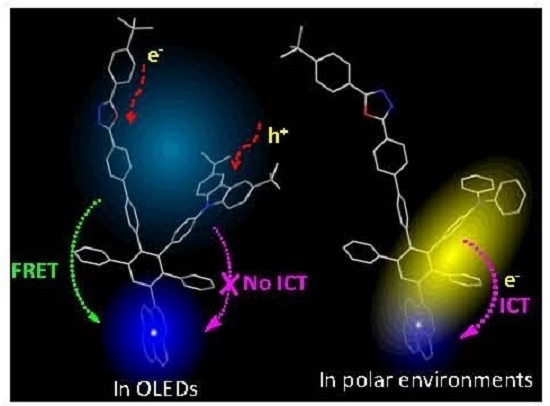
Blue Light Emitting Polyphenylene Dendrimers with Bipolar Charge Transport Moieties
Abstract
:1. Introduction
2. Results and Discussion
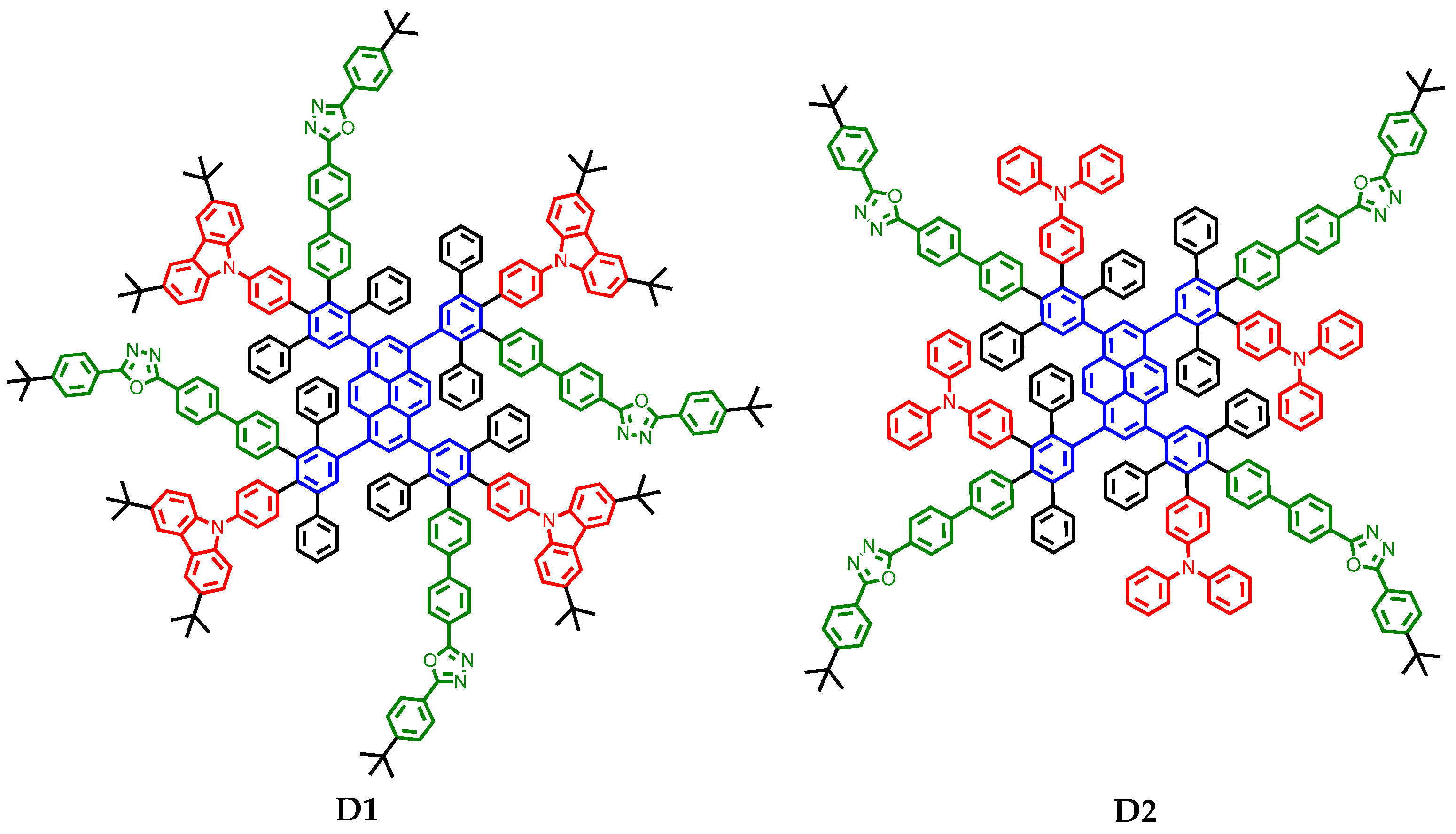
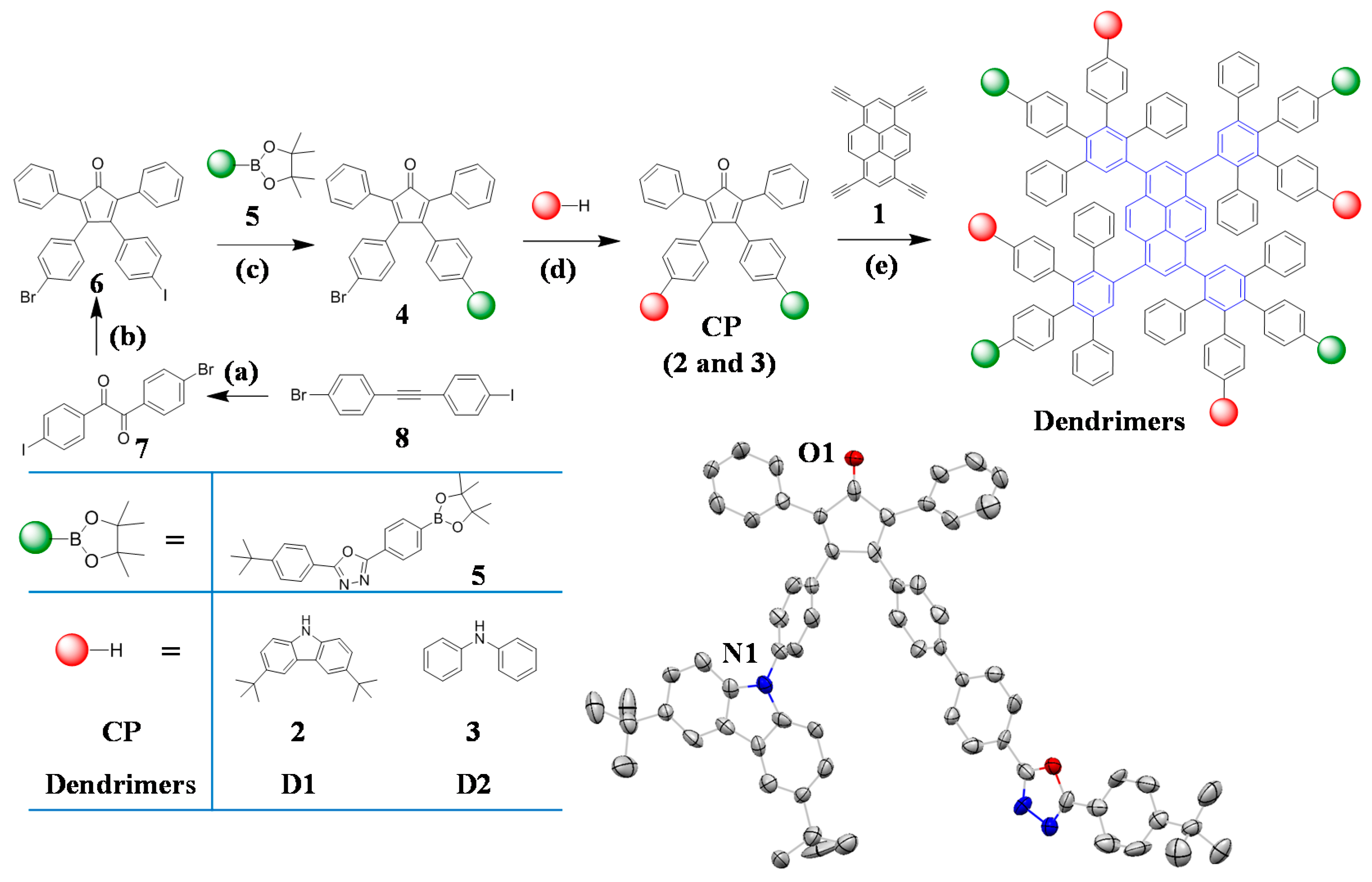
2.1. Synthesis and Structural Characterization
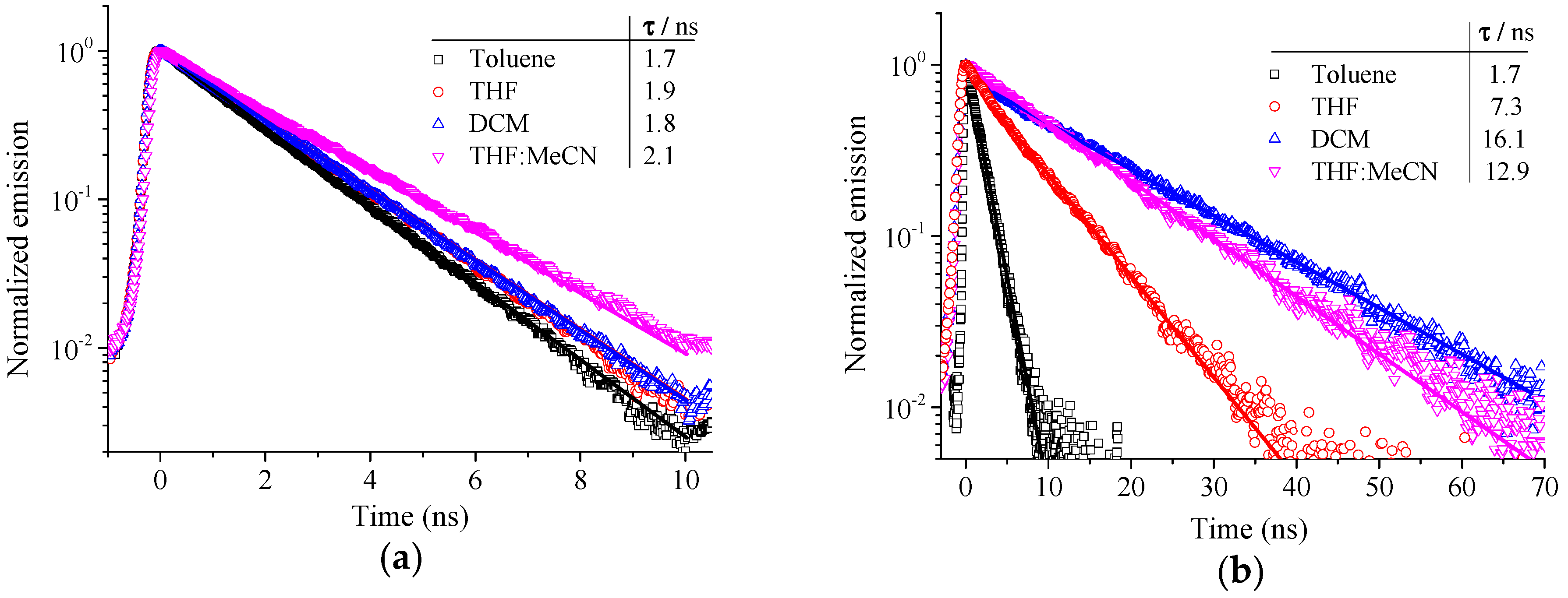
2.2. Photophysical Properties
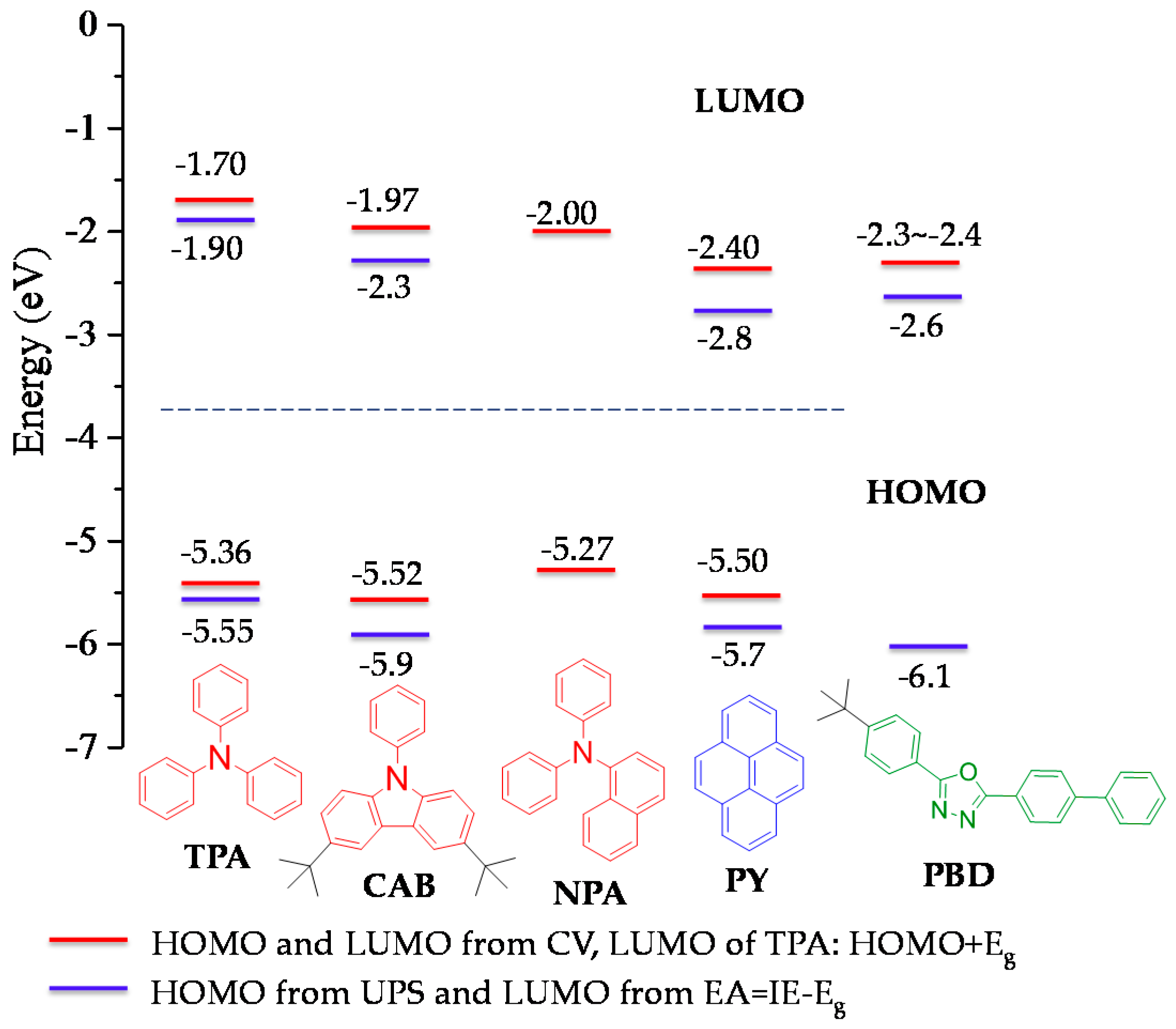
2.3. Electrochemistry
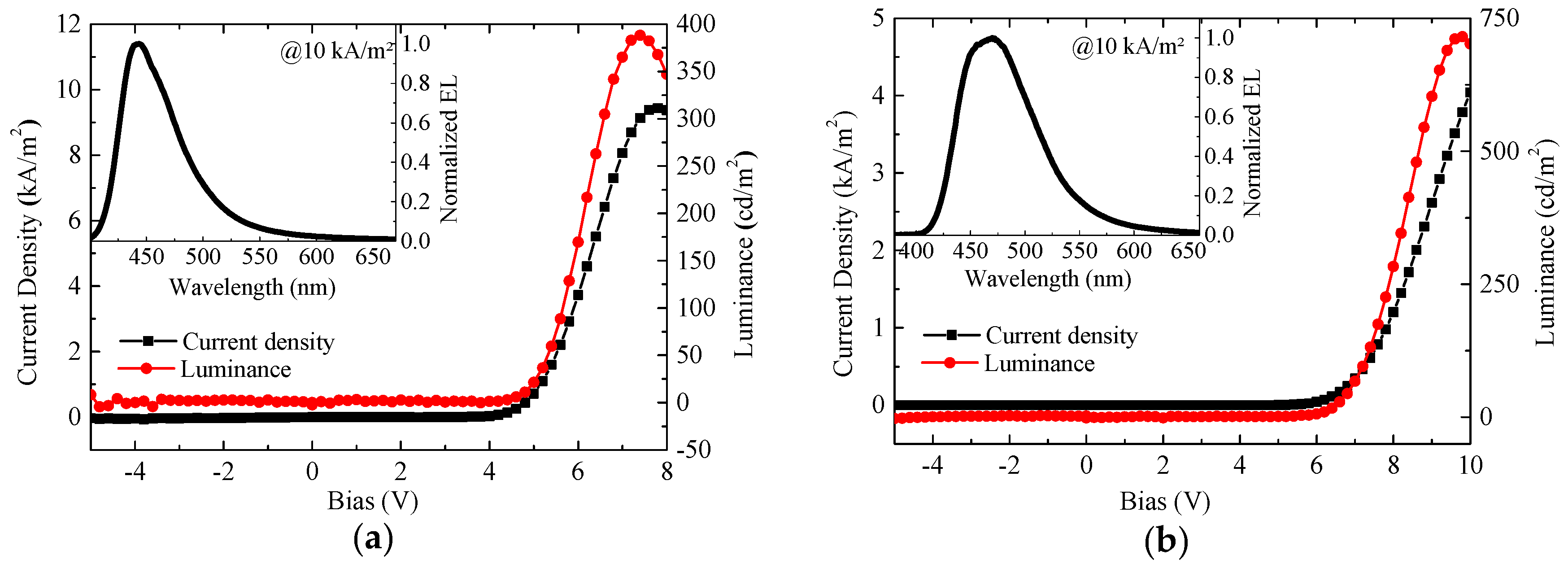
2.4. OLED Device Performances
3. Materials and Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- OLED-LG. Available online: http://www.oled-info.com/lg-oled (accessed on 1 September 2016).
- Samsung, O. Available online:. Available online: http://www.oled-info.com/samsung-oled (accessed on 1 September 2016).
- Fyfe, D. Organic Displays Come of Age. Nat. Photonics 2009, 3, 453–455. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Lee, J.; Forrest, S.R. Tenfold increase in the lifetime of blue phosphorescent organic light-emitting diodes. Nat. Commun. 2014, 5, 5008. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jeon, S.K.; Hwang, S.H.; Lee, J.Y. Stable Blue Thermally Activated Delayed Fluorescent Organic Light-Emitting Diodes with Three Times Longer Lifetime than Phosphorescent Organic Light-Emitting Diodes. Adv. Mater. 2015, 27, 2515–2520. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.O.; Jang, S.E.; Son, H.S.; Lee, J.Y. External Quantum Efficiency Above 20% in Deep Blue Phosphorescent Organic Light-Emitting Diodes. Adv. Mater. 2011, 23, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Hang, X.C.; Fleetham, T.; Turner, E.; Brooks, J.; Li, J. Highly Efficient Blue-Emitting Cyclometalated Platinum(II) Complexes by Judicious Molecular Design. Angew. Chem. Int. Ed. 2013, 52, 6753–6756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.S.; Li, B.; Huang, S.P.; Nomura, H.; Tanaka, H.; Adachi, C. Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence. Nat. Photonics 2014, 8, 326–332. [Google Scholar] [CrossRef]
- Wang, P.W.; Liu, Y.J.; Devadoss, C.; Bharathi, P.; Moore, J.S. Electroluminescent diodes from a single-component emitting layer of dendritic macromolecules. Adv. Mater. 1996, 8, 237–274. [Google Scholar] [CrossRef]
- Burn, P.L.; Lo, S.C.; Samuel, I.D.W. The development of light-emitting dendrimers for displays. Adv. Mater. 2007, 19, 1675–1688. [Google Scholar] [CrossRef]
- Lo, S.C.; Burn, P.L. Development of dendrimers: Macromolecules for use in organic light-emitting diodes and solar cells. Chem. Rev. 2007, 107, 1097–1116. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Moorefield, C.N.; Newkome, G.R. Dendritic macromolecules for organic light-emitting diodes. Chem. Soc. Rev. 2008, 37, 2543–2557. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.B.; Yang, X.L.; Zhao, J.; Zhou, G.J.; Wong, W.Y. Recent Advances in Solution-Processable Dendrimers for Highly Efficient Phosphorescent Organic Light-Emitting Diodes (PHOLEDs). Asian J. Org. Chem. 2015, 4, 394–429. [Google Scholar] [CrossRef]
- Halim, M.; Pillow, J.N.G.; Samuel, I.D.W.; Burn, P.L. Conjugated dendrimers for light-emitting diodes: Effect of generation. Adv. Mater. 1999, 11, 371–374. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, Y.; Luo, J.; Zhou, Y.; Zhou, J.H.; Wang, J.; Pei, J.; Cao, Y. Highly Efficient and Color-Stable Deep-Blue Organic Light-Emitting Diodes Based on a Solution-Processible Dendrimer. Adv. Mater. 2009, 21, 4854–4858. [Google Scholar] [CrossRef] [PubMed]
- Moonsin, P.; Prachumrak, N.; Namuangruk, S.; Jungsuttiwong, S.; Keawin, T.; Sudyoadsuk, T.; Promarak, V. Bifunctional oligofluorene-cored carbazole dendrimers as solution-processed blue emitters and hole transporters for electroluminescent devices. J. Mater. Chem. C 2014, 2, 5540–5552. [Google Scholar] [CrossRef]
- Liu, C.; Fu, Q.; Zou, Y.; Yang, C.L.; Ma, D.G.; Qin, J.G. Low Turn-on Voltage, High-Power-Efficiency, Solution-Processed Deep-Blue Organic Light-Emitting Diodes Based on Starburst Oligofluorenes with Diphenylamine End-Capper to Enhance the HOMO Level. Chem. Mater. 2014, 26, 3074–3083. [Google Scholar] [CrossRef]
- Lo, S.C.; Anthopoulos, T.D.; Namdas, E.B.; Burn, P.L.; Samuel, I.D.W. Encapsulated cores: Host-free organic light-emitting diodes based on solution-processible electrophosphorescent dendrimers. Adv. Mater. 2005, 17, 1945–1948. [Google Scholar] [CrossRef]
- Ding, J.Q.; Gao, J.; Cheng, Y.X.; Xie, Z.Y.; Wang, L.X.; Ma, D.G.; Jing, X.B.; Wang, F.S. Highly efficient green-emitting phosphorescent iridium dendrimers based on carbazole dendrons. Adv. Funct. Mater. 2006, 16, 575–581. [Google Scholar] [CrossRef]
- Kwok, C.C.; Wong, M.S. Synthesis and light-emitting properties of difunctional dendritic distyrylstilbenes. Macromolecules 2001, 34, 6821–6830. [Google Scholar] [CrossRef]
- Huang, W.S.; Lin, C.W.; Lin, J.T.; Huang, J.H.; Chu, C.W.; Wu, Y.H.; Lin, H.C. Highly branched green phosphorescent tris-cyclometalated iridium(III) complexes for solution-processed organic light-emitting diodes. Org. Electron. 2009, 10, 594–606. [Google Scholar] [CrossRef]
- Qin, T.S.; Ding, J.Q.; Baumgarten, M.; Wang, L.X.; Mullen, K. Red-Emitting Dendritic Iridium(III) Complexes for Solution Processable Phosphorescent Organic Light-Emitting Diodes. Macromol. Rapid Commun. 2012, 33, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.S.; Wiedemair, W.; Nau, S.; Trattnig, R.; Sax, S.; Winkler, S.; Vollmer, A.; Koch, N.; Baumgarten, M.; List, E.J.W.; et al. Core, Shell, and Surface-Optimized Dendrimers for Blue Light-Emitting Diodes. J. Am. Chem. Soc. 2011, 133, 1301–1303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Baumgarten, M.; Auer, M.; Trattnig, R.; List-Kratochvil, E.J.W.; Mullen, K. Core-and-Surface-Functionalized Polyphenylene Dendrimers for Solution-Processed, Pure-Blue Light-Emitting Diodes Through Surface-to-Core Energy Transfer. Macromol. Rapid Commun. 2014, 35, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Huang, W.S.; Lai, M.Y.; Tsao, W.C.; Lin, J.T.; Wu, Y.H.; Ke, T.H.; Chen, L.Y.; Wu, C.C. Versatile, Benzimidazole/Amine-Based Ambipolar Compounds for Electroluminescent Applications: Single-Layer, Blue, Fluorescent OLEDs, Hosts for Single-Layer, Phosphorescent OLEDs. Adv. Funct. Mater. 2009, 19, 2661–2670. [Google Scholar] [CrossRef]
- Kulkarni, A.P.; Kong, X.X.; Jenekhe, S.A. High-performance organic light-emitting diodes based on intramolecular charge-transfer emission from donor-acceptor molecules: Significance of electron-donor strength and molecular geometry. Adv. Funct. Mater. 2006, 16, 1057–1066. [Google Scholar] [CrossRef]
- Shih, P.I.; Tseng, Y.H.; Wu, F.I.; Dixit, A.K.; Shu, C.F. Stable and efficient white electroluminescent devices based on a single emitting layer of polymer blends. Adv. Funct. Mater. 2006, 16, 1582–1589. [Google Scholar] [CrossRef]
- Chen, C.H.; Lin, J.T.; Yeh, M.C.P. Nonconjugated red-emitting dendrimers with p-type and/or n-type peripheries. Org. Lett. 2006, 8, 2233–2236. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.F.; Zhu, W.H.; Li, S.F.; Xu, J.; Tian, H. Synthesis of carrier-transporting dendrimers with perylenebis(dicarboximide)s as a luminescent. Eur. J. Org. Chem. 2006, 2006, 986–1001. [Google Scholar] [CrossRef]
- Huang, T.H.; Lin, J.T.; Chen, L.Y.; Lin, Y.T.; Wu, C.C. Dipolar dibenzothiophene SS-dioxide derivatives containing diarylamine: Materials for single-layer organic light-emitting devices. Adv. Mater. 2006, 18, 602–606. [Google Scholar] [CrossRef]
- Hancock, J.M.; Gifford, A.P.; Zhu, Y.; Lou, Y.; Jenekhe, S.A. n-type conjugated oligoquinoline and oligoquinoxaline with triphenylamine endgroups: Efficient ambipolar light emitters for device applications. Chem. Mater. 2006, 18, 4924–4932. [Google Scholar] [CrossRef]
- Jenekhe, S.A.; Lu, L.D.; Alam, M.M. New conjugated polymers with donor-acceptor architectures: Synthesis and photophysics of carbazole-quinoline and phenothiazine-quinoline copolymers and oligomers exhibiting large intramolecular charge transfer. Macromolecules 2001, 34, 7315–7324. [Google Scholar] [CrossRef]
- Champion, R.D.; Cheng, K.F.; Pai, C.L.; Chen, W.C.; Jenekhe, S.A. Electronic properties and field-effect transistors of thiophene-based donor-acceptor conjugated copolymers. Macromol. Rapid Commun. 2005, 26, 1835–1840. [Google Scholar] [CrossRef]
- Grabowski, Z.R.; Rotkiewicz, K.; Rettig, W. Structural changes accompanying intramolecular electron transfer: Focus on twisted intramolecular charge-transfer states and structures. Chem. Rev. 2003, 103, 3899–4031. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.N.; Zhong, C.; Liu, Z.Y.; Yang, C.L.; Qin, J.G. New intramolecular through-space charge transfer emission: Tunable dual fluorescence of terfluorenes. Chem. Commun. 2010, 46, 6666–6668. [Google Scholar] [CrossRef] [PubMed]
- Linton, K.E.; Fisher, A.L.; Pearson, C.; Fox, M.A.; Palsson, L.O.; Bryce, M.R.; Petty, M.C. Colour tuning of blue electroluminescence using bipolar carbazole-oxadiazole molecules in single-active-layer organic light emitting devices (OLEDs). J. Mater. Chem. 2012, 22, 11816–11825. [Google Scholar] [CrossRef]
- Ding, J.Q.; Zhang, B.H.; Lu, J.H.; Xie, Z.Y.; Wang, L.X.; Jing, X.B.; Wang, F.S. Solution-Processable Carbazole-Based Conjugated Dendritic Hosts for Power-Efficient Blue-Electrophosphorescent Devices. Adv. Mater. 2009, 21. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.K.; Hussain, S.A.; Bhattacharjee, D. Photophysical characterizations of 2-(4-biphenylyl)-5-phenyl-1,3,4-oxadiazole in restricted geometry. J. Lumin. 2008, 128, 41–50. [Google Scholar] [CrossRef]
- Bansal, A.K.; Penzkofer, A. Linear and nonlinear optical spectroscopic characterisation of triphenylamine and 1,2,3-tris(3-methylphenylphenylamino)benzene. Chem. Phys. 2008, 352, 48–56. [Google Scholar] [CrossRef]
- Bernhardt, S.; Kastler, M.; Enkelmann, V.; Baumgarten, M.; Mullen, K. Pyrene as chromophore and electrophore: Encapsulation in a rigid polyphenylene shell. Chemistry 2006, 12, 6117–6128. [Google Scholar] [CrossRef] [PubMed]
- Sonar, P.; Soh, M.S.; Cheng, Y.H.; Henssler, J.T.; Sellinger, A. 1,3,6,8-Tetrasubstituted Pyrenes: Solution-Processable Materials for Application in Organic Electronics. Org. Lett. 2010, 12, 3292–3295. [Google Scholar] [CrossRef] [PubMed]
- Orita, A.; Miyamoto, K.; Nakashima, M.; Ye, F.; Otera, J. Double elimination protocol for convenient synthesis of dihalodiphenylacetylenes: Versatile building blocks for tailor-made phenylene-ethynylenes. Adv. Synth. Catal. 2004, 346, 767–776. [Google Scholar] [CrossRef]
- Orita, A.; Taniguchi, H.; Otera, J. One-shot double elimination process: A practical and concise protocol for diaryl acetylenes. Chem-Asian J. 2006, 1, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Stangenberg, R.; Wu, Y.Z.; Hedrich, J.; Kurzbach, D.; Wehner, D.; Weidinger, G.; Kuan, S.L.; Jansen, M.I.; Jelezko, F.; Luhmann, H.J.; et al. A Polyphenylene Dendrimer Drug Transporter with Precisely Positioned Amphiphilic Surface Patches. Adv. Healthc. Mater. 2015, 4, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Emmerling, F.; Orgzall, I.; Dietzel, B.; Schulz, B.; Larrucea, J. Ordering the amorphous—Structures in PBD LED materials. J. Mol. Struct 2012, 1030, 209–215. [Google Scholar] [CrossRef]
- Lin, Y.H.; Wu, H.H.; Wong, K.T.; Hsieh, C.C.; Lin, Y.C.; Chou, P.T. Modulate photoinduced electron transfer efficiency of bipolar dendritic systems. Org. Lett. 2008, 10, 3211–3214. [Google Scholar] [CrossRef] [PubMed]
- Galievsky, V.A.; Druzhinin, S.I.; Demeter, A.; Mayer, P.; Kovalenko, S.A.; Senyushkina, T.A.; Zachariasse, K.A. Ultrafast Intramolecular Charge Transfer with N-(4-Cyanophenyl)carbazole. Evidence for a LE Precursor and Dual LE plus ICT Fluorescence. J. Phys. Chem. A 2010, 114, 12622–12638. [Google Scholar] [CrossRef] [PubMed]
- Druzhinin, S.I.; Galievsky, V.A.; Demeter, A.; Kovalenko, S.A.; Senyushkina, T.; Dubbaka, S.R.; Knoche, P.; Mayer, P.; Grosse, C.; Stalke, D.; et al. Two-State Intramolecular Charge Transfer (ICT) with 3,5-Dimethyl-4(dimethylamino)benzonitrile (MMD) and Its Meta-Isomer mMMD. Ground State Amino Twist Not Essential for ICT. J. Phys. Chem. A 2015, 119, 11820–11836. [Google Scholar] [CrossRef] [PubMed]
- Zachariasse, K.A.; Grobys, M.; vonderHaar, T.; Hebecker, A.; Ilichev, Y.V.; Jiang, Y.B.; Morawski, O.; Kuhnle, W. Intramolecular charge transfer in the excited state. Kinetics and configurational changes. J. Photochem. Photobiol. A 1996, 102, 59–70. [Google Scholar] [CrossRef]
- Amthor, S.; Noller, B.; Lambert, C. UV/Vis/NIR spectral properties of triarylamines and their corresponding radical cations. Chem. Phys. 2005, 316, 141–152. [Google Scholar] [CrossRef]
- Gehrig, D.W.; Howard, I.A.; Laquai, F. Charge Carrier Generation Followed by Triplet State Formation, Annihilation, and Carrier Recreation in PBDTTT-C/PC60BM Photovoltaic Blends. J. Phys. Chem. C 2015, 119, 13509–13515. [Google Scholar] [CrossRef]
- Dimitrov, S.D.; Wheeler, S.; Niedzialek, D.; Schroeder, B.C.; Utzat, H.; Frost, J.M.; Yao, J.Z.; Gillett, A.; Tuladhar, P.S.; McCulloch, I.; et al. Polaron pair mediated triplet generation in polymer/fullerene blends. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Herbich, J.; Kapturkiewicz, A. Radiative Electron-Transfer in Aryl Derivatives of Dimethylanilines. Chem. Phys. 1993, 170, 221–233. [Google Scholar] [CrossRef]
- Wiessner, A.; Huttmann, G.; Kuhnle, W.; Staerk, H. Electron-Transfer, Solvation, and Amplified Stimulated-Emission of Pyrene-Dma and Anthracene-Dma. J. Phys. Chem. 1995, 99, 14923–14930. [Google Scholar] [CrossRef]
- Techert, S.; Schmatz, S.; Wiessner, A.; Staerk, H. Photophysical characteristics of directly linked pyrene-dimethylaniline derivatives. J. Phys. Chem. A 2000, 104, 5700–5710. [Google Scholar] [CrossRef]
- Dobkowski, J.; Rettig, W.; Waluk, J. Intramolecular charge-transfer properties of a molecule with a large donor group: the case of 4′-(pyren-1-yl) benzonitrile. Phys. Chem. Chem. Phys. 2002, 4, 4334–4339. [Google Scholar] [CrossRef]
- Hagopian, S.; Singer, L.A. Photophysical Studies on 1-(Para-Aminophenyl)Pyrene—Characterization of an Intramolecular Charge-Transfer State with Application to Proton-Transfer Dynamics. J. Am. Chem. Soc. 1985, 107, 1874–1880. [Google Scholar] [CrossRef]
- Farinola, G.M.; Ragni, R. Electroluminescent materials for white organic light emitting diodes. Chem. Soc. Rev. 2011, 40, 3467–3482. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Chellappan, V.; Webster, R.D.; Ke, L.; Zhang, T.F.; Liu, B.; Lai, Y.H. Bridged-triarylamine starburst oligomers as hole transporting materials for electroluminescent devices. J. Mater. Chem. 2012, 22, 15397–15404. [Google Scholar] [CrossRef]
- Tsai, M.H.; Ke, T.H.; Lin, H.W.; Wu, C.C.; Chiu, S.F.; Fang, F.C.; Liao, Y.L.; Wong, K.T.; Chen, Y.H.; Wu, C.I. Triphenylsilyl- and Trityl-Substituted Carbazole-Based Host Materials for Blue Electrophosphorescence. Acs Appl. Mater. Inter. 2009, 1, 567–574. [Google Scholar] [CrossRef]
- Chang, Y.T.; Chang, J.K.; Lee, Y.; Wang, P.S.; Wu, J.L.; Hsu, C.C.; Wu, I.W.; Tseng, W.H.; Pi, T.W.; Chen, C.I.; et al. High-Efficiency Small-Molecule-Based Organic Light Emitting Devices with Solution Processes and Oxadiazole-Based Electron Transport Materials. Acs Appl. Mater. Inter. 2013, 5, 10614–10622. [Google Scholar] [CrossRef]
- Janietz, S.; Wedel, A. Electrochemical redox behavior and electroluminescence in the mixed energy-sufficient system thianthrene and 2-(4-biphenyl)-5-(4-tert-butylphenyl)-1,3,4-oxadiazole. Adv. Mater. 1997, 9, 403–407. [Google Scholar] [CrossRef]
- Choulis, S.A.; Choong, V.E.; Patwardhan, A.; Mathai, M.K.; So, F. Interface modification to improve hole-injection properties in organic electronic devices. Adv. Funct. Mater. 2006, 16, 1075–1080. [Google Scholar] [CrossRef]
- Trattnig, R.; Pevzner, L.; Jager, M.; Schlesinger, R.; Nardi, M.V.; Ligorio, G.; Christodoulou, C.; Koch, N.; Baumgarten, M.; Müllen, K.; et al. Bright Blue Solution Processed Triple-Layer Polymer Light-Emitting Diodes Realized by Thermal Layer Stabilization and Orthogonal Solvents. Adv. Funct. Mater. 2013, 23, 4897–4905. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
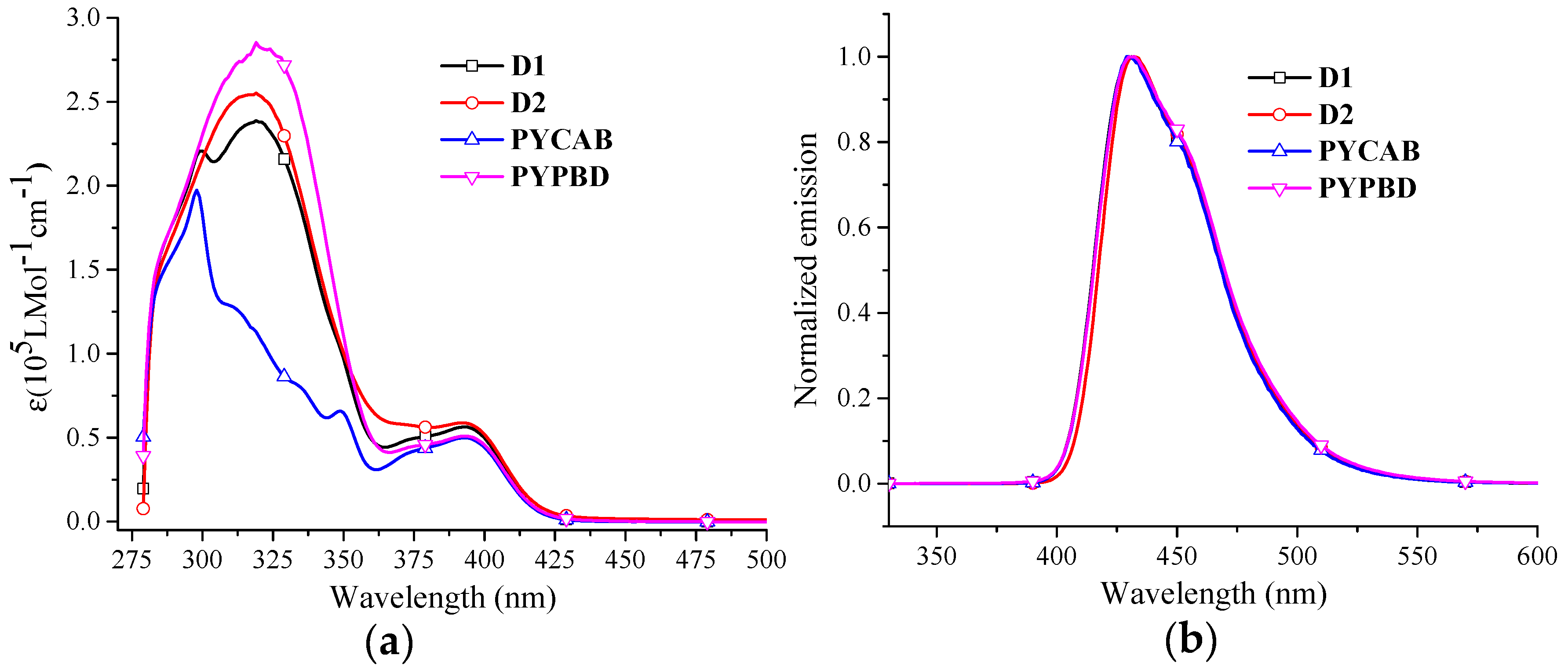



| Dendrimer | λab (nm) | λem (nm) | PLQY a | HOMO (eV) b | LUMO (eV) b | Eg (eV) c | |||
|---|---|---|---|---|---|---|---|---|---|
| Sol | Film | Sol | Film | Excitation | |||||
| Surface | Core | ||||||||
| D1 | 299, 319, 392 | 300, 316, 391 | 431 | 446 | 0.80 | 0.93 | −5.52 | −2.45 | 2.95 |
| D2 | 319, 392 | 313, 393 | 432 | 440 | 0.59 | 0.85 | −5.34 | −2.44 | 2.95 |
| PYPBD | 320, 392 | 320, 393 | 431 | 453 | 0.66 | 0.78 | −5.50 | −2.52 | 2.97 |
| Dendrimer | Von (V) | Η (cd/A) | Lmax (cd/m2) | CIE (x, y) |
|---|---|---|---|---|
| D1 | 4 | 0.048 | 388 | 0.16, 0.10 a |
| 5.5 | 0.13 | 1866 | 0.16, 0.12 b | |
| 4.4 | 0.21 | 2701 | 0.16, 0.12 c | |
| D2 | 4.8 | 0.02 | 203 | 0.20, 0.18 b |
| PYPBD | 9.7 | 0.04 | 277 | 0.18, 0.18 a |
| 7 | 0.17 | 854 | 0.18, 0.18 b | |
| 7 | 0.24 | 1288 | 0.18, 0.18 c | |
| PYNPA | 5.2 | 0.24 | 715 | 0.16, 0.20 a |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Auer-Berger, M.; Gehrig, D.W.; Blom, P.W.M.; Baumgarten, M.; Schollmeyer, D.; List-Kratochvil, E.J.W.; Müllen, K. Blue Light Emitting Polyphenylene Dendrimers with Bipolar Charge Transport Moieties. Molecules 2016, 21, 1400. https://doi.org/10.3390/molecules21101400
Zhang G, Auer-Berger M, Gehrig DW, Blom PWM, Baumgarten M, Schollmeyer D, List-Kratochvil EJW, Müllen K. Blue Light Emitting Polyphenylene Dendrimers with Bipolar Charge Transport Moieties. Molecules. 2016; 21(10):1400. https://doi.org/10.3390/molecules21101400
Chicago/Turabian StyleZhang, Guang, Manuel Auer-Berger, Dominik W. Gehrig, Paul W. M. Blom, Martin Baumgarten, Dieter Schollmeyer, E. J. W. List-Kratochvil, and Klaus Müllen. 2016. "Blue Light Emitting Polyphenylene Dendrimers with Bipolar Charge Transport Moieties" Molecules 21, no. 10: 1400. https://doi.org/10.3390/molecules21101400