Comparative Studies of Interactions between Fluorodihydroquinazolin Derivatives and Human Serum Albumin with Fluorescence Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
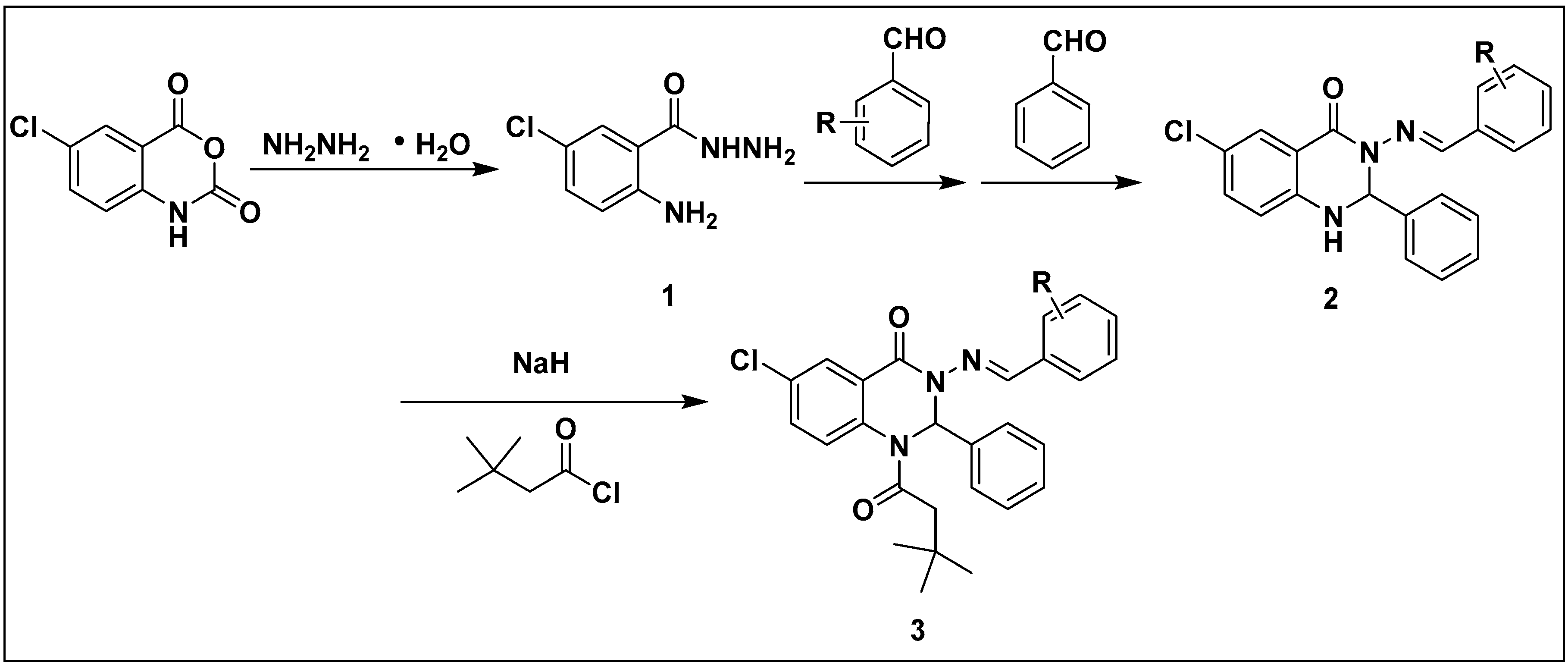
2.1. Synthesis
2.2. Fluorescence Quenching Mechanism
2.3. Binding Sites and Identification of Binding Sites on HSA
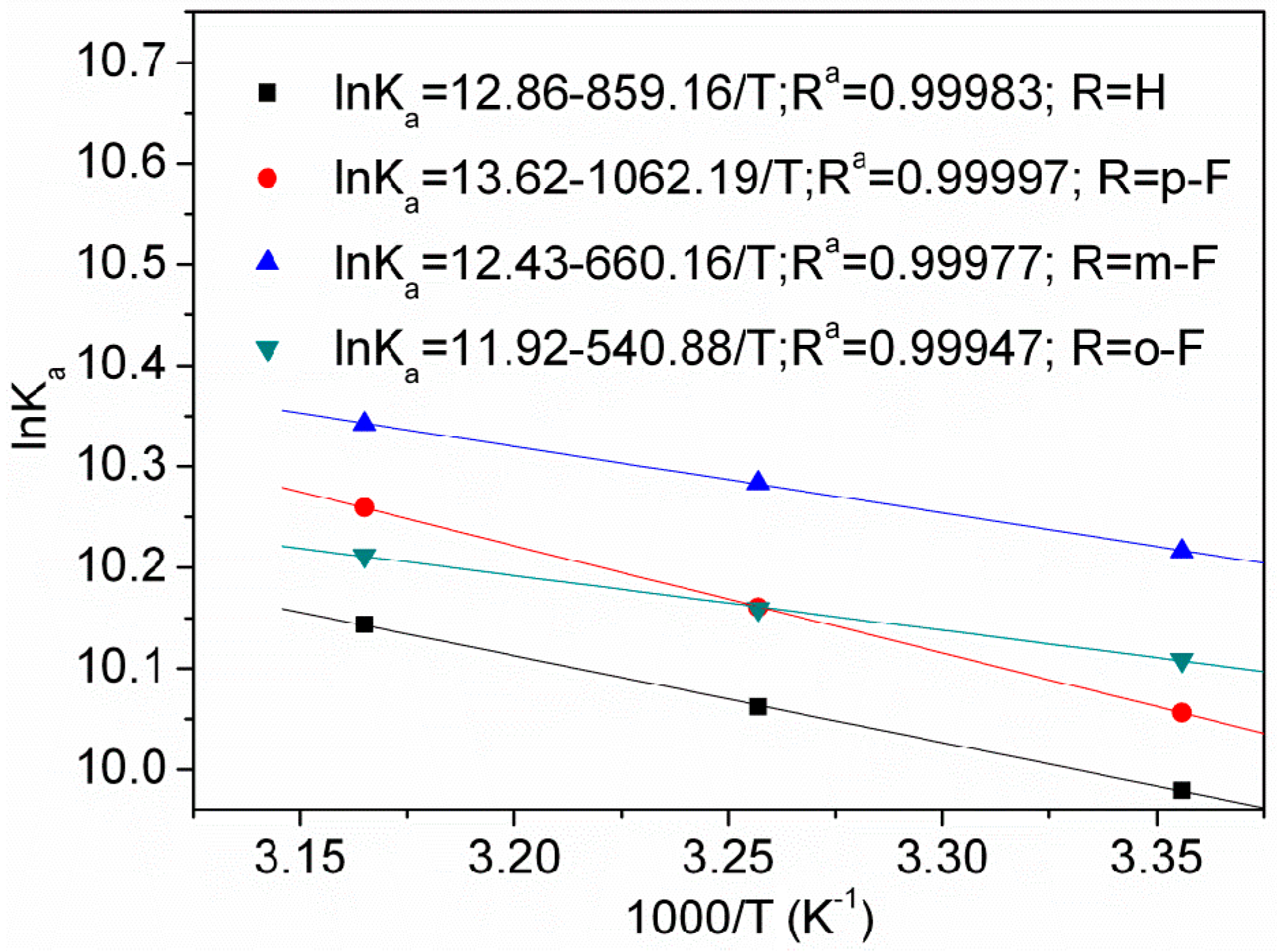
2.4. Thermodynamic Parameters and Binding Modes
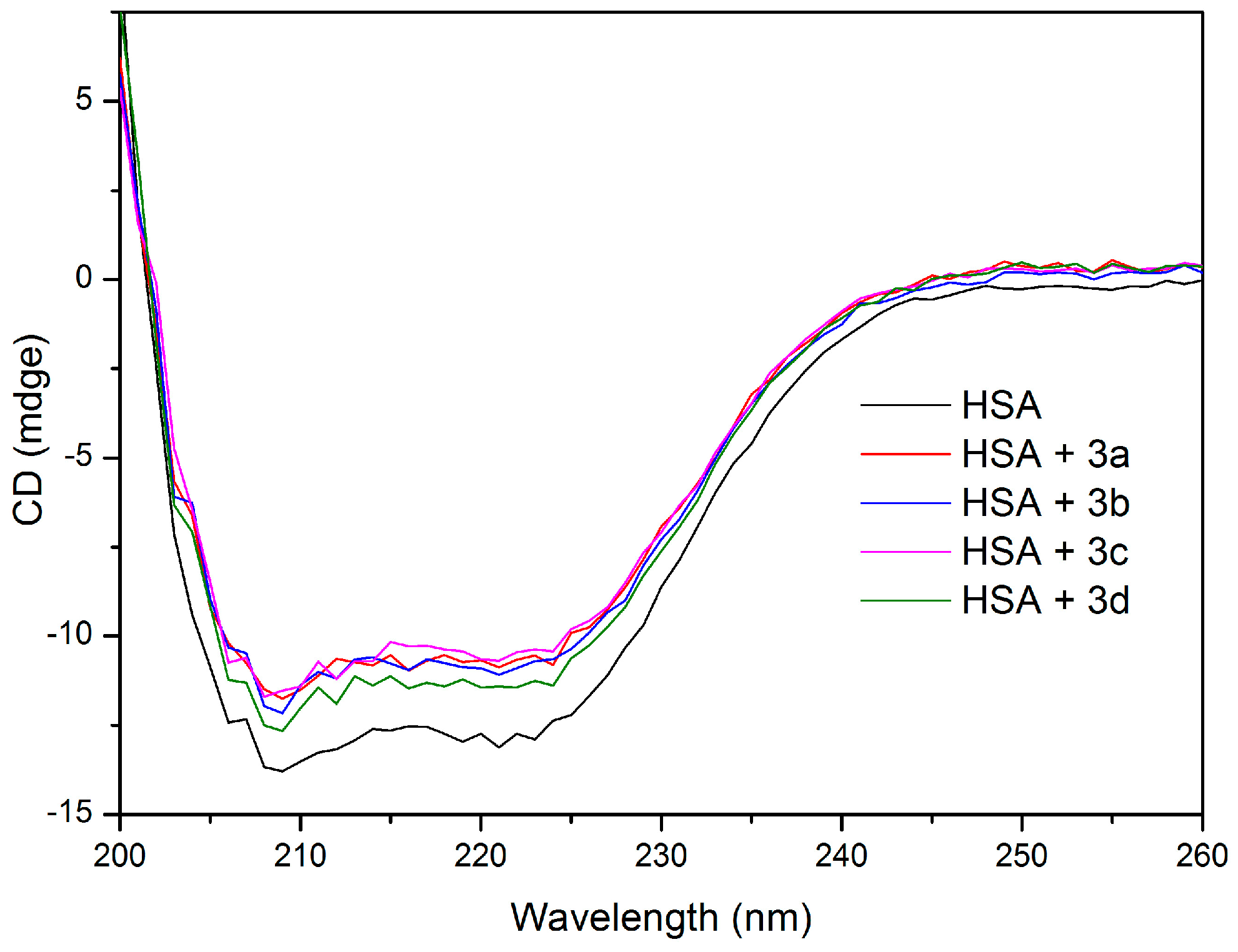
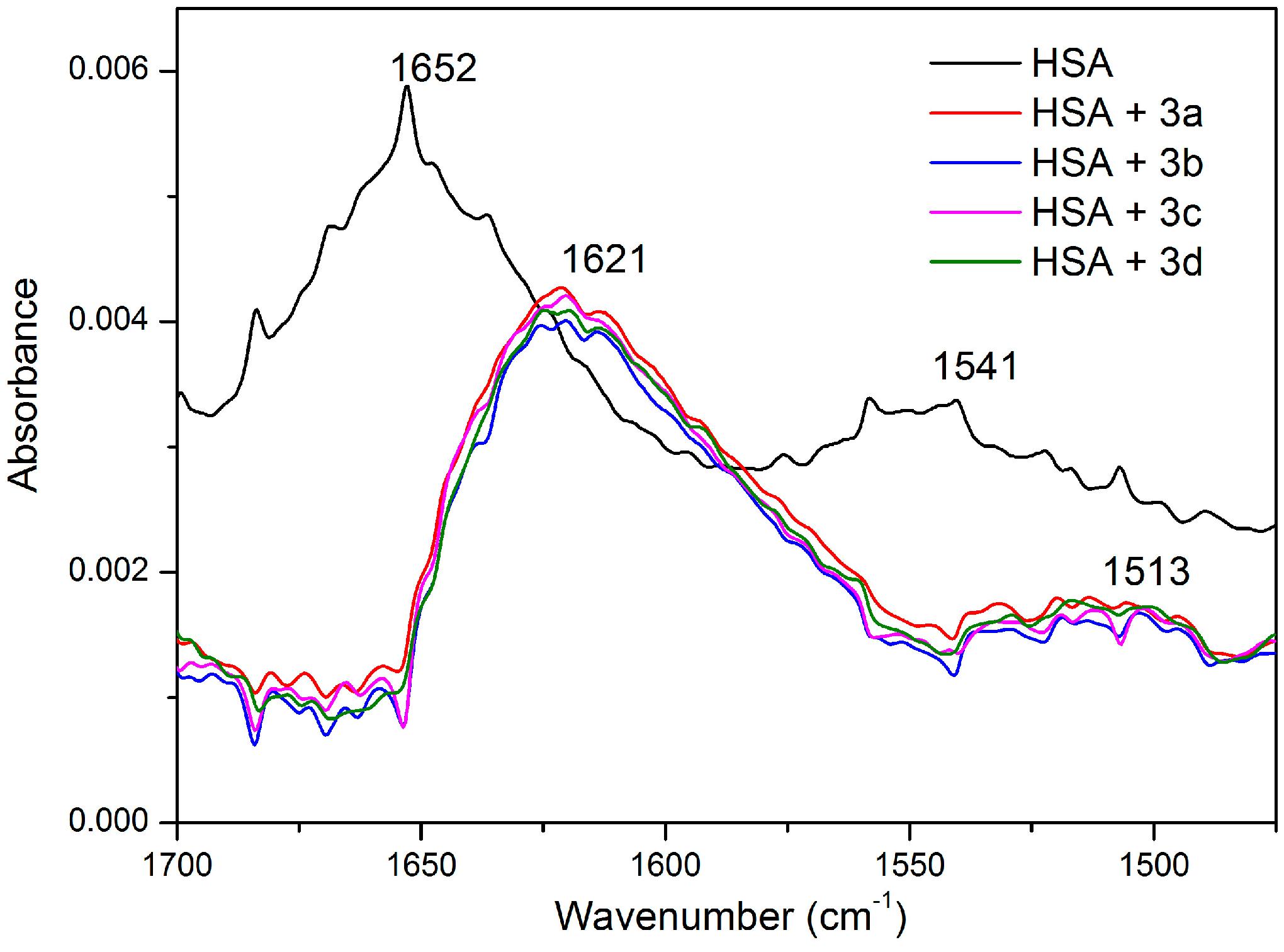
2.5. HSA Conformational Change by CD and FT-IR Measurements
3. Experimental Section
3.1. General Methods and Materials
3.2. Fluorescence Titration Experiments
3.3. Site Marker Competitive Experiments
3.4. Circular Dichroism Spectra Studies
3.5. Fourier Transform Infrared (FT-IR) Measurements
3.6. Synthesis of (un)Substituted Phenyl-2,3-dihydroquinazolin-4(1H)-one Derivatives FDQL 3a–d
4. Concluding Remarks
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kandagal, P.B.; Ashoka, S.; Seetharamappa, J.; Shaikh, S.M.T.; Jadegoud, Y.; Ijare, O.B. Study of the interaction of an anticancer drug with human and bovine serum albumin: Spectroscopic approach. J. Pharm. Biomed. Anal. 2006, 41, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, Y.; Liu, S. Spectroscopic studies on the interaction of human serum albumin and water-soluble carboxyl carbon nanotubes. Spectrosc. Spect. Anal. 2016, 36, 1109–1115. [Google Scholar]
- Ma, X.; Yan, J.; Xu, K.; Guo, L.; Li, H. Binding mechanism of trans-n-caffeoyltyramine and human serum albumin: Investigation by multi-spectroscopy and docking simulation. Bioorg. Chem. 2016, 66, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Rohacova, J.; Marin, M.L.; Miranda, M.A. Complexes between fluorescent cholic acid derivatives and human serum albumin. A photophysical approach to investigate the binding behavior. J. Phys. Chem. B 2010, 114, 4710–4716. [Google Scholar] [CrossRef] [PubMed]
- Housaindokht, M.R.; Rouhbakhsh Zaeri, Z.; Bahrololoom, M.; Chamani, J.; Bozorgmehr, M.R. Investigation of the behavior of hsa upon binding to amlodipine and propranolol: Spectroscopic and molecular modeling approaches. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 85, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Zhang, L.; Diao, J.; Li, X.; Ma, L.; Sun, Y. Human serum albumin stability and toxicity of anthraquinone dye alizarin complexone: An albumin-dye model. Ecotoxicol. Environ. Saf. 2012, 79, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Peng, W. Biophysical evaluation of protein structural flexibility for ligand biorecognition in solid solution. Phys. Chem. Chem. Phys. 2016, 18, 6595–6606. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Abdelhameed, A.S.; Rajpoot, R.K.; Khan, R.H. Interplay of multiple interaction forces: Binding of tyrosine kinase inhibitor nintedanib with human serum albumin. J. Photochem. Photobiol. B 2016, 157, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Du, Y. Study on the binding of chiral drug duloxetine hydrochloride to human serum albumin. Eur. J. Med. Chem. 2010, 45, 4043–4049. [Google Scholar] [CrossRef] [PubMed]
- Abdollahpour, N.; Asoodeh, A.; Saberi, M.R.; Chamani, J. Separate and simultaneous binding effects of aspirin and amlodipine to human serum albumin based on fluorescence spectroscopic and molecular modeling characterizations: A mechanistic insight for determining usage drugs doses. J. Lumin. 2011, 131, 1885–1899. [Google Scholar] [CrossRef]
- Wang, T.; Xiang, B.; Wang, Y.; Chen, C.; Dong, Y.; Fang, H.; Wang, M. Spectroscopic investigation on the binding of bioactive pyridazinone derivative to human serum albumin and molecular modeling. Colloids Surf. B Biointerfaces 2008, 65, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Li, N.; Chen, G.; Xu, Y.; Chen, Y.; Hu, S.; Hu, Z. Binding studies of costunolide and dehydrocostuslactone with hsa by spectroscopy and atomic force microscopy. J. Lumin. 2011, 131, 2063–2071. [Google Scholar] [CrossRef]
- Bijari, N.; Shokoohinia, Y.; Ashrafi-Kooshk, M.R.; Ranjbar, S.; Parvaneh, S.; Moieni-Arya, M.; Khodarahmi, R. Spectroscopic study of interaction between osthole and human serum albumin: Identification of possible binding site of the compound. J. Lumin. 2013, 143, 328–336. [Google Scholar] [CrossRef]
- Afrin, S.; Riyazuddeen; Rabbani, G.; Khan, R.H. Spectroscopic and calorimetric studies of interaction of methimazole with human serum albumin. J. Lumin. 2014, 151, 219–223. [Google Scholar] [CrossRef]
- Mabkhot, Y.N.; Al-Har, M.S.; Barakat, A.; Aldawsari, F.D.; Aldalbahi, A.; Ul-Haq, Z. Synthesis, anti-microbial and molecular docking studies of quinazolin-4(3H)-one derivatives. Molecules 2014, 19, 8725–8739. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Alsaid, M.S.; Al-Dosari, M.S.; El-Gazzar, M.G.; Parvez, M.K. Design, synthesis and anticancer evaluation of novel quinazoline-sulfonamide hybrids. Molecules 2016, 21, 189. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.R.; Khan, S.I.; Singh, S.; Khan, I.A.; Vishwakarma, R.A.; Bharate, S.B. Synthesis, antimalarial and antitubercular activities of meridianin derivatives. Eur. J. Med. Chem. 2015, 98, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, M.; Yao, X.; Li, Y.; Tan, J.; Wang, L.; Qiao, W.; Geng, Y.; Liu, Y.; Wang, Q. Design, synthesis and antiviral activity of novel quinazolinones. Eur. J. Med. Chem. 2012, 53, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Farghaly, T.A.R.; Edrees, M.M.; Mosselhi, M.A. Synthesis, tautomeric structure and antimicrobial activity of 3-arylhydrazono-4-phenyl-[1,2,4]-triazepino[2,3-a]quinazoline-2,7(1h)-diones. Molecules 2012, 17, 8483–8493. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, A.A.M.; Abou-Zeid, L.A.; ElTahir, K.E.H.; Mohamed, M.A.; Abu El-Enin, M.A.; El-Azab, A.S. Design, synthesis of 2,3-disubstituted 4(3H)-quinazolinone derivatives as anti-inflammatory and analgesic agents: Cox-1/2 inhibitory activities and molecular docking studies. Bioorg. Med. Chem. 2016, 24, 3818–3828. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, A.A.M.; Abou-Zeid, L.A.; El Tahir, K.E.H.; Ayyad, R.R.; El-Sayed, M.A.A.; El-Azab, A.S. Synthesis, anti-inflammatory, analgesic, cox-1/2 inhibitory activities and molecular docking studies of substituted 2-mercapto-4(3H)-quinazolinones. Eur. J. Med. Chem. 2016, 121, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Georgey, H.; Abdel-Gawad, N.; Abbas, S. Synthesis and anticonvulsant activity of some quinazolin-4-(3H)-one derivatives. Molecules 2008, 13, 2557–2569. [Google Scholar] [CrossRef] [PubMed]
- Khattab, S.N.; Haiba, N.S.; Asal, A.M.; Bekhit, A.A.; Amer, A.; Abdel-Rahman, H.M.; El-Faham, A. Synthesis and evaluation of quinazoline amino acid derivatives as mono amine oxidase (mao) inhibitors. Bioorg. Med. Chem. 2015, 23, 3574–3585. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, R.; Kumar, A.; Yadav, R. Synthesis and biological activity of peptide derivatives of iodoquinazolinones/nitroimidazoles. Molecules 2008, 13, 958–976. [Google Scholar] [CrossRef] [PubMed]
- Alagarsamy, V.; Sharma, H.K.; Parthiban, P.; Singh, J.C.H.; Murugan, S.T.; Solomon, V.R. Cheminform abstract: 4-(3-Methoxyphenyl)-1-substituted-4H-[1,2,4]triazolo [4,3-a]quinazolin-5-ones: New class of h 1-antihistaminic agents. Cheminform 2009, 40, 5–9. [Google Scholar] [CrossRef]
- Bandurco, V.T.; Wong, E.M.; Levine, S.D.; Hajos, Z.G. Antihypertensive pyrrolo[1,2-c]quinazolines and pyrrolo[1,2-c]quinazolinones. J. Med. Chem. 1981, 24, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, Y.; Lv, C.; Wang, L.; Ou, J.; Wang, M.; Liu, S. Impact of halogen substituents on interactions between 2-phenyl-2,3-dihydroqulinazolin-4(1H)-one derivatives and human serum albumin. Molecules 2012, 17, 2000. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. (Ed.) Mechanisms and dynamics of fluorescence quenching. In Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; pp. 331–351.
- Lakowicz, J.R.; Weber, G. Quenching of fluorescence by oxygen. Probe for structural fluctuations in macromolecules. Biochemistry 1973, 12, 4161–4170. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Green, R.J.; Frazier, R.A. Interaction of flavonoids with bovine serum albumin: A fluorescence quenching study. J. Agric. Food Chem. 2005, 53, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Ware, W.R. Oxygen quenching of fluorescence in solution: An experimental study of the diffusion process. J. Phys. Chem. 1962, 66, 316–320. [Google Scholar] [CrossRef]
- Chaves, O.A.; Amorim, A.P.O.; Castro, L.H.E.; Sant′Anna, C.M.R.; de Oliveira, M.C.C.; Cesarin-Sobrinho, D.; Netto-Ferreira, J.C.; Ferreira, A.B.B. Fluorescence and docking studies of the interaction between human serum albumin and pheophytin. Molecules 2015, 20, 19526–19539. [Google Scholar] [CrossRef] [PubMed]
- Cacita, N.; Nikolaou, S. Studying the interaction between trinuclear ruthenium complexes and human serum albumin by means of fluorescence quenching. J. Lumin. 2016, 169, 115–120. [Google Scholar] [CrossRef]
- Cheng, X.; Fan, X.; Jiang, F.; Liu, Y.; Lei, K. Resonance energy transfer, ph-induced folded states and the molecular interaction of human serum albumin and icariin. Luminescence 2015, 30, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Hong, M.; Chang, G.; Li, X.; Li, Z. A comparative study of cytotoxicity and interaction with DNA/protein of five transition metal complexes with schiff base ligands. J. Photochem. Photobiol. B 2015, 148, 232–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Q.; Wang, F.; Yuan, L.; Xu, Z.; Jiang, F.; Liu, Y. Comparison of interactions between human serum albumin and silver nanoparticles of different sizes using spectroscopic methods. Luminescence 2015, 30, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Liu, R.; Jiang, X.; Xu, Q. The interaction between cepharanthine and two serum albumins: Multiple spectroscopic and chemometric investigations. Luminescence 2014, 29, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, V.D.; Vorum, H.; Honore, B.; Qasim, M.A. Molecular basis of indomethacin-human serum albumin interaction. J. Pharm. Pharmacol. 1999, 51, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Fan, J.; Li, J.; Hu, Z. Interactions between 1-benzoyl-4-p-chlorophenyl thiosemicarbazide and serum albumin: Investigation by fluorescence spectroscopy. Bioorg. Med. Chem. 2004, 12, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Qin, Y.; Lin, A.; Hu, X.; Zou, G. Interaction of daunomycin antibiotic with human serum albumin: Investigation by resonant mirror biosensor technique, fluorescence spectroscopy and molecular modeling methods. J. Pharm. Biomed. Anal. 2005, 39, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Wang, Y.; Li, Z.; Dong, C. Fluorescence study on the interaction of human serum albumin with bromsulphalein. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 65, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Sjöholm, I.; Ekman, B.; Kober, A.; Ljungstedt-Påhlman, I.; Seiving, B.; Sjödin, T. Binding of drugs to human serum albumin: Xi. The specificity of three binding sites as studied with albumin immobilized in microparticles. Mol. Pharmacol. 1979, 16, 767–777. [Google Scholar] [PubMed]
- Leckband, D. Measuring the forces that control protein interactions. Annu. Rev. Biophys. Biomol. Struct. 2003, 29, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.M.; Jess, T.J.; Price, N.C. How to study proteins by circular dichroism. Biochim biophys acta. Biochim. Biophys. Acta 2005, 1751, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Sun, Y.; Dong, Q.; Liu, R.; Yan, X.; Zhang, Y.; Liu, J. Interaction of human serum albumin with novel imidazole derivatives studied by spectroscopy and molecular docking. Luminescence 2016, 31, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Sircar, J.C.; Capiris, T.; Kesten, S.J.; Herzig, D.J. Pyrazolo[5,1-b]quinazolin-9-ones: A new series of antiallergic agents. J. Med. Chem. 1981, 24, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Fulop, F.; Simeonov, M.; Pihlaja, K. Formation of 1,2-dihydroquinazolin-4(3H)-ones. Reinvestigation of a recently reported 1,3,4-benzotriazepine synthesis. Tetrahedron 1992, 48, 531–538. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from authors.
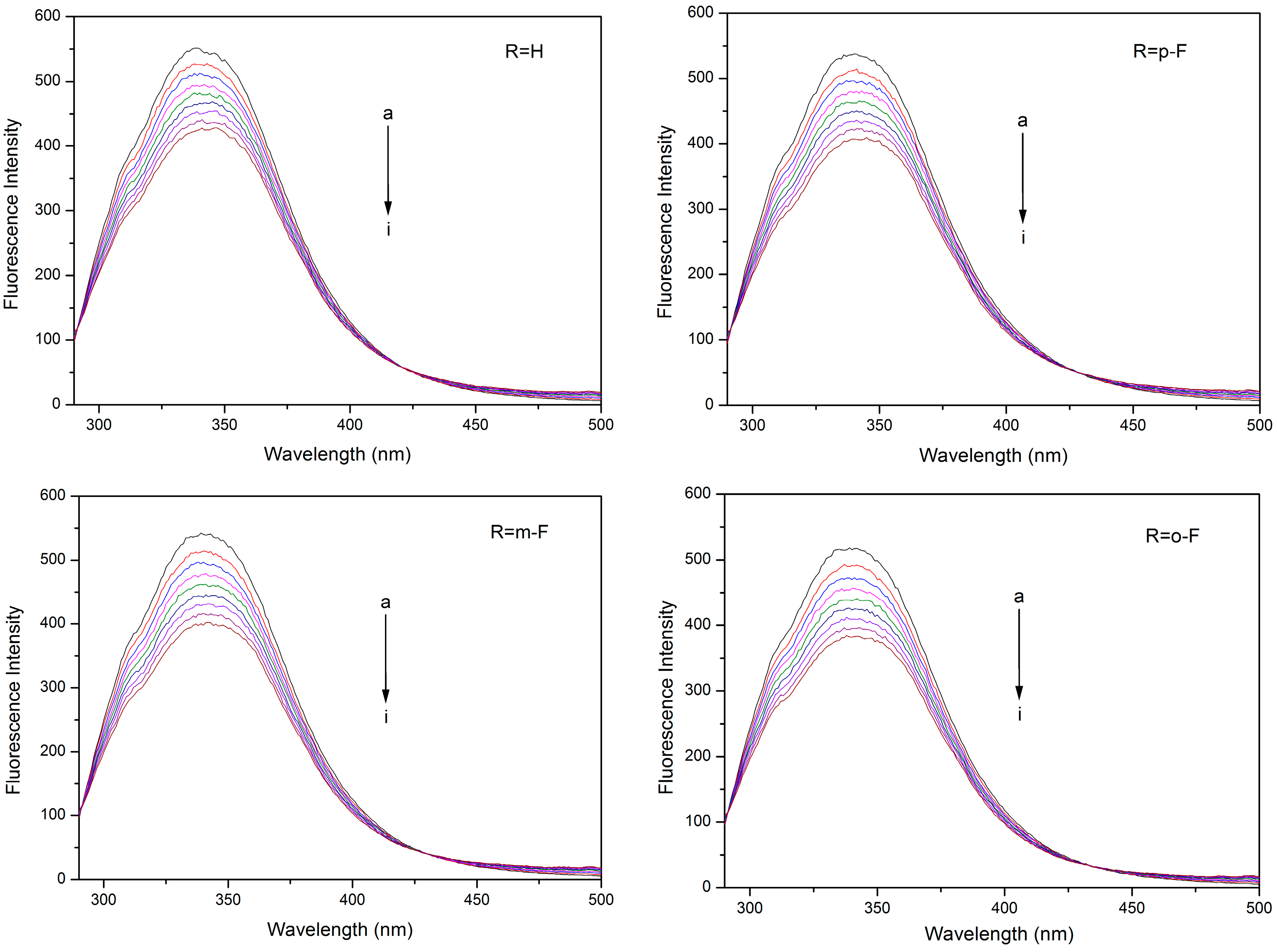
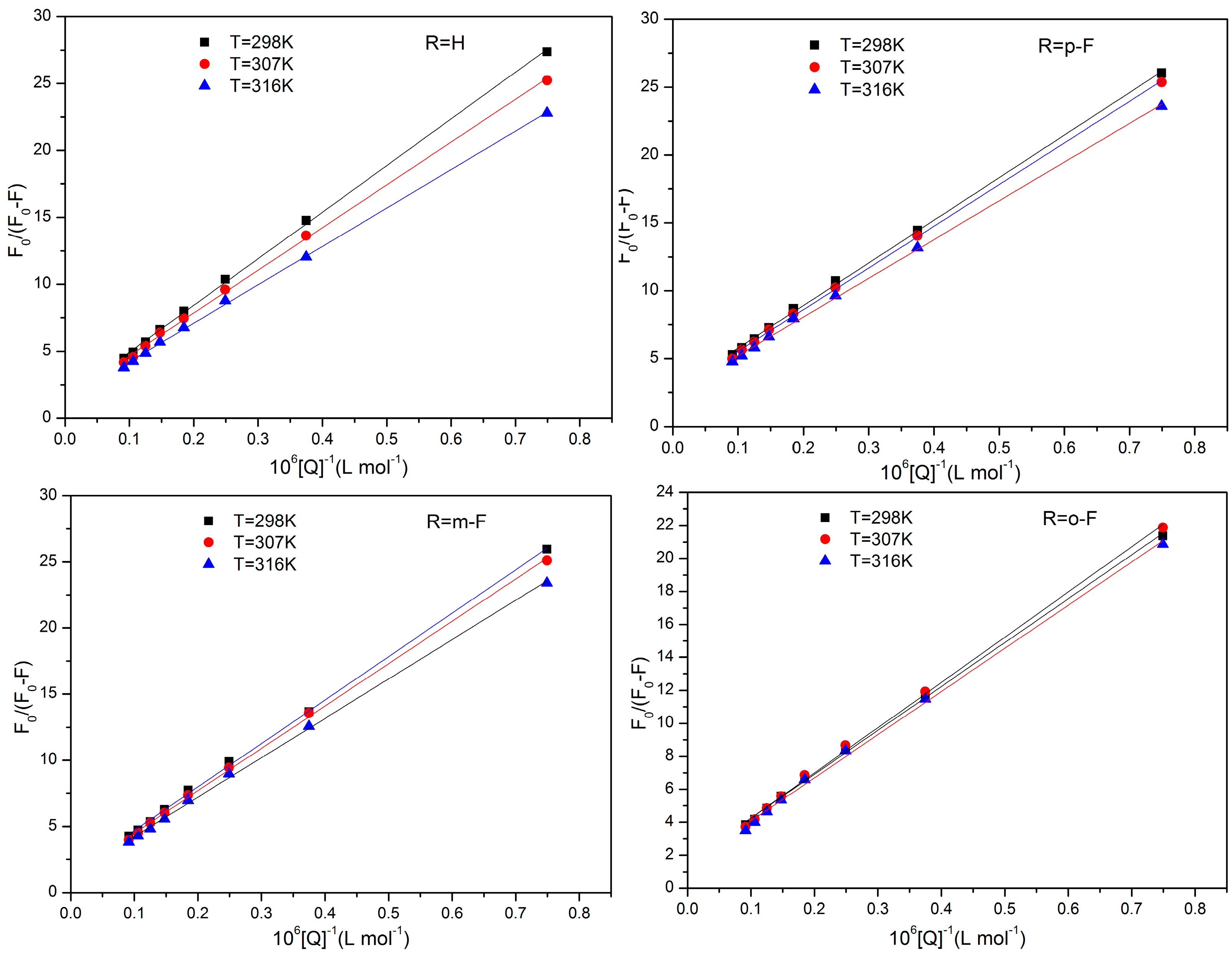
| Compound | T (K) | KSV (×104 M−1) | Kq (×1012 M−1·s−1) | R a | SD b |
|---|---|---|---|---|---|
| R = H | 298 | 2.77 | 2.77 | 0.99916 | 0.00605 |
| 307 | 2.98 | 2.98 | 0.99898 | 0.00677 | |
| 316 | 3.23 | 3.23 | 0.99919 | 0.00513 | |
| R = p-F | 298 | 3.11 | 3.11 | 0.99931 | 0.00451 |
| 307 | 3.25 | 3.25 | 0.99933 | 0.00466 | |
| 316 | 3.52 | 3.52 | 0.99946 | 0.00453 | |
| R = m-F | 298 | 3.24 | 3.24 | 0.99941 | 0.00436 |
| 307 | 3.44 | 3.44 | 0.99889 | 0.00633 | |
| 316 | 3.63 | 3.63 | 0.99924 | 0.00553 | |
| R = o-F | 298 | 3.30 | 3.30 | 0.99973 | 0.00299 |
| 307 | 3.36 | 3.36 | 0.99942 | 0.00449 | |
| 316 | 3.40 | 3.40 | 0.99907 | 0.00573 |
| Compound | T (K) | Ka (×104 M−1) | R a | ΔH (kJ·mol−1) | ΔG (kJ·mol−1) | ΔS (J·mol−1·K−1) |
|---|---|---|---|---|---|---|
| R = H | 298 | 2.16 | 0.99949 | 7.14 | −24.72 | 106.92 |
| 307 | 2.34 | 0.99969 | −25.68 | |||
| 316 | 2.54 | 0.99963 | −26.65 | |||
| R = p-F | 298 | 2.33 | 0.99945 | 8.83 | −24.92 | 113.24 |
| 307 | 2.59 | 0.99967 | −25.93 | |||
| 316 | 2.85 | 0.99967 | −26.95 | |||
| R = m-F | 298 | 2.73 | 0.99979 | 5.49 | −25.31 | 103.36 |
| 307 | 2.92 | 0.99960 | −26.24 | |||
| 316 | 3.10 | 0.99967 | −27.17 | |||
| R = o-F | 298 | 2.46 | 0.99956 | 4.50 | −25.04 | 99.13 |
| 307 | 2.58 | 0.99949 | −25.93 | |||
| 316 | 2.72 | 0.99912 | −26.83 |
| Compound | T (K) | n | R a | SD b |
|---|---|---|---|---|
| R = H | 298 | 0.99314 | 0.99945 | 0.01088 |
| 307 | 0.98423 | 0.99947 | 0.01059 | |
| 316 | 0.97492 | 0.99928 | 0.01220 | |
| R = p-F | 298 | 0.97047 | 0.99914 | 0.01329 |
| 307 | 0.98121 | 0.99942 | 0.01100 | |
| 316 | 0.98160 | 0.99947 | 0.01054 | |
| R = m-F | 298 | 0.98976 | 0.99949 | 0.01043 |
| 307 | 1.00621 | 0.99926 | 0.01276 | |
| 316 | 0.99960 | 0.99942 | 0.01126 | |
| R = o-F | 298 | 0.94907 | 0.99959 | 0.00895 |
| 307 | 0.97208 | 0.99936 | 0.01150 | |
| 316 | 0.98093 | 0.99884 | 0.01562 |
| Compound | Site Marker | Ka (×104 M−1) | R a | SD b |
|---|---|---|---|---|
| R = H | Blank | 2.16 | 0.99949 | 0.26651 |
| PB | 1.88 | 0.99962 | 0.27020 | |
| FA | 1.04 | 0.99948 | 0.25962 | |
| Dig | 2.18 | 0.99938 | 0.24163 | |
| R = p-F | Blank | 2.33 | 0.99945 | 0.23559 |
| PB | 2.44 | 0.99960 | 0.23309 | |
| FA | 2.10 | 0.99976 | 0.22148 | |
| Dig | 2.43 | 0.99954 | 0.20943 | |
| R = m-F | Blank | 2.73 | 0.99979 | 0.14653 |
| PB | 2.08 | 0.99931 | 0.26689 | |
| FA | 1.65 | 0.99969 | 0.26159 | |
| Dig | 2.78 | 0.99955 | 0.18883 | |
| R = o-F | Blank | 2.46 | 0.99956 | 0.18871 |
| PB | 2.25 | 0.99922 | 0.25466 | |
| FA | 1.72 | 0.99972 | 0.22793 | |
| Dig | 2.54 | 0.99914 | 0.24946 |
| Compound | ΔΔH (kJ·mol−1) | ΔΔS (J·mol−1·K−1) |
|---|---|---|
| R = p-F | 1.69 | 6.32 |
| R = m-F | −1.65 | −3.56 |
| R = o-F | −2.64 | −7.79 |
| T (K) | R = p-F | R = m-F | R = o-F | |
|---|---|---|---|---|
| ΔΔG (kJ·mol−1) | 298 | −0.20 | −0.59 | −0.32 |
| 307 | −0.25 | −0.56 | −0.25 | |
| 316 | −0.30 | −0.52 | −0.18 |
| Sample | Secondary Structure (%) | |||
|---|---|---|---|---|
| α-Helix | β-Sheet | β-Turn | Random Coil | |
| HSA | 38.8 | 23.6 | 11.0 | 26.6 |
| HSA + 3a (1:2) | 35.8 | 22.7 | 14.2 | 27.2 |
| HSA + 3b (1:2) | 36.8 | 17.6 | 17.0 | 28.6 |
| HSA + 3c (1:2) | 35.1 | 16.3 | 19.7 | 28.9 |
| HSA + 3d (1:2) | 38.0 | 23.9 | 12.5 | 25.5 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhu, M.; Liu, F.; Wu, X.; Pan, D.; Liu, J.; Fan, S.; Wang, Z.; Tang, J.; Na, R.; et al. Comparative Studies of Interactions between Fluorodihydroquinazolin Derivatives and Human Serum Albumin with Fluorescence Spectroscopy. Molecules 2016, 21, 1373. https://doi.org/10.3390/molecules21101373
Wang Y, Zhu M, Liu F, Wu X, Pan D, Liu J, Fan S, Wang Z, Tang J, Na R, et al. Comparative Studies of Interactions between Fluorodihydroquinazolin Derivatives and Human Serum Albumin with Fluorescence Spectroscopy. Molecules. 2016; 21(10):1373. https://doi.org/10.3390/molecules21101373
Chicago/Turabian StyleWang, Yi, Meiqing Zhu, Feng Liu, Xiangwei Wu, Dandan Pan, Jia Liu, Shisuo Fan, Zhen Wang, Jun Tang, Risong Na, and et al. 2016. "Comparative Studies of Interactions between Fluorodihydroquinazolin Derivatives and Human Serum Albumin with Fluorescence Spectroscopy" Molecules 21, no. 10: 1373. https://doi.org/10.3390/molecules21101373






