Antidermatophytic Action of Resorcinol Derivatives: Ultrastructural Evidence of the Activity of Phenylethyl Resorcinol against Microsporum gypseum
Abstract
:1. Introduction
2. Results
2.1. Antifungal Activity
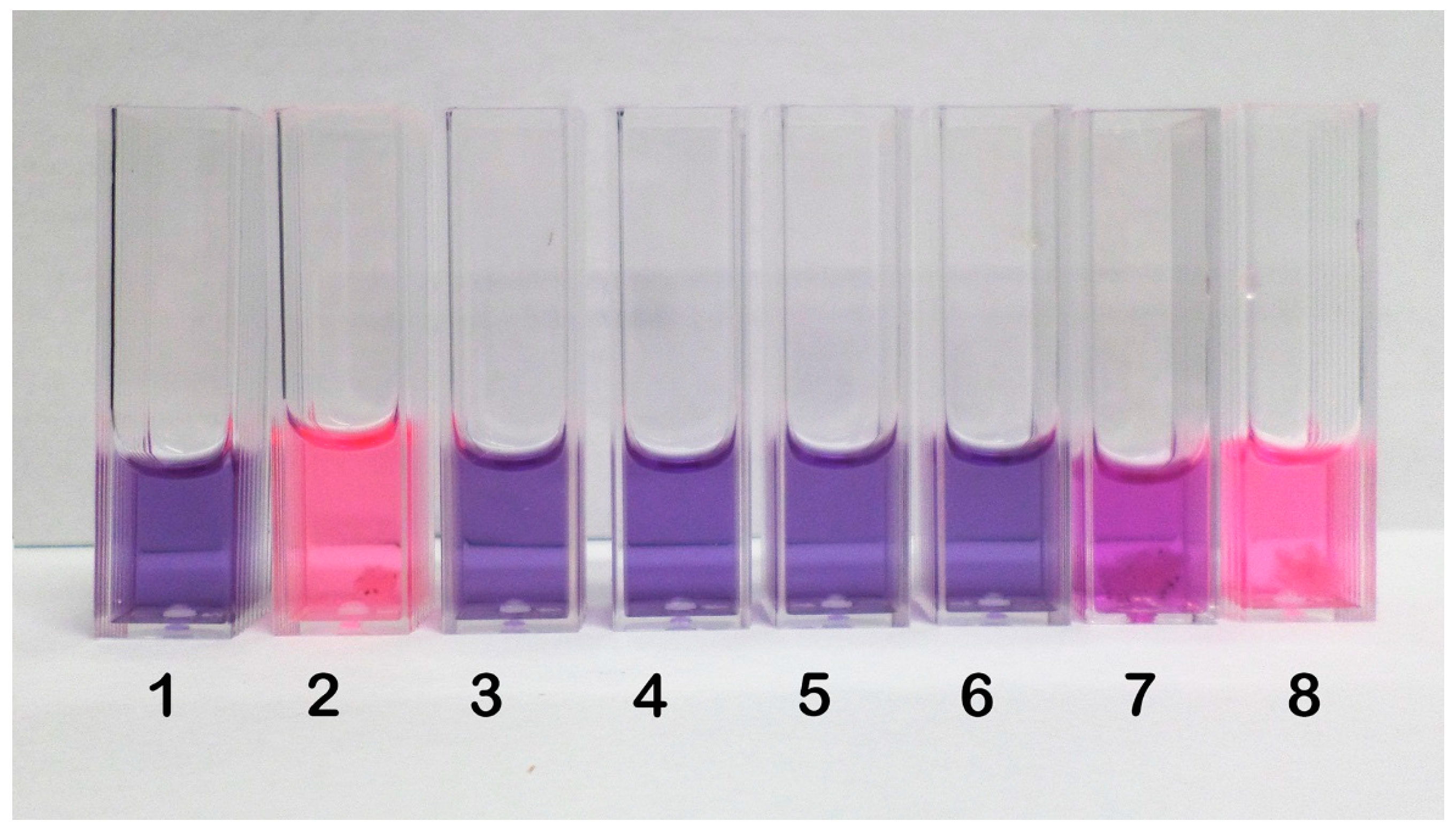
2.2. Inhibition of Spore Germination by Resazurin Assay
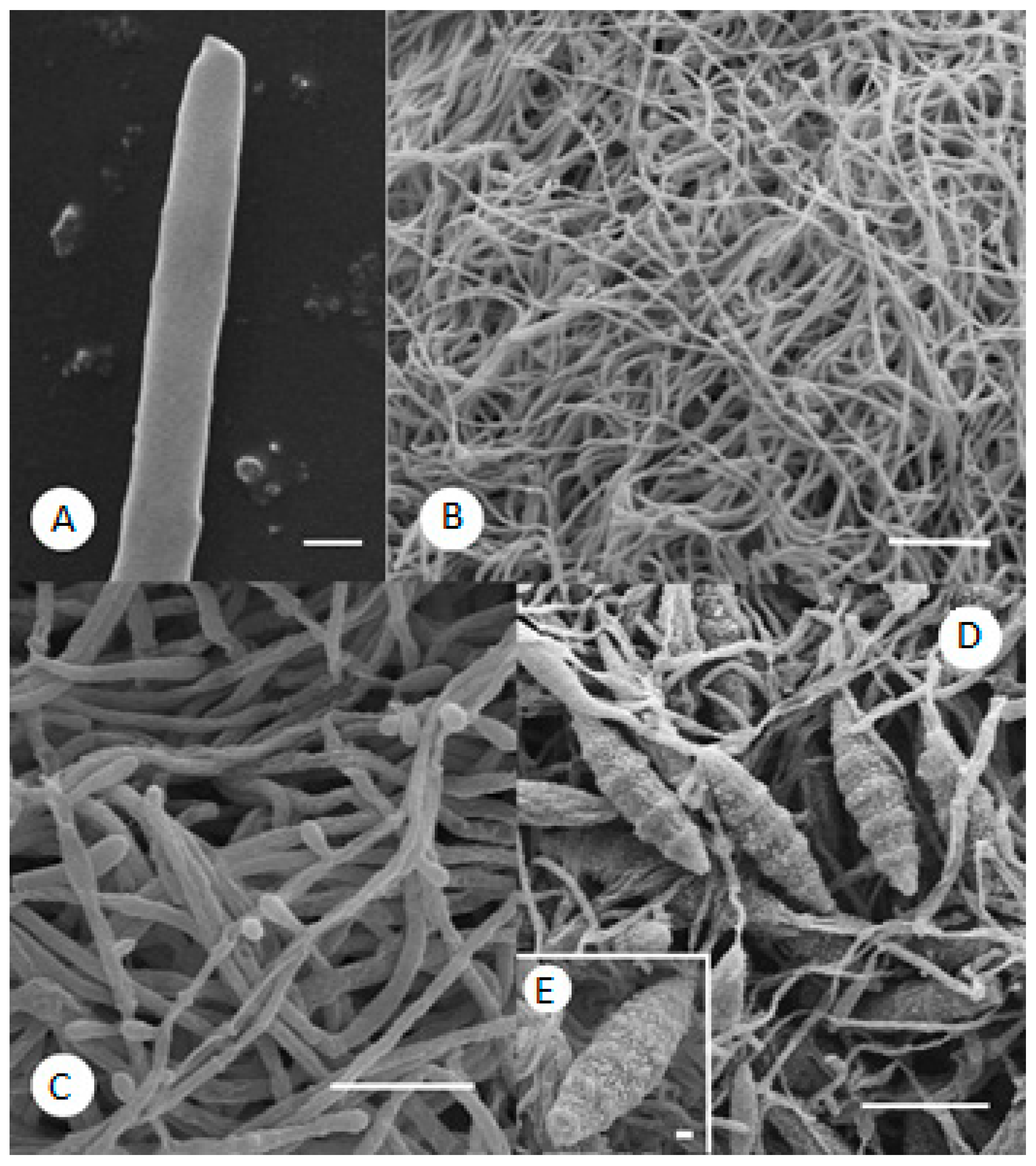
2.3. SEM Observations
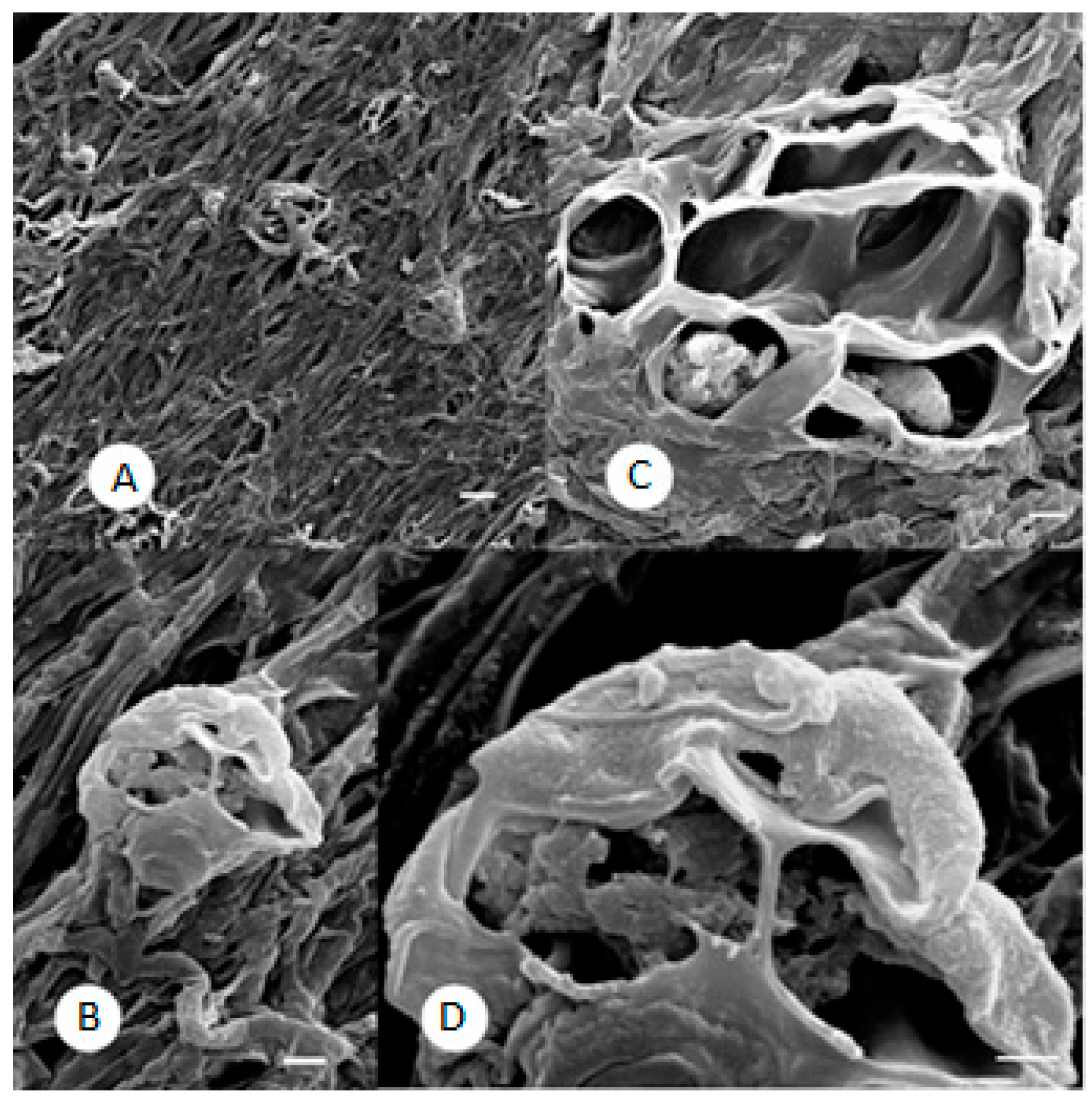
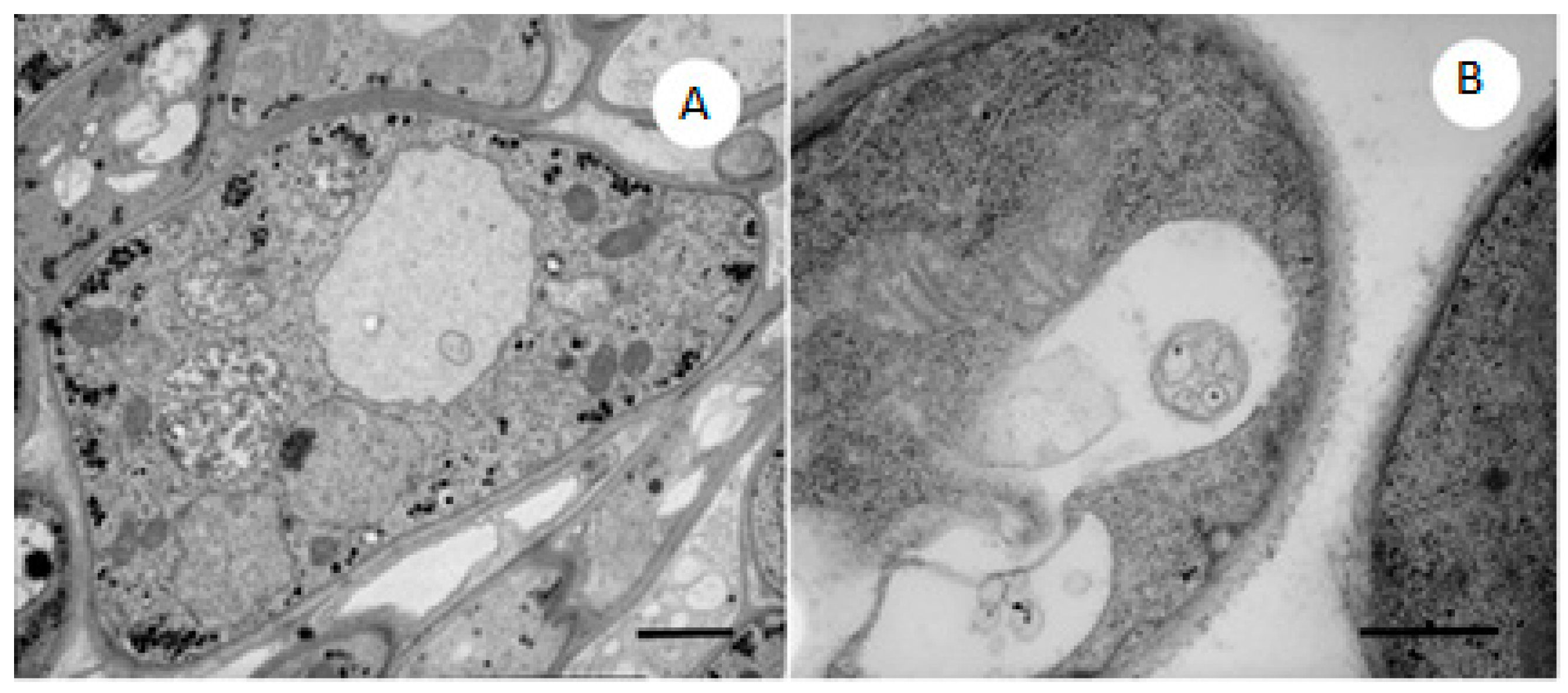
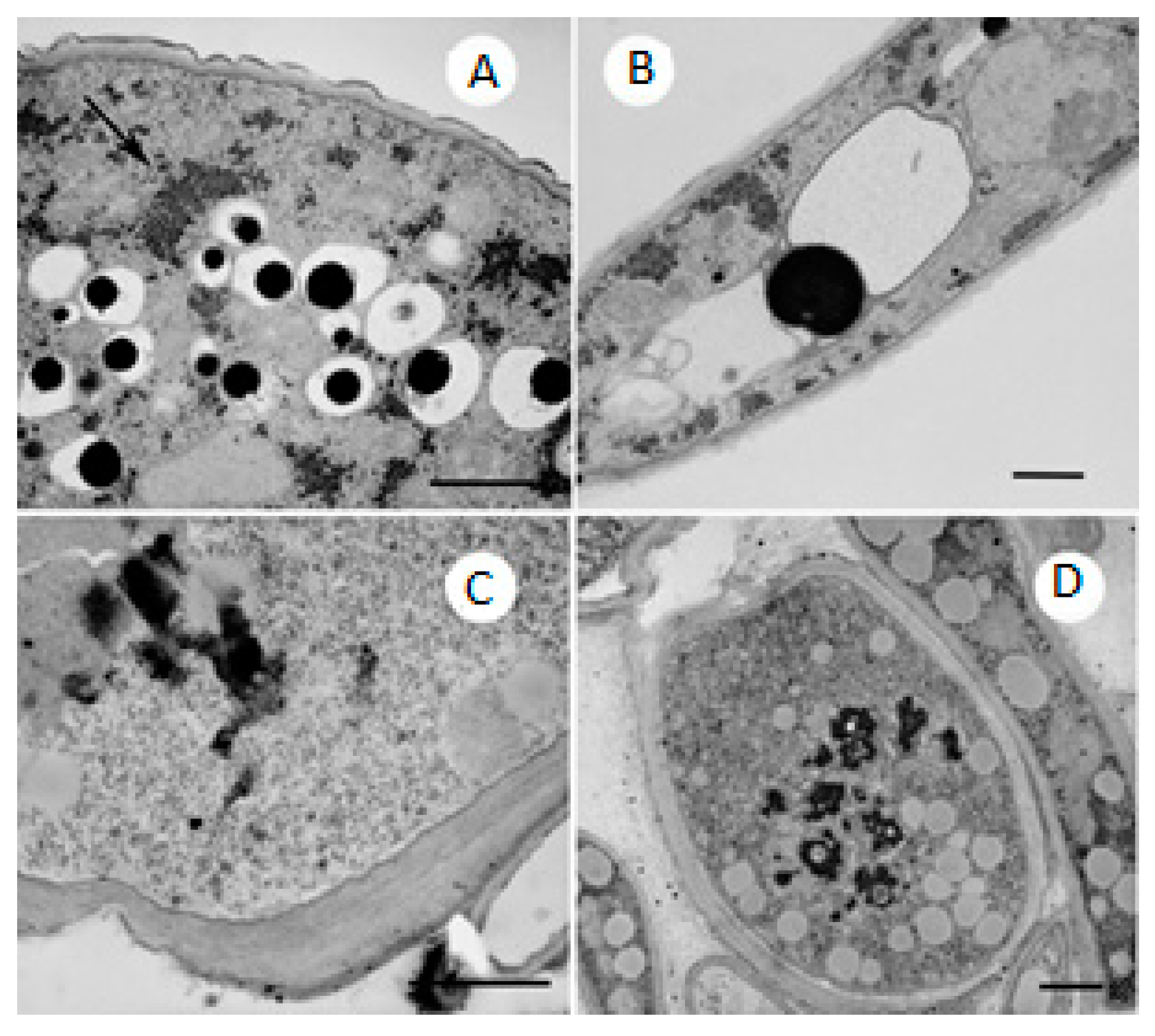
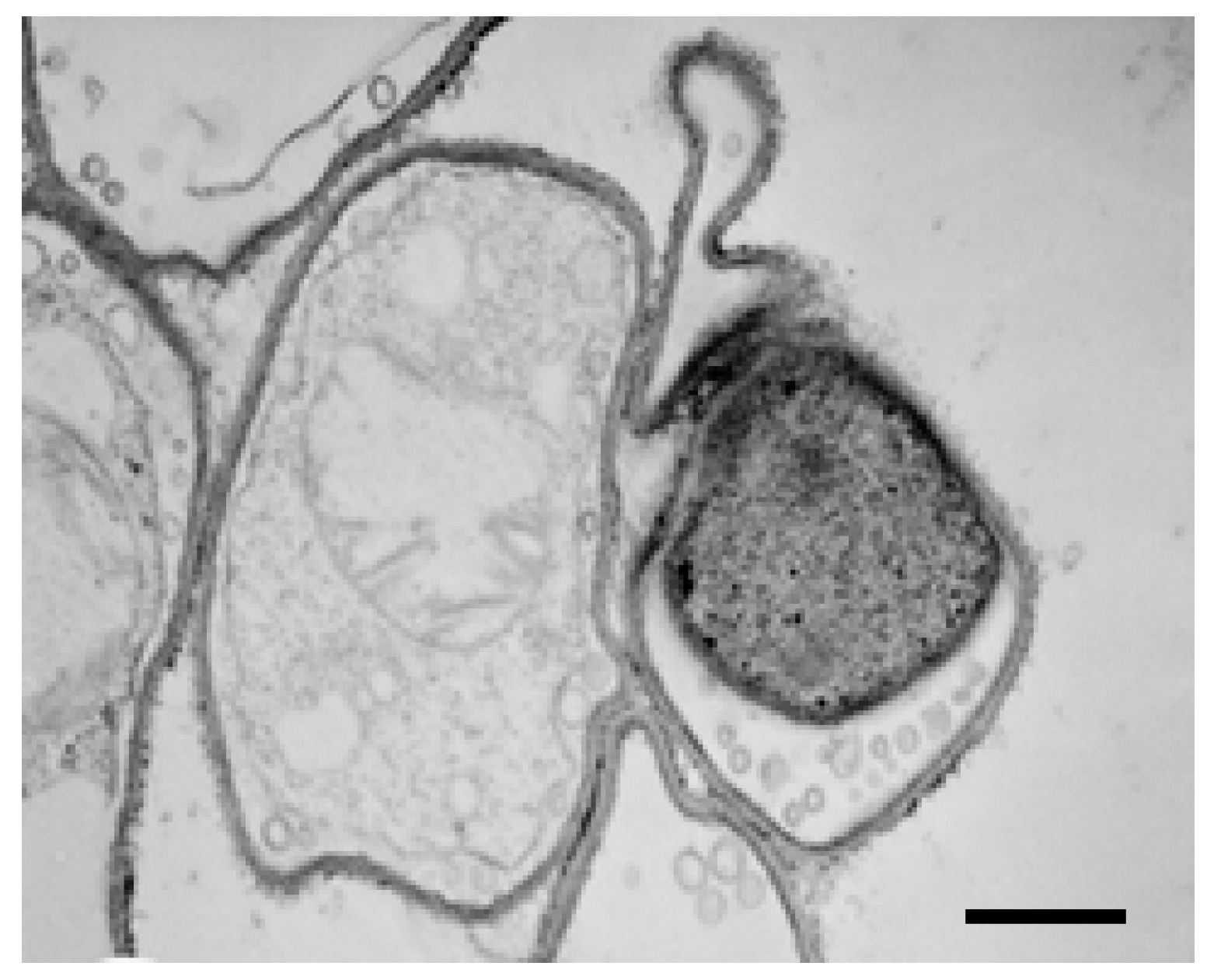
2.4. TEM Observations
3. Experimental Section
3.1. Microorganisms
3.2. Chemicals
3.3. Growth Inhibition
3.4. Spore Germination Assay
3.4.1. Evaluation of Spore Germination Inhibition
3.4.2. TEM and SEM
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Romagnoli, C.; Baldisserotto, A.; Malisardi, G.; Vicentini, C.B.; Mares, D.; Andreotti, E.; Vertuani, S.; Manfredini, S. A multi-target approach toward the development of novel candidates for antidermatophytic activity: Ultrastructural evidence on α-bisabolol-Treated Microsporum gypseum. Molecules 2015, 20, 11765–11776. [Google Scholar] [CrossRef] [PubMed]
- Martindale, W. The Extra Pharmacopoeia, 28th ed.; Pharmaceutical Press: London, UK, 1989; pp. 1637–1638. [Google Scholar]
- Birch, C.A. Castellani’s paint. Sir Aldo Castellani. (1877–1971). Practitioner 1974, 212, 895–896. [Google Scholar] [PubMed]
- Kozubek, A.; Tyman, J.H.P. Resorcinolic lipids, the natural non-isoprenoid phenolic amphiphiles and their biological activity. Chem. Rev. 1999, 99. [Google Scholar] [CrossRef]
- Adawadkar, P.D.; Elsohly, M.A. Isolation, purification and antimicrobial activity of anacardic acid from Ginko biloba fruits. Fitoterapia 1981, 52, 129–135. [Google Scholar]
- Stasiuk, M.; Kozubek, A. Biological activity of phenolic lipids. Cell. Mol. Life Sci. 2010, 67, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Tasaka, K.; Kamei, C.; Nakano, S.; Takeuchi, Y.; Yamato, M. Effects of certain resorcinol derivatives on the tyrosinase activity and the growth of melanoma cells. Methods Find. Exp. Clin. Pharmacol. 1998, 20, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Jimenez, A.; Teruel-Puche, J.A.; Ortiz-Ruiz, C.V.; Berna, J.; Tudela, J.; Garcia-Canovas, F. 4-n-Butylresorcinol, a depigmenting agent used in cosmetics, reacts with tyrosinase. IUBMB Life 2016, 68, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, R.; Riesco, J.M.; Pasqual, A.M. Dermatophyte morphology: A scanning electron microscopy study. Scanning Microsc. 1990, 4, 363–374. [Google Scholar]
- Ajello, L. The dermatophyte, Microsporum gypseum, as a saprophyte and parasite. J. Investig. Dermatol. 1953, 21, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, S.; Sawada, M.; Suzaki, R.; Kobayashi, K.; Ninomiya, J.; Tanaka, M.; Harada, T.; Kawana, S.; Uchida, H. Tineafaciei by Microsporum gypseum mimicking allergic reaction following cosmetic tattooing of the eyebrows. Med. Mycol. J. 2012, 53, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Mares, D. Fungal morphogenesis induced by natural and synthetic substances: Herniarin-induced alterations in the dermatophyte Microsporum cookie Ajello. Plant Biosyst. 2005, 139, 323–334. [Google Scholar] [CrossRef]
- Mares, D.; Romagnoli, C.; Sacchetti, G.; Vicentini, C.B.; Bruni, A. Morphological study of Trichophyton rubrum: Ultrastructural findings after treatment with 4-amino-3-methyl-1-phenylpyrazolo-(3,4-c)isothiazole. Med. Mycol. 1998, 36, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Pollack, J.K.; Harris, S.D.; Marten, M.R. Autophagy in filamentous fungi. Fungal Genet. Biol. 2009, 46, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Palmer, G.E.; Askew, D.S.; Williamson, P.R. The diverse roles of autophagy in medically important fungi. Autophagy 2008, 4, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Osherov, N.; Yarden, O. The cell wall of filamentous fungi. In Cellular and Molecular Biology of Filamentous Fungi; Borkovich, K.A., Ebbole, D.J., Eds.; ASM Press: Washington, DC, USA, 2010; pp. 224–237. [Google Scholar]
- Wilson, C.L.; Jumper, G.A.; Mason, D.L. Vacuole dynamics in fungal plant pathogens. Phytopathology 1980, 70, 783–788. [Google Scholar] [CrossRef]
- Kozubek, A.; Demel, R.A. The effect of 5-(n-alk(en)yl)resorcinols from rye on membrane structure. Biochim. Biophys. Acta 1981, 642, 242–251. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
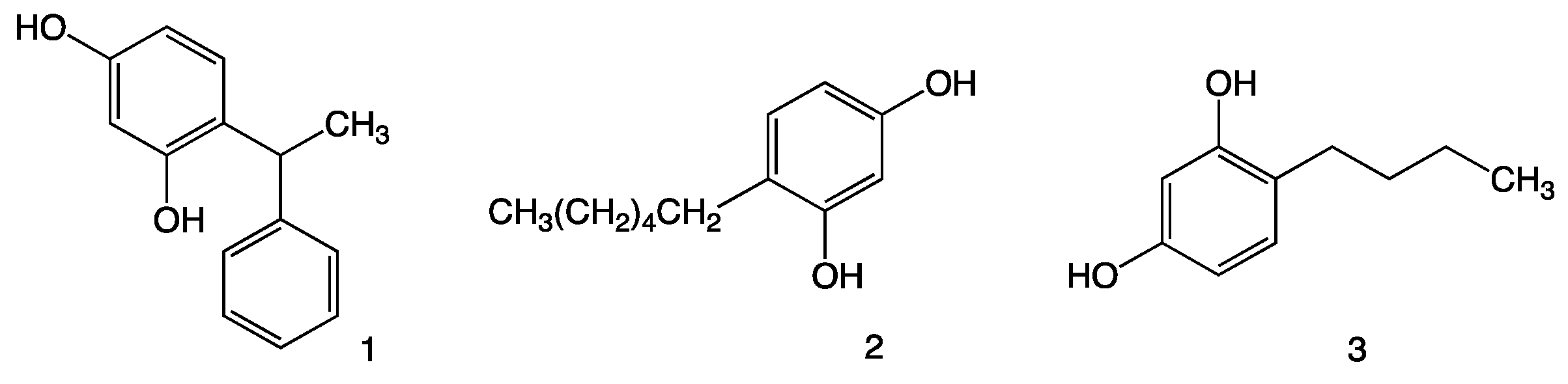
| Compound | 5 μg/mL | 10 μg/mL | 20 μg/mL | 50 μg/mL | 100 μg/mL | 200 μg/mL |
|---|---|---|---|---|---|---|
| M. gypseum | ||||||
| 4-Hexylresorcinol | 5.5 | 21.12 | 71.5 | 88.32 | 93.46 | 94.57 |
| Phenylethylresorcinol | 20.99 | 34.16 | 54.73 | 75.31 | 98.51 | 99.50 |
| 4-Butylresorcinol | 25.00 | 33.33 | ||||
| M. canis | ||||||
| 4-Hexylresorcinol | 17.5 | 32.5 | 82.5 | 85 | 92.68 | 97.5 |
| Phenylethylresorcinol | + | + | 10 | 47.5 | 94.74 | 95 |
| 4-Butylresorcinol | + | + | ||||
| T. violaceum | ||||||
| 4-Hexylresorcinol | 12.46 | 21.45 | 37.02 | 59.21 | 63.16 | 66.09 |
| Phenylethylresorcinol | 0.97 | 3.38 | 5.80 | 31.40 | 68.92 | 100 |
| 4-Butylresorcinol | + | + | ||||
| A. cajetani | ||||||
| 4-Hexylresorcinol | 18.11 | 43.31 | 69.29 | 74.02 | 78.13 | 82.29 |
| Phenylethylresorcinol | + | 13.77 | 39.86 | 67.39 | 84.56 | 100 |
| 4-Butylresorcinol | + | + | ||||
| T. mentagrophytes | ||||||
| 4-Hexylresorcinol | 31.48 | 43.52 | 81.08 | 82.41 | 87.04 | 88.89 |
| Phenylethylresorcinol | 5.88 | 10.92 | 36.13 | 93.28 | 99.16 | 100 |
| 4-Butylresorcinol | 16.67 | 45 | ||||
| E. floccosum | ||||||
| 4-Hexylresorcinol | 5.73 | 20.31 | 50.00 | 69.27 | 78.13 | 86.40 |
| Phenylethylresorcinol | 22.42 | 24.66 | 36.32 | 47.09 | 66.28 | 85.82 |
| 4-Butylresorcinol | 12.61 | 19.82 | ||||
| N. gypsea | ||||||
| 4-Hexylresorcinol | 13.61 | 19.73 | 53.74 | 76.19 | 83.67 | 85.47 |
| Phenylethylresorcinol | 11.56 | 16.76 | 31.21 | 50.87 | 82.66 | 97.78 |
| 4-Butylresorcinol | + | 21.43 | ||||
| T. rubrum | ||||||
| 4-Hexylresorcinol | 28.95 | 38.16 | 59.65 | 73.68 | 78.95 | 81.90 |
| Phenylethylresorcinol | 18.41 | 26.37 | 39.30 | 65.17 | 70.12 | 81.33 |
| 4-Butylresorcinol | + | 5.86 | ||||
| T. tonsurans | ||||||
| 4-Hexylresorcinol | 4.6875 | 18.75 | 75 | 85.94 | 92.19 | 93.10 |
| Phenylethylresorcinol | 12.5 | 14.0625 | 37.5 | 89.0625 | 100 | 100 |
| 4-Butylresorcinol | + | + | ||||
| Compound | 5 μg/mL | 10 μg/mL | 20 μg/mL | 50 μg/mL | 100 μg/mL | 200 μg/mL |
|---|---|---|---|---|---|---|
| M. gypseum | ||||||
| Fluconazole | 12.34 | 34.54 | 55.12 | 76.01 | 80.23 | 85.40 |
| Phenylethyl resorcinol | 19.65 | 35.48 | 58.12 | 74.07 | 97.98 | 99.02 |
| M. canis | ||||||
| Fluconazole | 17.12 | 26.48 | 39.95 | 61.87 | 80.43 | 88.51 |
| Phenylethyl resorcinol | + | + | 11.32 | 46.80 | 93.54 | 96.11 |
| T. violaceum | ||||||
| Fluconazole | 36.76 | 67.65 | 83.78 | 81.33 | 97.08 | 94.65 |
| Phenylethylresorcinol | 1.13 | 5.43 | 6.02 | 27.58 | 69.05 | 100 |
| A. cajetani | ||||||
| Fluconazole | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 8.67 |
| Phenylethylresorcinol | + | 13.77 | 39.86 | 67.39 | 84.56 | 100 |
| T. mentagrophytes | ||||||
| Fluconazole | 62.46 | 79.67 | 100 | 100 | 100 | 100 |
| Phenylethylresorcinol | 7.31 | 11.96 | 35.56 | 94.00 | 98.00 | 100 |
| E. floccosum | ||||||
| Fluconazole | 98.00 | 100 | 100 | 100 | 100 | 100 |
| Phenylethylresorcinol | 21.79 | 25.11 | 39.87 | 46.87 | 68.53 | 87.67 |
| N. gypsea | ||||||
| Fluconazole | 0.0 | 0.0 | 0.0 | 0.0 | 1.09 | 5.78 |
| Phenylethylresorcinol | 13.43 | 18.99 | 35.11 | 50.95 | 83.74 | 98.89 |
| T. rubrum | ||||||
| Fluconazole | 13.13 | 25.66 | 40.44 | 58.76 | 68.68 | 76.06 |
| Phenylethylresorcinol | 17.15 | 28.55 | 39.92 | 64.69 | 72.34 | 84.48 |
| T. tonsurans | ||||||
| Fluconazole | 27.77 | 48.56 | 44.12 | 72.33 | 71.33 | 79.90 |
| Phenylethylresorcinol | 13.17 | 16.44 | 35.57 | 89.74 | 100 | 100 |
| Dermatophyte | 4-Hexylresorcinol | Phenylethylresorcinol |
|---|---|---|
| IC50 μg/mL | ||
| M. gypseum | 18.77 | 11.42 |
| M. canis | 14.02 | 43.47 |
| T. violaceum | 48.39 | 54.60 |
| A. cajetani | 16.22 | 30.87 |
| T. mentagrophytes | 12.97 | 24.68 |
| E. floccosum | 29.42 | 37.40 |
| N. gypsea | 24.72 | 33.64 |
| T. rubrum | 16.22 | 31.42 |
| T. tonsurans | 20.09 | 23.24 |
| Compound | Concentration | % Inhibition of Spore Germination |
|---|---|---|
| 4-Hexylresorcinol | 100 μg/mL | 100 |
| 200 μg/mL | 100 | |
| Phenylethylresorcinol | 100 μg/mL | 100 |
| 200 μg/mL | 100 | |
| 4-Butylresorcinol | 100 μg/mL | 13.5 ± 0.8 |
| 200 μg/mL | 80.2 ± 1.1 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romagnoli, C.; Baldisserotto, A.; Vicentini, C.B.; Mares, D.; Andreotti, E.; Vertuani, S.; Manfredini, S. Antidermatophytic Action of Resorcinol Derivatives: Ultrastructural Evidence of the Activity of Phenylethyl Resorcinol against Microsporum gypseum. Molecules 2016, 21, 1306. https://doi.org/10.3390/molecules21101306
Romagnoli C, Baldisserotto A, Vicentini CB, Mares D, Andreotti E, Vertuani S, Manfredini S. Antidermatophytic Action of Resorcinol Derivatives: Ultrastructural Evidence of the Activity of Phenylethyl Resorcinol against Microsporum gypseum. Molecules. 2016; 21(10):1306. https://doi.org/10.3390/molecules21101306
Chicago/Turabian StyleRomagnoli, Carlo, Anna Baldisserotto, Chiara B. Vicentini, Donatella Mares, Elisa Andreotti, Silvia Vertuani, and Stefano Manfredini. 2016. "Antidermatophytic Action of Resorcinol Derivatives: Ultrastructural Evidence of the Activity of Phenylethyl Resorcinol against Microsporum gypseum" Molecules 21, no. 10: 1306. https://doi.org/10.3390/molecules21101306






