul, K.; Hankey, G.J.; Norman, P.; Jamrozik, K.; Flicker, L. Homocysteine and depression in later life. Arch. Gen. Psychiatr. 2008, 65, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, N.; Piperi, C.; Salonicioti, A.; Psarra, V.; Gazi, F.; Papadimitriou, A.; Lea, R.W.; Kalofoutis, A. Correlation of folate, vitamin B12 and homocysteine plasma levels with depression in an elderly Greek population. Clin. Biochem. 2007, 40, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Akabane, E.R.; Nanami, M.; Kiyobayashi, Y.; Moriguchi, R.; Hasuike, Y.; Otaki, Y.; Miyagawa, K.; Itahana, R.; Izumi, M. Comparison of cytotoxicity of cysteine and homocysteine for renal epithelial cells. Nephron. Exp. Nephrol. 2005, 100, e11–e20. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Caldwell, R.B.; Li-Masters, T.; Caldwell, R.W. Homocysteine induces endothelial dysfunction via inhibition of arginine transport. J. Physiol. Pharmacol. 2007, 58, 191–206. [Google Scholar] [PubMed]
- Zieminska, E.; Lazarewicz, J.W. Excitotoxic neuronal injury in chronic homocysteine neurotoxicity studied in vitro: The role of NMDA and group I metabotropic glutamate receptors. Acta Neurobiol. Exp. (Wars.) 2006, 66, 301–309. [Google Scholar] [PubMed]
- Quadri, P.; Fragiacomo, C.; Pezzati, R.; Zanda, E.; Tettamanti, M.; Lucca, U. Homocysteine and B vitamins in mild cognitive impairment and dementia. Clin. Chem. Lab. Med. 2005, 43, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Smith, A.D.; Jobst, K.A.; Refsum, H.; Sutton, L.; Ueland, P.M. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch. Neurol. 1998, 55, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Ferri, R.; Bella, R.; Alagona, G.; Carnemolla, A.; Pennisi, G. Homocysteine, vitamin B12 and folate in vascular dementia and in Alzheimer disease. Clin. Chem. Lab. Med. 2004, 42, 1032–1035. [Google Scholar] [CrossRef] [PubMed]
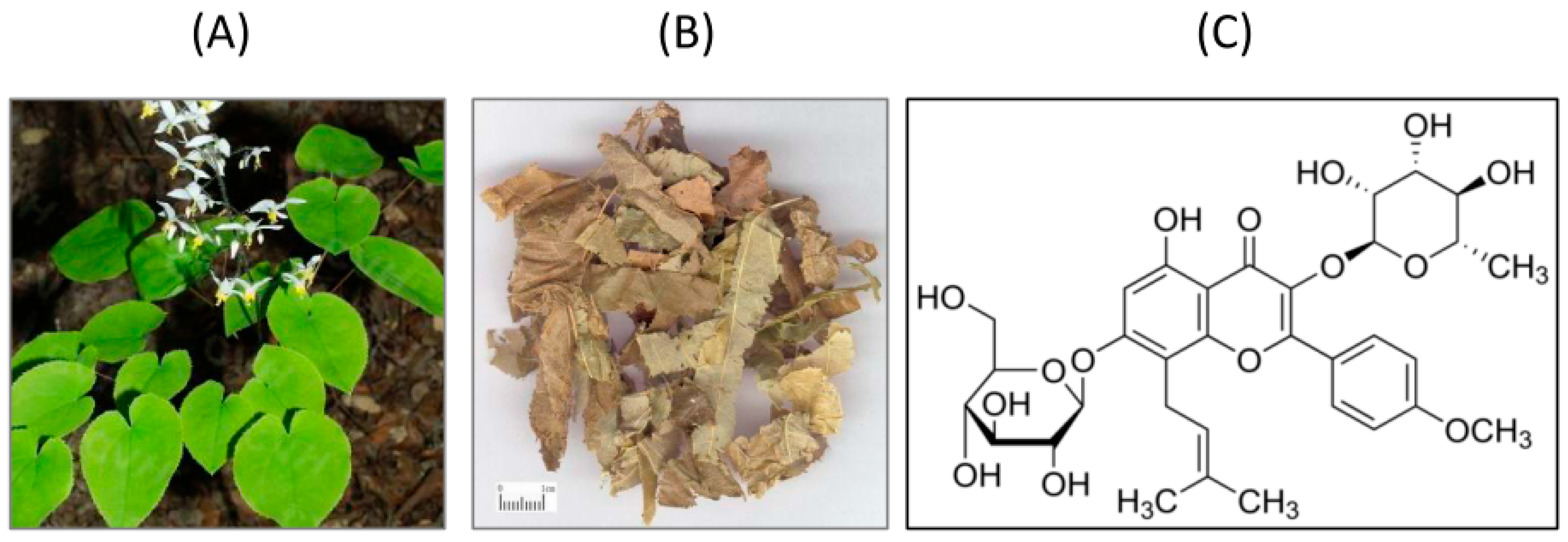
- Wu, H.; Lien, E.J.; Lien, L.L. Chemical and pharmacological investigations of Epimedium species: A survey. Prog. Drug Res. 2003, 60, 1–57. [Google Scholar] [PubMed]
- Liang, H.R.; Vuorela, P.; Vuorela, H.; Hiltunen, R. Isolation and immunomodulatory effect of flavonol glycosides from Epimedium hunanense. Planta Med. 1997, 63, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Makarova, M.N.; Pozharitskaya, O.N.; Shikov, A.N.; Tesakova, S.V.; Makarov, V.G.; Tikhonov, V.P. Effect of lipid-based suspension of Epimedium koreanum Nakai extract on sexual behavior in rats. J. Ethnopharmacol. 2007, 114, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, J.C.; Chen, Y.; Huang, F.; Yang, M.S.; Xiao, P.G. Comparison of antioxidative and antitumor activities of six flavonoids from Epimedium koreanum. Zhongguo Zhong Yao Za Zhi. 2007, 32, 715–718. (In Chinese) [Google Scholar] [PubMed]
- Bao, J.L.; Hong, Y.Z.; Chang, Q.X.; Guang, Y.; Jiang, T.; Jian, H.H.; Jin, F.W.; Xiao, H.D.; Yu, X.C.; Jing, C.D. Neuroprotective effects of icariin on corticosterone-induced apoptosis in primary cultured rat hippocampal neurons. Brain Res. 2011, 1375, 59–67. [Google Scholar]
- Luo, Y.; Nie, J.; Gong, Q.H.; Lu, Y.F.; Wu, Q.; Shi, J.S. Protective effects of icariin against learning and memory deficits induced by aluminium in rats. Clin. Exp. Pharmacol. Physiol. 2007, 34, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, F.; Wu, Q.; Gong, Q.; Lu, Y.; Shi, J. Protective effects of icariin on brain dysfunction induced by lipopolysaccharide in rats. Phytomedicine 2010, 17, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Gong, Q.H.; Xu, Y.S.; Wang, L.N.; Jin, H.; Li, F.; Li, L.S.; Ma, Y.M.; Shi, J.S. Icariin, a phoshphodiesterase-5 inhibitor, improves learning and memory in APP/PS1 transgenic mice by stimulation of NO/cGMP signaling. Int. J. Neuropsychopharmacol. 2014, 17, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, Y.; Yang, M.; Shen, Z.; Zhang, X.; Zhang, W.; Li, H. LC–MS/MS method for the simultaneous determination of icariin and its major metabolites in rat plasma. J. Pharm. Biomed. Anal. 2007, 45, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Janke, C.; Bulinski, J.C. Post-translational regulation of the microtubule cytoskeleton: Mechanisms and function. Nat. Rev. Mol. Cell Biol. 2011, 12, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.E.; Tian, Q.; Wei, W.; Peng, J.H.; Liu, G.P.; Zhou, X.W.; Wang, Q.; Wang, D.W.; Wang, J.Z. Homocysteine induces tau phosphorylation by inactivating protein phosphatase 2A in rat hippocampus. Neurobiol. Aging 2007, 29, 1654–1665. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhou, X.; Yang, X.; Wang, J.Z. Homocysteine induces tau hyperphosphorylation in rats. Neuroreport 2007, 18, 2005–2008. [Google Scholar] [CrossRef] [PubMed]
- Sontag, E.; Nunbhakdi-Craig, V.; Sontag, J.M.; az-Arrastia, R.; Ogris, E.; Dayal, S.; Lentz, S.R.; Arning, E.; Bottiglieri, T. Protein phosphatase 2A methyltransferase links homocysteine metabolism with tau and amyloid precursor protein regulation. J. Neurosci. 2007, 27, 2751–2759. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Hasegawa, M.; Jakes, R.; Lawler, S.; Cuenda, A.; Cohen, P. Phosphorylation of microtubule-associated protein tau by stress-activated protein kinases. FEBS Lett. 1997, 409, 57–62. [Google Scholar] [CrossRef]
- Reynolds, C.H.; Utton, M.A.; Gibb, G.M.; Yates, A.; Anderton, B.H. Stress-activated protein kinase/c-jun N-terminal kinase phosphorylates tau protein. J. Neurochem. 1997, 68, 1736–1744. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.H.; Betts, J.C.; Blackstock, W.P.; Nebreda, A.R.; Anderton, B.H. Phosphorylation sites on tau identified by nanoelectrospray mass spectrometry: Differences in vitro between the mitogen-activated protein kinases ERK2, c-Jun N-terminal kinase and P38, and glycogen synthase kinase-3β. J. Neurochem. 2000, 74, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, B.; Wu, J.; Xu, C.; Tao, J.; Duan, X.; Cao, Y.; Dong, J. Icariin inhibits corticosterone-induced apoptosis in hypothalamic neurons via the PI3-K/Akt signaling pathway. Mol. Med. Rep. 2012, 6, 967–972. [Google Scholar] [PubMed]
- Golde, T.D. Alzheimer disease therapy: Can the amyloid cascade be halted? J. Clin. Investig. 2003, 111, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, L.C.; Doody, R.; Clark, C. Disease-modifying therapies for Alzheimer disease. Neurology 2007, 69, 1622–1634. [Google Scholar]
- Pacheco-Quinto, J.; de Turco, E.B.R.; DeRosa, S.; Howard, A.; Cruz-Sanchez, F.; Sambamurti, K.; Refolo, L.; Petanceska, S.; Pappolla, M.A. Hyperhomocysteinemia Alzheimer’s mouse model of amyloidosis shows increased brain amyloid β peptide levels. Neurobiol. Dis. 2006, 22, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, B.; Polvikoski, T.; Kivipelto, M.; Tanskanen, M.; Myllykangas, L.; Erkinjuntti, T.; Mäkelä, M.; Oinas, M.; Paetau, A.; Scheltens, P.; et al. Plasma homocysteine, Alzheimer and cerebrovascular pathology: A population-based autopsy study. Brain 2013, 136, 2707–2716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.E.; Wei, W.; Liu, Y.H.; Peng, J.H.; Tian, Q.; Liu, G.P.; Zhang, Y.; Wang, J.Z. Hyperhomocysteinemia increases β-amyloid by enhancing expression of gamma-secretase and phosphorylation of amyloid precursor protein in rat brain. Am. J. Pathol. 2009, 174, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Badding, M.A.; Dean, D.A. Highly acetylated tubulin permits enhanced interactions with and trafficking of plasmids along microtubules. Gene Ther. 2013, 20, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Wang, Z.; Liu, L.; Jian, X. Inhibiting histone deacetylase 6 partly protects cultured rat cortical neurons from oxygen-glucose deprivation-induced necroptosis. Mol. Med. Rep. 2015, 12, 2661–2667. [Google Scholar] [CrossRef] [PubMed]
- Saragoni, L.; Hernández, P.; Maccioni, R.B. Differential association of tau with subsets of microtubules containing posttranslationally-modified tubulin variants in neuroblastoma cells. Neurochem. Res. 2000, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.F.; Courtneidge, S.A. The ADAMs family of metalloproteases: Multidomain proteins with multiple functions. Genes Dev. 2003, 17, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L.; Chen, Z.-B.; Wu, J.Y.; Zhang, X.; Xu, Y. Icariin enhances neuronal survival after oxygen and glucose deprivation by increasing SIRT1. Eur. J. Pharmacol. 2009, 609, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Chaterjee, P.; Sharma, P.K.; Singh, A.K.; Gupta, A.; Gill, K.; Tripathi, M.; Dey, A.B.; Dey, S. Sirtuin 1: A promising serum protein marker for early detection of Alzheimer’s disease. PLoS ONE 2013, 8, e61560. [Google Scholar]
- Sample Availability: The herb is commercially available.


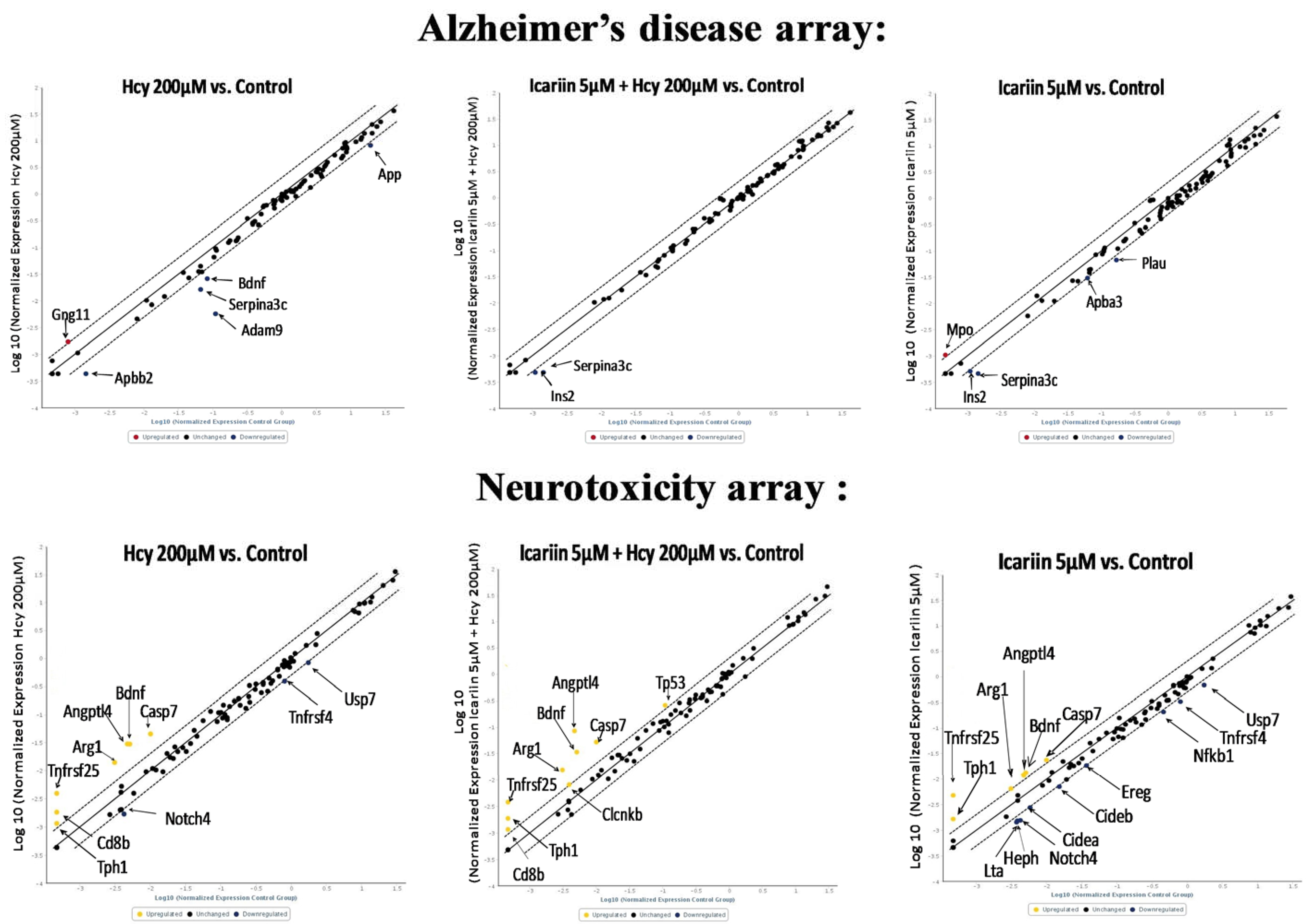
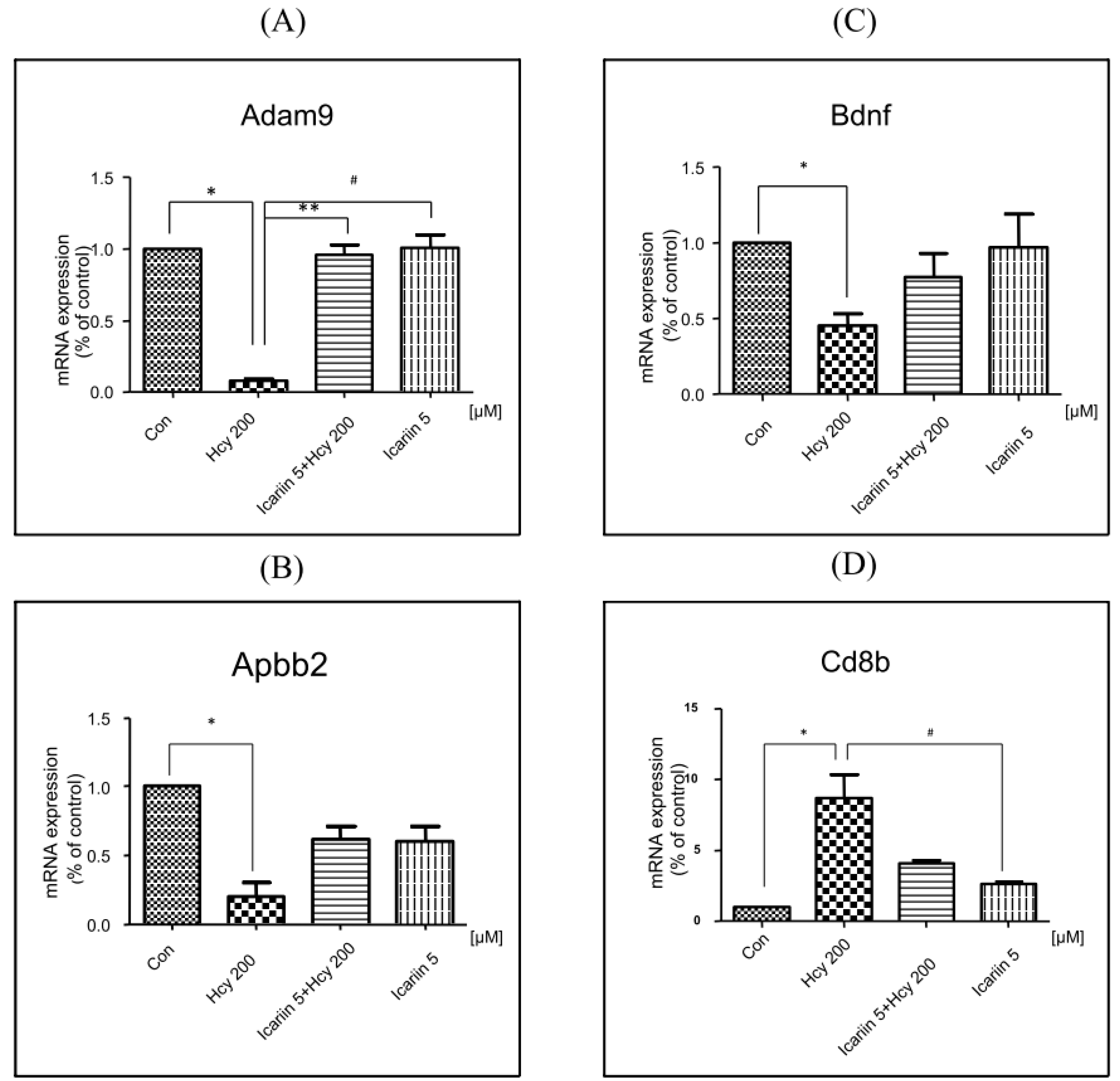
| Alzheimer’s Disease Array (Fold Change) | |||
| Gene Name | Hcy | Icariin + Hcy | Icariin |
| Gng11 | 2.61 | 1 | 1 |
| Adam9 | −15.66 | 1 | 1 |
| Apbb2 | −3.32 | 1 | 1 |
| Serpina3c | −2.74 | −3.08 | −2.7 |
| Bdnf | −2.6 | 1 | 1 |
| Ins2 | 1 | −2.35 | 1 |
| Mpo | 1 | 1 | 2.59 |
| Plau | 1 | 1 | −2.15 |
| Neurotoxicity Array (Fold Change) | |||
| Gene Name | Hcy | Icariin + Hcy | Icariin |
| Angptl4 | 6.35 | 17.98 | 2.49 |
| Arg1 | 4.49 | 4.92 | 2.08 |
| Bdnf | 5.83 | 6.57 | 2.57 |
| Casp7 | 4.50 | 5.17 | 2.32 |
| Cd8b | 3.90 | 2.48 | 1 |
| Tnfrsf25 | 8.42 | 8.12 | 10.20 |
| Tph1 | 2.47 | 4.00 | 3.52 |
| Notch4 | −2.49 | 1 | −2.71 |
| Trpm4 | −2.07 | 1 | −2.45 |
| Usp7 | −2.11 | 1 | −2.57 |
| Clcnkb | 1 | 2.09 | 1 |
| Tp53 | 1 | 2.38 | 1 |
| Cidea | 1 | 1 | −2.10 |
| Cideb | 1 | 1 | −2.14 |
| Ereg | 1 | 1 | −2.04 |
| Heph | 1 | 1 | −2.51 |
| Lta | 1 | 1 | −2.61 |
| Nfkb1 | 1 | 1 | −2.24 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.-A.; HO, Y.-S.; Chen, L.; Hsiao, W.L.W. The Protective Effects of Icariin against the Homocysteine-Induced Neurotoxicity in the Primary Embryonic Cultures of Rat Cortical Neurons. Molecules 2016, 21, 1557. https://doi.org/10.3390/molecules21111557
Li X-A, HO Y-S, Chen L, Hsiao WLW. The Protective Effects of Icariin against the Homocysteine-Induced Neurotoxicity in the Primary Embryonic Cultures of Rat Cortical Neurons. Molecules. 2016; 21(11):1557. https://doi.org/10.3390/molecules21111557
Chicago/Turabian StyleLi, Xiao-Ang, Yuen-Shan HO, Lei Chen, and W.L. Wendy Hsiao. 2016. "The Protective Effects of Icariin against the Homocysteine-Induced Neurotoxicity in the Primary Embryonic Cultures of Rat Cortical Neurons" Molecules 21, no. 11: 1557. https://doi.org/10.3390/molecules21111557





