Mono-PEGylation of Alpha-MMC and MAP30 from Momordica charantia L.: Production, Identification and Anti-Tumor Activity
Abstract
:1. Introduction
2. Results and Discussion
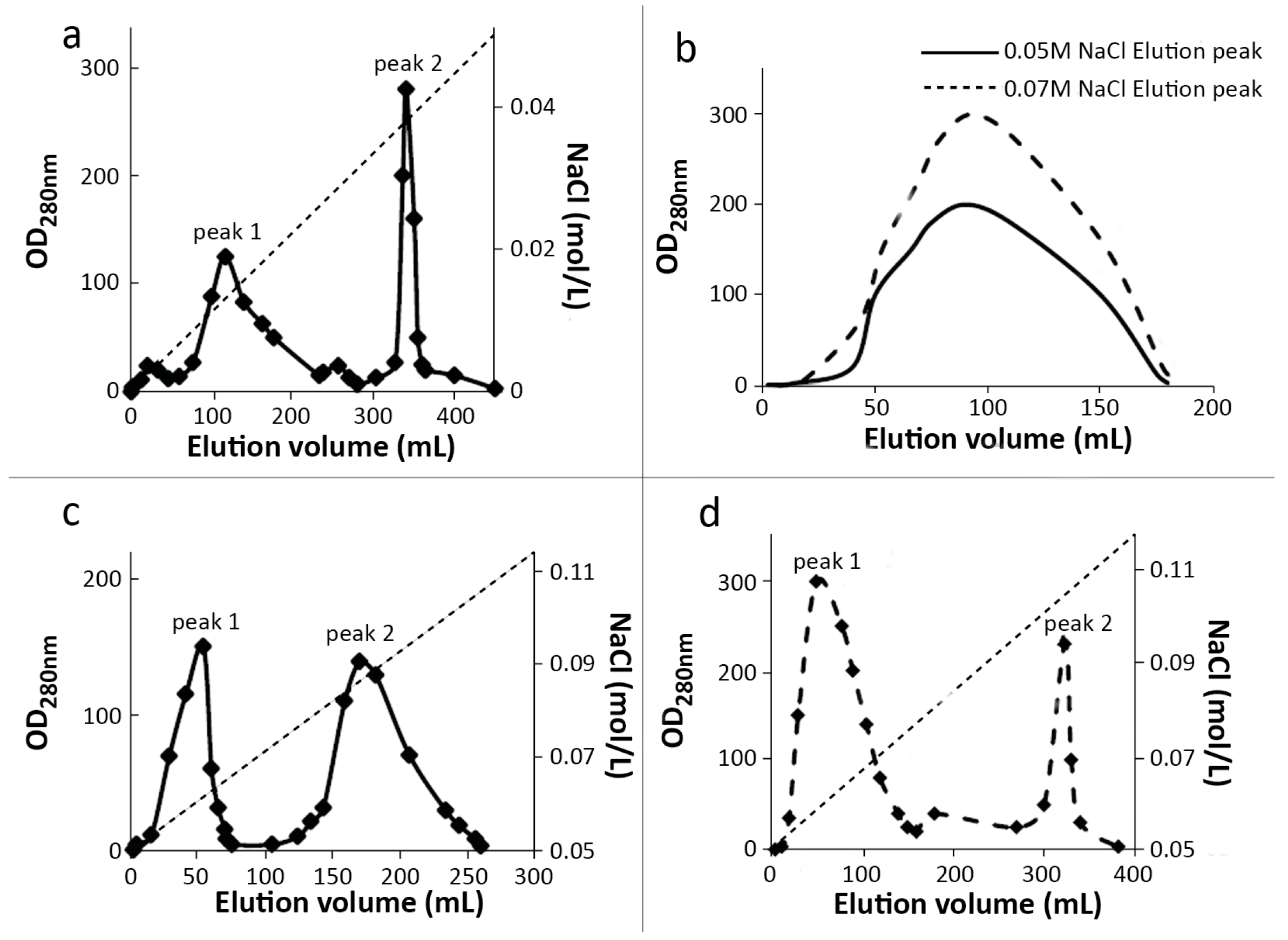
2.1. Preparation and Purification of mPEG-SC-RIPs
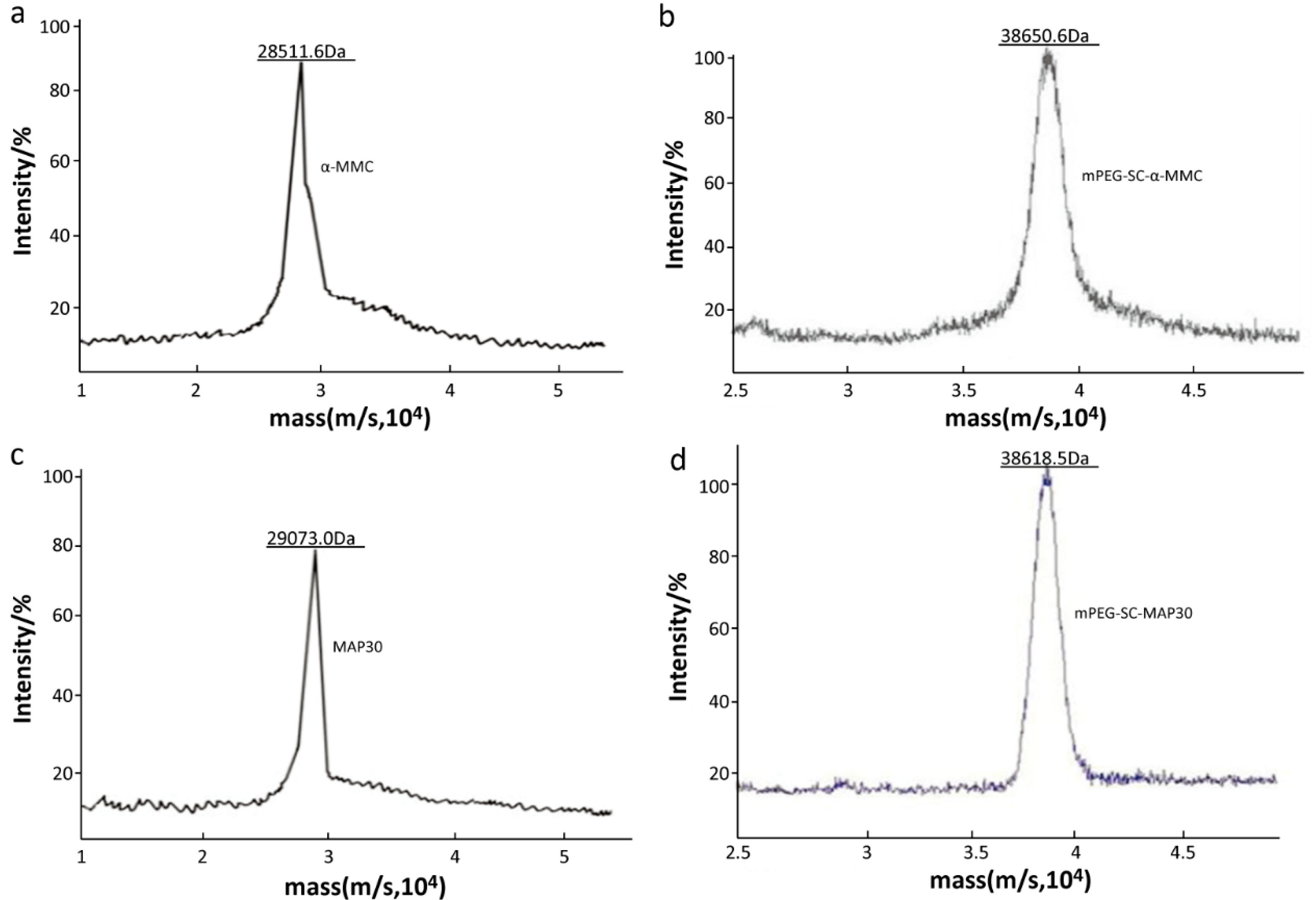
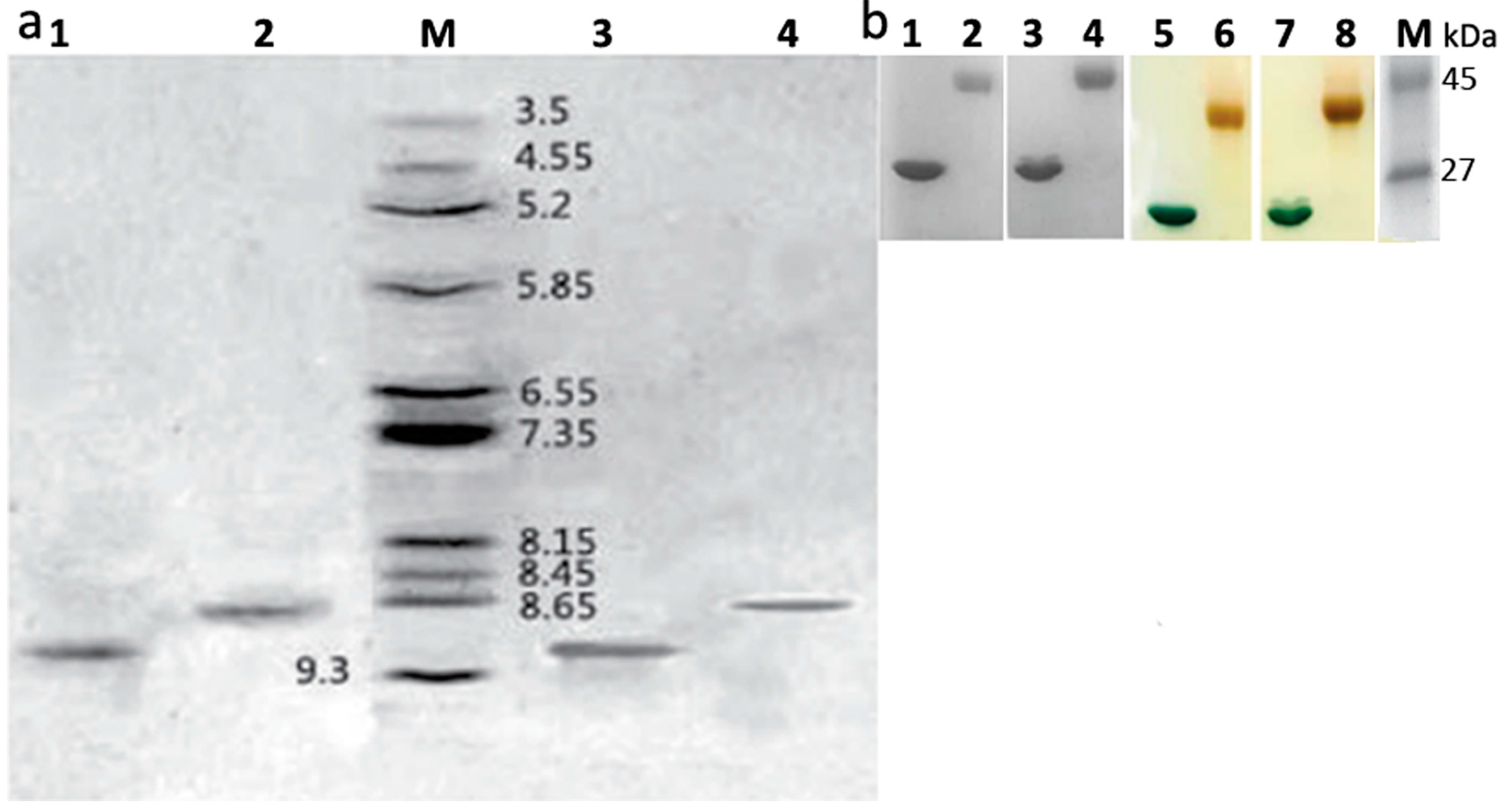
2.2. Identification of Mono-PEGylated RIPs
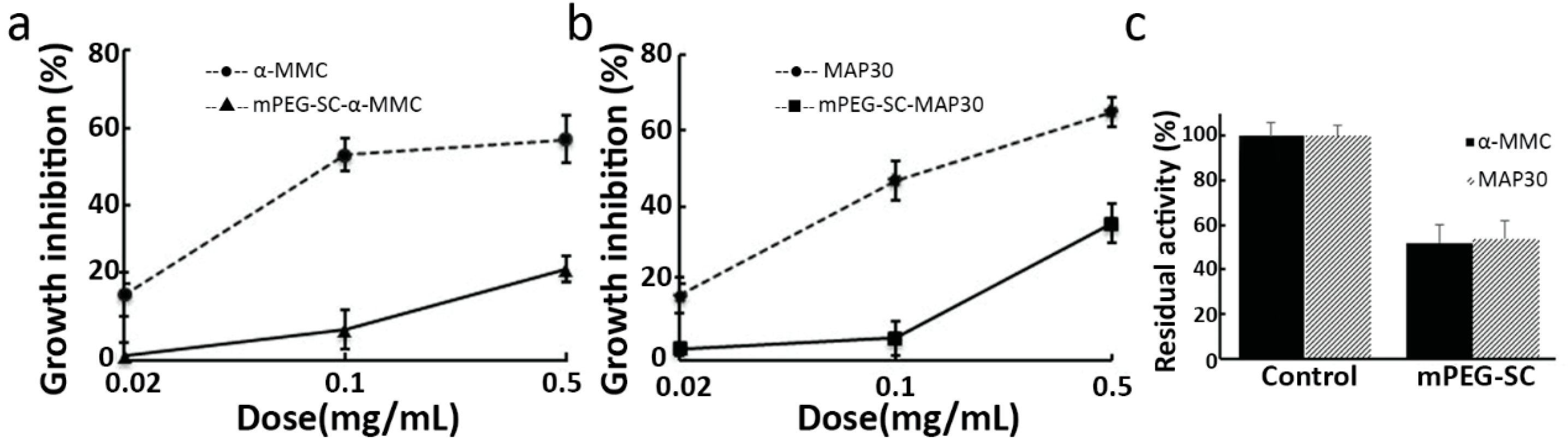
2.3. Inhibitory effect of RIPs and mPEGylated RIPs on A549 Cells
3. Materials and Methods
3.1. Materials and Reagents
3.2. Chemical Synthetic Procedure of Mono-mPEG-RIPs
3.3. Isolation and Purification of Mono-mPEGylated
3.3.1. MacroCap SP
3.3.2. SP-Sepharose FF
3.4. Identification of mPEGylated RIPs
3.4.1. Determination of Protein Content
3.4.2. SDS-PAGE in the Absence of α-Mercaptoethanol
3.4.3. Iodine Staining for Detecting Residual PEG
3.4.4. Determination of Isoelectric Points of Samples on IEF-PAGE
3.4.5. The Precise Molecular Weight of Mono-mPEGylated RIPs by MALDI-TOF/TOF
3.5. Inhibitory Effect of Mono-mPEGylated RIPs on A549 Cells
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stirpe, F.; Barbieri, L.; Battelli, M.G.; Soria, M.; Lappi, D.A. Ribosome-inactivating proteins from plants: Present status and future prospects. Nat. Biotechnol. 1992, 10, 405–412. [Google Scholar] [CrossRef]
- De Virgilio, M.; Lombardi, A.; Caliandro, R.; Fabbrini, M.S. Ribosome-inactivating proteins: From plant defense to tumor attack. Toxins (Basel) 2010, 2, 2699–2737. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.C.; Singh, V. Current status of ribosome inactivating proteins. Indian J. Biochem. Biophys. 2000, 37, 1–5. [Google Scholar] [PubMed]
- Fong, W.P.; Wong, R.N.; Go, T.T.; Yeung, H.W. Minireview: Enzymatic properties of ribosome-inactivating proteins (RIPs) and related toxins. Life Sci. 1991, 49, 1859–1869. [Google Scholar] [CrossRef]
- Meng, Y.; Lin, S.; Liu, S.; Fan, X.; Li, G.; Meng, Y. A novel method for simultaneous production of two ribosome-inactivating proteins, alpha-MMC and MAP30, from Momordica charantia L. PLoS ONE 2014, 9, e101998. [Google Scholar]
- Wang, L.; Shen, F.; Zhang, M.; He, Q.; Zhao, H.; Yu, X.; Yang, S.; Liu, Y.; Deng, N.; Zheng, J.; Zhu, L.; Liu, X. Cytotoxicity mechanism of alpha-MMC in normal liver cells through LRP1 mediated endocytosis and JNK activation. Toxicology 2016, 357–358, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Gupta, R.C.; Puri, M. Ribosome inactivating proteins from plants inhibiting viruses. Virol. Sin. 2011, 26, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Shen, F.; Chen, Y.; Wang, B.; Deng, M.; Meng, Y. PEGylation of alpha-momorcharin: Synthesis and characterization of novel anti-tumor conjugates with therapeutic potential. Biotechnol. Lett. 2010, 32, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Liu, S.; Li, J.; Meng, Y.; Zhao, X. Preparation of an antitumor and antivirus agent: Chemical modification of α-MMC and MAP30 from Momordica charantia L. with covalent conjugation of polyethyelene glycol. Int. J. Nanomed. 2012, 7, 3133–3142. [Google Scholar]
- Shang, C.; Rougé, P.; van Damme, E.J. Ribosome Inactivating Proteins from Rosaceae. Molecules 2016, 21, 1105. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Yang, X.Z.; Fu, L.Y.; Lu, Y.T.; Lu, Y.H.; Zhao, J.; Wang, F.J. Recombinant expression and purification of a MAP30-cell penetrating peptide fusion protein with higher anti-tumor bioactivity. Protein Expr. Purif. 2015, 111, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Lee-Huang, S.; Huang, P.L.; Nara, P.L.; Chen, H.C.; Kung, H.F.; Huang, P.; Huang, H.I.; Huang, P.L. MAP 30: A new inhibitor of HIV-1 infection and replication. FEBS Lett. 1990, 272, 12–18. [Google Scholar] [CrossRef]
- Yao, X.; Li, J.; Deng, N.; Wang, S.; Meng, Y.; Shen, F. Immunoaffinity purification of α-momorcharin from bitter melon seeds (Momordica charantia). J. Sep. Sci. 2011, 21, 3092–3098. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, Y.; Liu, Z.; Shen, F.; Bian, X.; Meng, Y. Anti-tumor activity and immunological modification of ribosome-inactivating protein (RIP) from Momordica charantia by covalent attachment of polyethylene glycol. Acta. Biochim. Biophys. Sin. 2009, 41, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Lowery, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1971, 227, 680–685. [Google Scholar] [CrossRef]
- Niepmann, M.; Zheng, J. Discontinuous native protein gel electrophoresis. Electrophoresis 2006, 27, 3949–3951. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Pi, K. Effect of separation dimensions on resolution and throughput using very narrow-range IEF for 2-DE after solution phase isoelectric fractionation of a complex proteome. J. Sep. Sci. 2009, 32, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Budilova´, J.; Pazourek, J.; Kra´sensky, P.; Pospı´chal, J. Continuous mode of operation for large volume dosing in analytical carrier ampholyte-free isoelectric focusing of proteins applied to off-line detection of fractions. J. Sep. Sci. 2006, 29, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Roy, P. Purification and identification of trichloroethylene induced proteins from Stenotrophomonas maltophilia PM102 by immunoaffinity- chromatography and MALDI-TOF Mass spectrometry. SpringerPlus 2013, 1, 207. [Google Scholar] [CrossRef] [PubMed]
- Dozier, J.K.; Distefano, M.D. Site-Specific PEGylation of Therapeutic proteins. Int. J. Mol. Sci. 2015, 16, 25831–25864. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, E.; Di, M.A. A new age for biomedical applications of Ribosome Inactivating Proteins (RIPs): From Bioconjugate to nanoconstructs. J. Biomed. Sci. 2016, 23, 54. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, H. Current status and prospects of polyethyleneglycol-modified medicines. Biol. Pharm Bull. 2013, 36, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.

| Separation Methods | mPEGylation Rate (%) | Yields (%) | ||
|---|---|---|---|---|
| α-MMC | MAP30 | α-MMC | MAP30 | |
| Macro-Cap SP | 37.4 ± 2.0 | 50.1 ± 1.9 | 31.7 ± 2.5 | 53.88 ± 1.8 |
| SP-Sepharose FF | 43.7 ± 1.9 | 48.9 ± 2.4 | 42.87 ± 2.3 | 56.37 ± 2.0 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Sun, F.; Li, J.; Wu, M.; Fan, X.; Meng, Y.; Meng, Y. Mono-PEGylation of Alpha-MMC and MAP30 from Momordica charantia L.: Production, Identification and Anti-Tumor Activity. Molecules 2016, 21, 1457. https://doi.org/10.3390/molecules21111457
Sun Y, Sun F, Li J, Wu M, Fan X, Meng Y, Meng Y. Mono-PEGylation of Alpha-MMC and MAP30 from Momordica charantia L.: Production, Identification and Anti-Tumor Activity. Molecules. 2016; 21(11):1457. https://doi.org/10.3390/molecules21111457
Chicago/Turabian StyleSun, Yun, Fenghui Sun, Jianlong Li, Minlu Wu, Xiang Fan, Yanfa Meng, and Yao Meng. 2016. "Mono-PEGylation of Alpha-MMC and MAP30 from Momordica charantia L.: Production, Identification and Anti-Tumor Activity" Molecules 21, no. 11: 1457. https://doi.org/10.3390/molecules21111457





