Thymol Mitigates Cadmium Stress by Regulating Glutathione Levels and Reactive Oxygen Species Homeostasis in Tobacco Seedlings
Abstract
:1. Introduction
2. Results
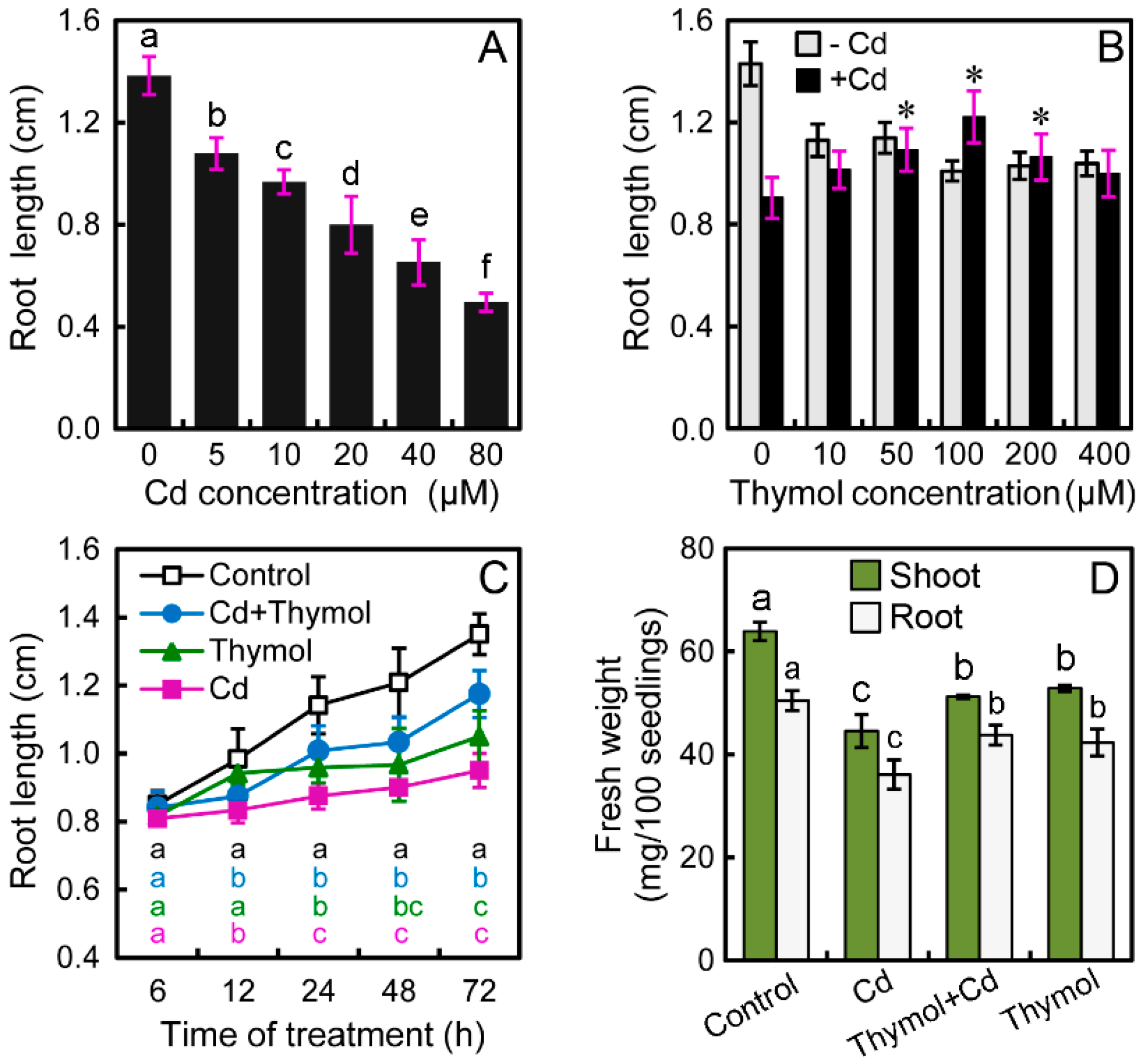
2.1. Thymol Significantly Alleviated Cd-Induced Growth Inhibition of Tobacco Seedlings
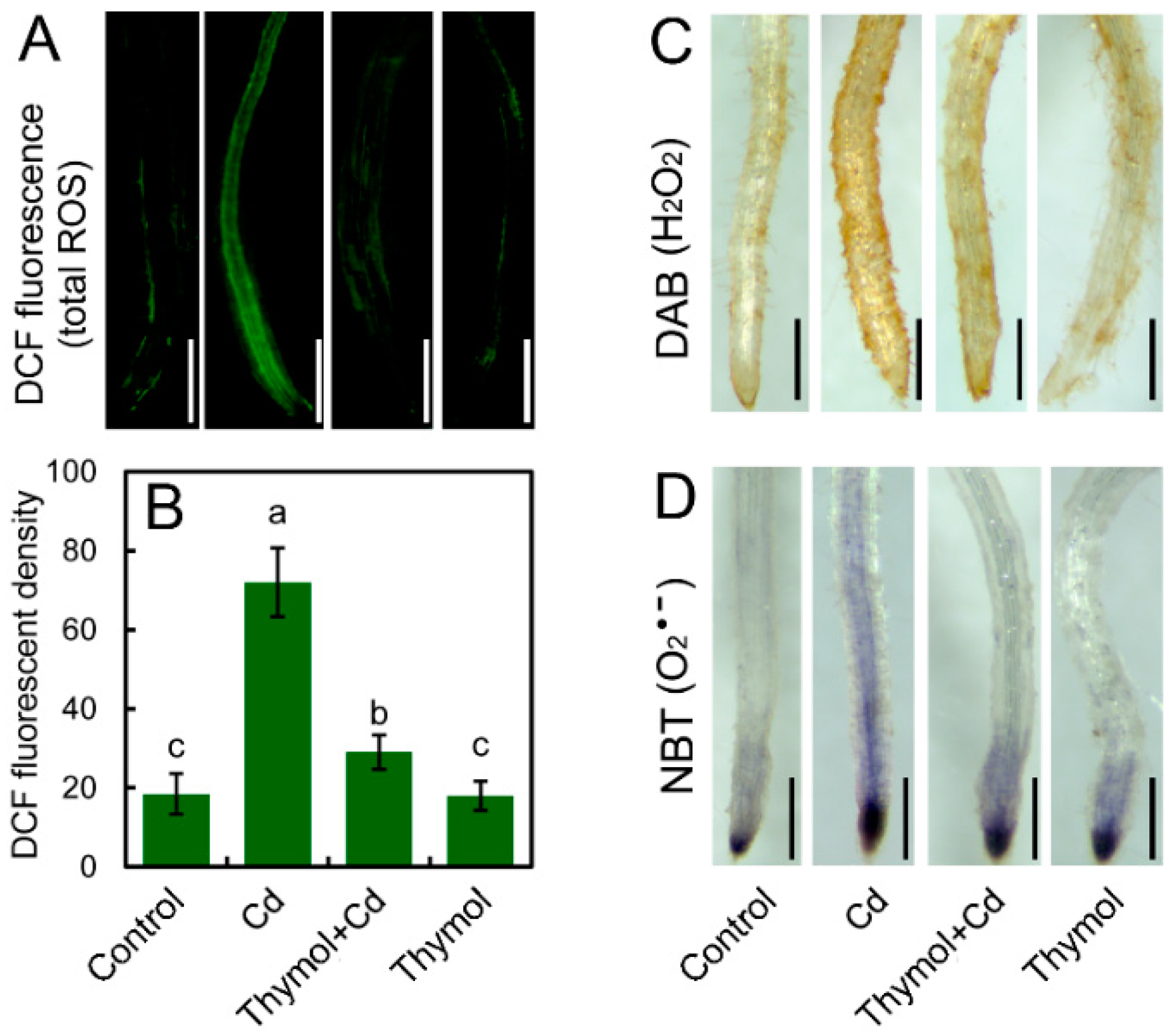
2.2. Thymol Blocked Cd-Induced ROS Accumulation in Tobacco Seedlings
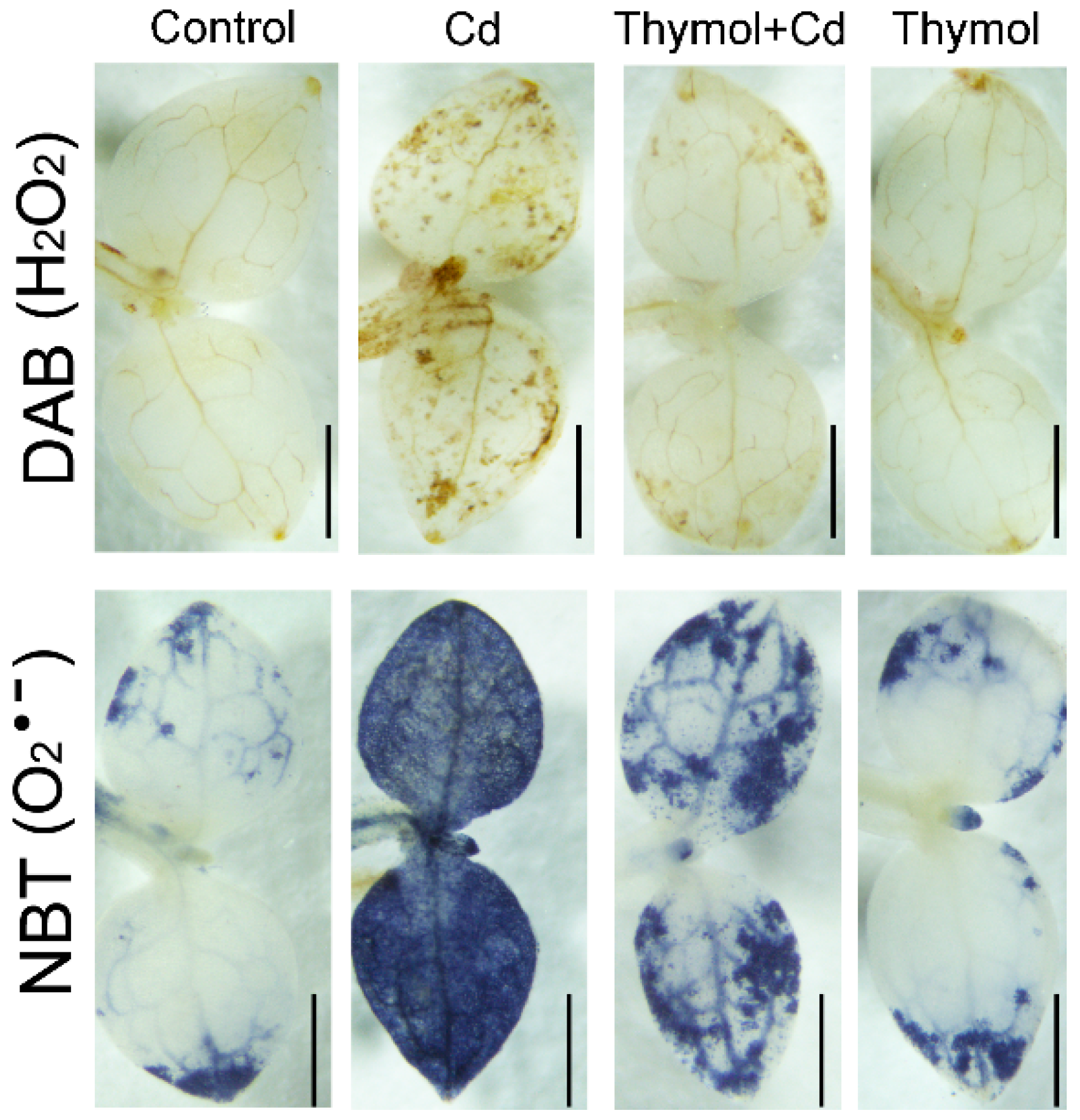
2.3. Thymol Ameliorate Cd-Induced Oxidative Injury in Tobacco Seedlings
2.4. Thymol Alleviated Cd-Induced Cell Death in Tobacco Seedlings
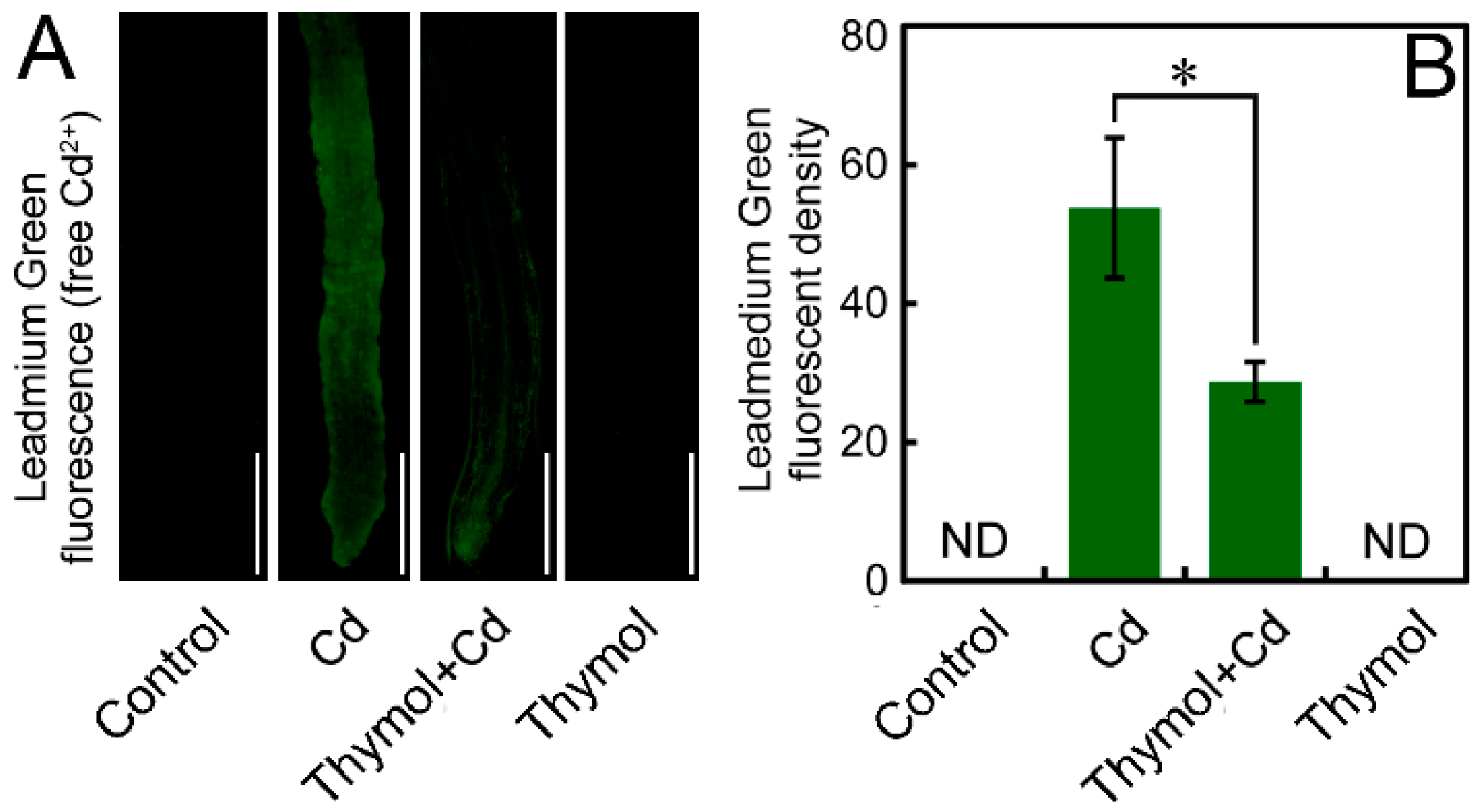
2.5. Thymol Decreased Free Cd2+ in the Roots of N. tabacum Seedlings
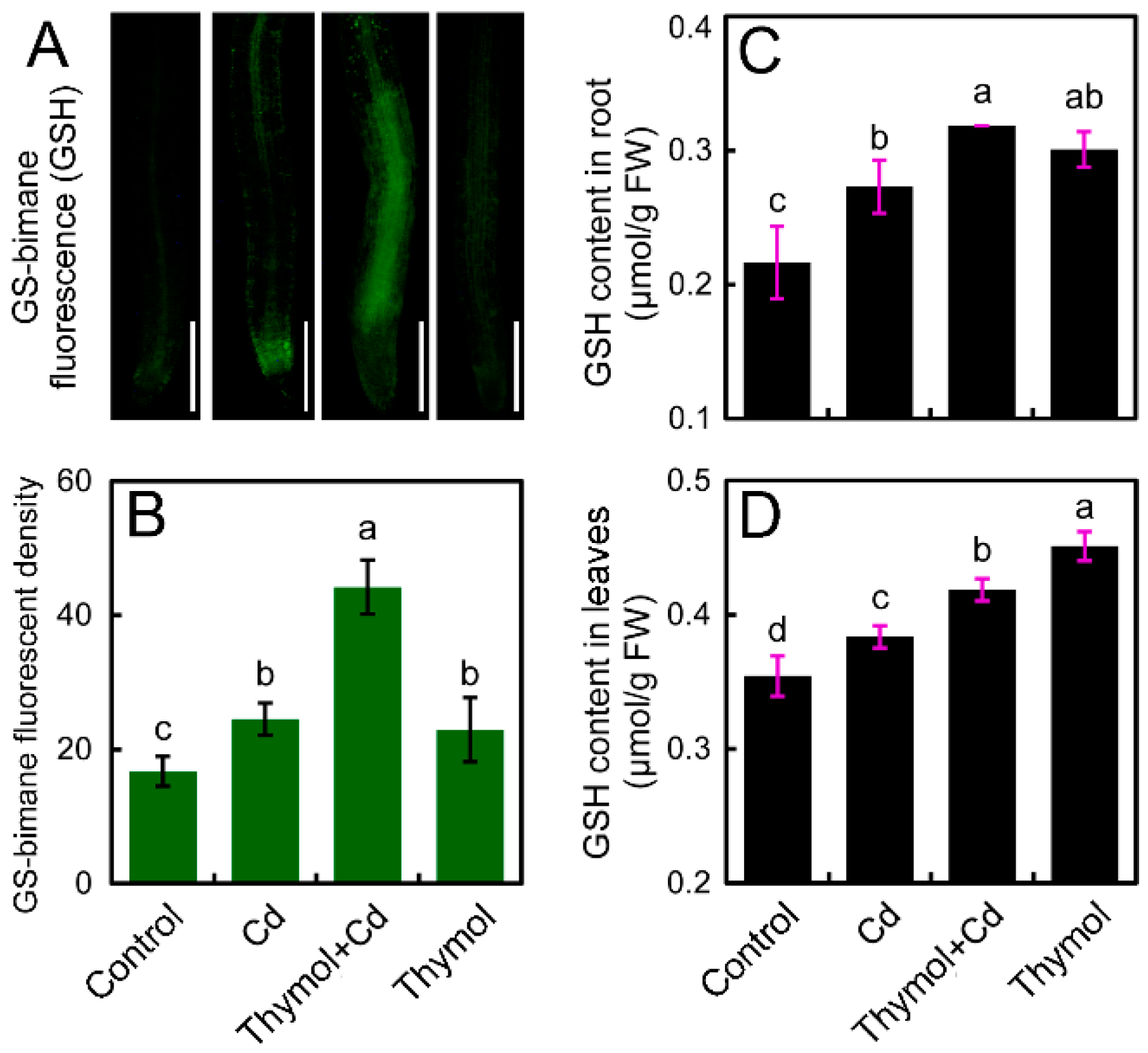
2.6. Thymol Enhanced the Content of GSH in the Roots of Cd-Treated Tobacco Seedlings
2.7. Thymol Stimulated the Expressin of GSH1 and PCS1 in the Roots of Cd-Treated Tobacco Seedlings
3. Discussion
4. Materials and Methods
4.1. Plant Culture and Treatment
4.2. Histochemical Analysis of Total ROS in Roots
4.3. Histochemical Detection of Intracellular H2O2 in Roots and Leaves
4.4. Histochemical Detection of Intracellular O2•− in Roots and Leaves
4.5. Histochemical Detection of Lipid Peroxidation in Roots and Leaves
4.6. Determination of TBARS Content
4.7. Histochemical Detection of Cell Death in Roots and Leaves
4.8. Histochemical Detection of Free Cd2+ in Roots
4.9. Histochemical Detection of GSH in Roots
4.10. Determination of GSH Content in Roots and Leaves
4.11. Analysis of Gene Expression
4.12. Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Samantaray, S.; Rout, G.R. Studies on cadmium toxicity in plants: A review. Environ. Pollut. 1997, 98, 29–36. [Google Scholar] [CrossRef]
- Chmielowska-Bąk, J.; Gzyl, J.; Rucińska-Sobkowiak, R.; Arasimowicz-Jelonek, M.; Deckert, J. The new insights into cadmium sensing. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef]
- Hernández, L.E.; Sobrino-Plata, J.; Montero-Palmero, M.B.; Carrasco-Gil, S.; Flores-Cáceres, M.L.; Ortega-Villasante, C.; Escobar, C. Contribution of glutathione to the control of cellular redox homeostasis under toxic metal and metalloid stress. J. Exp. Bot. 2015, 66, 2901–2911. [Google Scholar] [CrossRef] [PubMed]
- Pickering, I.J.; Prince, R.C.; George, G.N.; Rauser, W.E.; Wickramasinghe, W.A.; Watson, A.A.; Dameron, C.T.; Dance, I.G.; Fairlie, D.P.; Salt, D.E. X-ray absorption spectroscopy of cadmium phytochelatin and model systems. Biochim. Biophys. Acta (BBA)—Protein Struct. Mol. Enzymol. 1999, 1429, 351–364. [Google Scholar] [CrossRef]
- Vestergaard, M.; Matsumoto, S.; Nishikori, S.; Shiraki, K.; Hirata, K.; Takagi, M. Chelation of cadmium ions by phytochelatin synthase: role of the cysteine-rich C-terminal. Anal. Sci. 2008, 24, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, L.A.; Ronzhina, D.A.; Ivanov, L.A.; Stroukova, L.V.; Peuke, A.D.; Rennenberg, H. Over-expression of GSH1 in the cytosol affects the photosynthetic apparatus and improves the performance of transgenic poplars on heavy metal-contaminated soil. Plant Biol. Stuttg. 2011, 13, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, P.; Zanella, L.; Proia, A.; de Paolis, A.; Falasca, G.; Altamura, M.M.; Sanita di Toppi, L.; Costantino, P.; Cardarelli, M. Cadmium tolerance and phytochelatin content of Arabidopsis seedlings over-expressing the phytochelatin synthase gene AtPCS1. J. Exp. Bot. 2011, 62, 5509–5519. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Aarts, M.G.; Thomine, S.; Verbruggen, N. Plant science: The key to preventing slow cadmium poisoning. Trends Plant Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Parihar, P.; Singh, R.; SINGH, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics and ionomics. Front. Plant Sci. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Ulbricht, C.; Hammerness, P.; Bevins, A.; Sollars, D. Thyme (Thymus vulgaris L.), thymol. J. Herb. Pharmacother. 2004, 4, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Akthar, M.S.; Degaga, B.; Azam, T. Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms: A review. Issues Biol. Sci. Pharm. Res. 2014, 2, 1–7. [Google Scholar]
- Liang, D.; Li, F.; Fu, Y.; Cao, Y.; Song, X.; Wang, T.; Wang, W.; Guo, M.; Zhou, E.; Li, D.; et al. Thymol inhibits LPS-stimulated inflammatory response via down-regulation of NF-κB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation 2014, 37, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Riella, K.R.; Marinho, R.R.; Santos, J.S.; Pereira-Filho, R.N.; Cardoso, J.C.; Albuquerque-Junior, R.L.C.; Thomazzi, S.M. Anti-inflammatory and cicatrizing activities of thymol, a monoterpene of the essential oil from Lippia gracilis, in rodents. J. Ethnopharmacol. 2012, 143, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Nagoor Meeran, M.F.; Jagadeesh, G.S.; Selvaraj, P. Thymol, a dietary monoterpene phenol abrogates mitochondrial dysfunction in β-adrenergic agonist induced myocardial infarcted rats by inhibiting oxidative stress. Chem.-Biol. Interact. 2016, 244, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Castillo, S.; Pérez-Alfonso, C.O.; Martínez-Romero, D.; Guillén, F.; Serrano, M.; Valero, D. The essential oils thymol and carvacrol applied in the packing lines avoid lemon spoilage and maintain quality during storage. Food Control 2014, 35, 132–136. [Google Scholar] [CrossRef]
- González-Aguilar, G.; Ansorena, M.; Viacava, G.; Roura, S.; Ayala-Zavala, J. Plant Essential Oils as Antifungal Treatments on the Postharvest of Fruit and Vegetables. In Antifungal Metabolites from Plants; Razzaghi-Abyaneh, M., Rai, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 429–446. [Google Scholar]
- USEPA/IRIS Integrated Risk Information System. Available online: http://www.epa.gov/iris/ (accessed on 7 November 2014).
- National Library of Medicine Hazardous Substances Data Bank. Available online: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB (accessed on 7 November 2014).
- Kim, Y.S.; Hwang, J.W.; Kang, S.H.; Kim, E.H.; Jeon, Y.J.; Jeong, J.H.; Kim, H.R.; Moon, S.H.; Jeon, B.T.; Park, P.J. Thymol from Thymus quinquecostatus Celak. protects against tert-butyl hydroperoxide-induced oxidative stress in Chang cells. J. Nat. Med. 2014, 68, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Koul, O.; Walia, S.; Dhaliwal, G.S. Essential oils as green pesticides: Potential and constraints. Biopestic. Int. 2008, 4, 63–84. [Google Scholar]
- Abd-Elhakim, Y.M.; Mohamed, W.A.M. Assessment of the role of thymol in combating chromium (VI)-induced oxidative stress in isolated rat erythrocytes in vitro. Toxicol. Environ. Chem. 2015. [Google Scholar] [CrossRef]
- Shettigar, N.B.; Das, S.; Rao, N.B.; Rao, S.B. Thymol, a monoterpene phenolic derivative of cymene, abrogates mercury-induced oxidative stress resultant cytotoxicity and genotoxicity in hepatocarcinoma cells. Environ. Toxicol. 2015, 30, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Garnier, L.; Simon-Plas, F.; Thuleau, P.; Agnel, J.-P.; Blein, J.-P.; Ranjeva, R.; Montillet, J.-L. Cadmium affects tobacco cells by a series of three waves of reactive oxygen species that contribute to cytotoxicity. Plant Cell Environ. 2006, 29, 1956–1969. [Google Scholar] [CrossRef] [PubMed]
- Cho, U.-H.; Seo, N.-H. Oxidative stress in Arabidopsis thaliana exposed to cadmium is due to hydrogen peroxide accumulation. Plant Sci. 2005, 168, 113–120. [Google Scholar] [CrossRef]
- Pérez-Chaca, M.V.; RodrÍGuez-Serrano, M.; Molina, A.S.; Pedranzani, H.E.; Zirulnik, F.; Sandalio, L.M.; Romero-Puertas, M.C. Cadmium induces two waves of reactive oxygen species in Glycine max (L.) roots. Plant Cell Environ. 2014, 37, 1672–1687. [Google Scholar] [CrossRef] [PubMed]
- Van Breusegem, F.; Dat, J.F. Reactive oxygen species in plant cell death. Plant Physiol. 2006, 141, 384–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montillet, J.L.; Chamnongpol, S.; Rusterucci, C.; Dat, J.; van de Cotte, B.; Agnel, J.P.; Battesti, C.; Inze, D.; van Breusegem, F.; Triantaphylides, C. Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol. 2005, 138, 1516–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Kruk, I.; Michalska, T.; Lichszteld, K.; Kładna, A.; Aboul-Enein, H.Y. The effect of thymol and its derivatives on reactions generating reactive oxygen species. Chemosphere 2000, 41, 1059–1064. [Google Scholar] [CrossRef]
- Javan, A.J.; Javan, M.J. Electronic structure of some thymol derivatives correlated with the radical scavenging activity: Theoretical study. Food Chem. 2014, 165, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 2012. [Google Scholar] [CrossRef]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Xiang, C.; Werner, B.L.; Christensen, E.M.; Oliver, D.J. The biological functions of glutathione revisited in Arabidopsis transgenic plants with altered glutathione levels. Plant Physiol. 2001, 126, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Marino, D.; Dunand, C.; Puppo, A.; Pauly, N. A burst of plant NADPH oxidases. Trends Plant Sci. 2012, 17, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, D.M.; Umbach, A.L.; Subbaiah, C.C.; Siedow, J.N. Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 2006, 141, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Heyno, E.; Klose, C.; Krieger-Liszkay, A. Origin of cadmium-induced reactive oxygen species production: Mitochondrial electron transfer versus plasma membrane NADPH oxidase. New Phytol. 2008, 179, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Miller, G.; Morales, J.; Shulaev, V.; Torres, M.A.; Mittler, R. Respiratory burst oxidases: The engines of ROS signaling. Curr. Opin. Plant Biol. 2011, 14, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Deng, M.; Gong, M. Cd2+ stress induces two waves of H2O2 accumulation associated with ROS-generating system and ROS-scavenging system in cultured tobacco cells. Aust. J. Crop Sci. 2012, 6, 846–853. [Google Scholar]
- Kavoosi, G.; Teixeira da Silva, J.A.; Saharkhiz, M.J. Inhibitory effects of Zataria multiflora essential oil and its main components on nitric oxide and hydrogen peroxide production in lipopolysaccharide-stimulated macrophages. J. Pharm. Pharmacol. 2012, 64, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef] [PubMed]
- Zunino, M.P.; Zygadlo, J.A. Effect of monoterpenes on lipid oxidation in maize. Planta 2004, 219, 303–309. [Google Scholar] [PubMed]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Kim, J.H.; Jeong, C.Y.; Hong, S.W.; Lee, H. Inhibition of histone deacetylation alters Arabidopsis root growth in response to auxin via PIN1 degradation. Plant Cell Rep. 2013, 32, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.S.; Wang, S.J.; Yang, Z.M. Biological detection and analysis of mercury toxicity to alfalfa (Medicago sativa) plants. Chemosphere 2008, 70, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Frahry, G.; Schopfer, P. NADH-stimulated, cyanide-resistant superoxide production in maize coleoptiles analyzed with a tetrazolium-based assay. Planta 2001, 212, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Yang, Z.M. Nitric oxide reduces aluminum toxicity by preventing oxidative stress in the roots of Cassia tora L. Plant Cell Physiol. 2005, 46, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fan, F.; Zhuo, R.; Ma, F.; Gong, Y.; Wan, X.; Jiang, M.; Zhang, X. Expression of the laccase gene from a white rot fungus in Pichia pastoris can enhance the resistance of this yeast to H2O2-mediated oxidative stress by stimulating the glutathione-based antioxidative system. Appl. Environ. Microbiol. 2012, 78, 5845–5854. [Google Scholar] [CrossRef] [PubMed]
- Kellermeier, F.; Chardon, F.; Amtmann, A. Natural variation of Arabidopsis root architecture reveals complementing adaptive strategies to potassium starvation. Plant Physiol. 2013, 161, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhang, W.; Li, B.; Wang, Y.; Li, K.; Sodmergen; Han, C.; Zhang, Y.; Li, X. An endoplasmic reticulum response pathway mediates programmed cell death of root tip induced by water stress in Arabidopsis. New Phytol. 2010, 186, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, P.; Zanella, L.; De Paolis, A.; Di Litta, D.; Cecchetti, V.; Falasca, G.; Barbieri, M.; Altamura, M.M.; Costantino, P.; Cardarelli, M. Cadmium-inducible expression of the ABC-type transporter AtABCC3 increases phytochelatin-mediated cadmium tolerance in Arabidopsis. J. Exp. Bot. 2015, 66, 3815–3829. [Google Scholar] [CrossRef] [PubMed]
- Liso, R.; De Tullio, M.C.; Ciraci, S.; Balestrini, R.; La Rocca, N.; Bruno, L.; Chiappetta, A.; Bitonti, M.B.; Bonfante, P.; Arrigoni, O. Localization of ascorbic acid, ascorbic acid oxidase, and glutathione in roots of Cucurbita maxima L. J. Exp. Bot. 2004, 55, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Bi, J.; Wang, X.; Jiang, T.; Wu, C.; Tian, F.; Gao, X.; Wan, X.; Zheng, H. GLP-2 prevents intestinal mucosal atrophy and improves tissue antioxidant capacity in a mouse model of total parenteral nutrition. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.L.; Carezzano, M.; Giuliano, M.; Daghero, J.; Zygadlo, J.; Bogino, P.; Giordano, W.; Demo, M. Antimicrobial activity of essential oils of Thymus vulgaris and Origanum vulgare on phytopathogenic strains isolated from soybean. Plant Biol. Stuttg. 2015, 17, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Zhou, H.; Zhou, W.; Hu, L.; Chen, J.; Shi, Z. The fungicidal activity of thymol against Fusarium graminearum via inducing lipid peroxidation and disrupting ergosterol biosynthesis. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of thymol are available from the authors.



© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, X.; Ling, T.; Xue, Y.; Xu, C.; Zhou, W.; Hu, L.; Chen, J.; Shi, Z. Thymol Mitigates Cadmium Stress by Regulating Glutathione Levels and Reactive Oxygen Species Homeostasis in Tobacco Seedlings. Molecules 2016, 21, 1339. https://doi.org/10.3390/molecules21101339
Ye X, Ling T, Xue Y, Xu C, Zhou W, Hu L, Chen J, Shi Z. Thymol Mitigates Cadmium Stress by Regulating Glutathione Levels and Reactive Oxygen Species Homeostasis in Tobacco Seedlings. Molecules. 2016; 21(10):1339. https://doi.org/10.3390/molecules21101339
Chicago/Turabian StyleYe, Xiefeng, Tianxiao Ling, Yanfeng Xue, Cunfa Xu, Wei Zhou, Liangbin Hu, Jian Chen, and Zhiqi Shi. 2016. "Thymol Mitigates Cadmium Stress by Regulating Glutathione Levels and Reactive Oxygen Species Homeostasis in Tobacco Seedlings" Molecules 21, no. 10: 1339. https://doi.org/10.3390/molecules21101339




