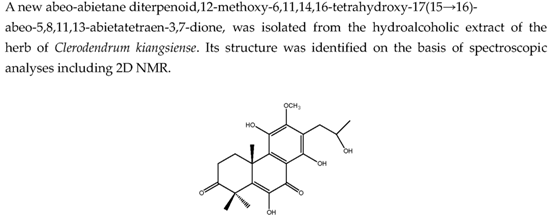
Bioactive Diterpenoids from Clerodendrum kiangsiense
Abstract
:1. Introduction
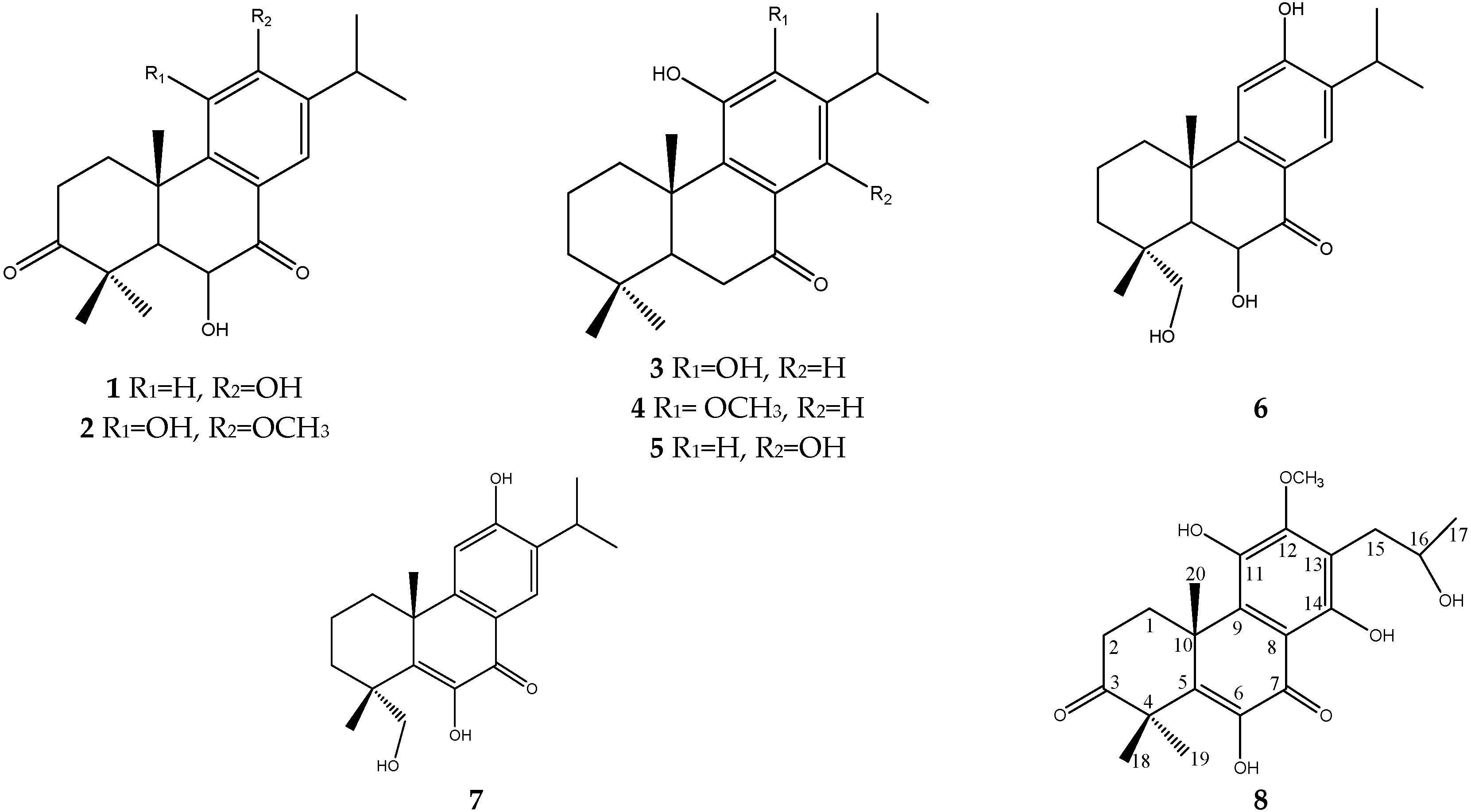
2. Results and Discussion

| NO | δH (J in Hz) | δC | HMBC |
|---|---|---|---|
| 1 | 1.80, m, α 3.32, m, β | 26.9, CH2 | C-2, C-3, C-10, C-20 |
| 2 | 2.71, m, α 2.73, m, β | 33.2, CH2 | C-1, C-3, C-4, C-10 |
| 3 | 214.0, qC | ||
| 4 | 48.8, qC | ||
| 5 | 140.3, qC | ||
| 6 | 142.4, qC | ||
| 7 | 183.6, qC | ||
| 8 | 109.5, qC | ||
| 9 | 139.3, qC | ||
| 10 | 40.6, qC | ||
| 11 | 132.9, qC | ||
| 12 | 152.6, qC | ||
| 13 | 118.7, qC | ||
| 14 | 155.2, qC | ||
| 15 | 2.91, dd (13.8, 4.0) a 2.83, dd (13.7, 8.3) a | 32.9, CH2 | C-12, C-13, C-14, C-16, C-17 |
| 16 | 4.18, m | 67.8, CH | C-15, C-17 |
| 17 | 1.28, d (6.2) | 23.8, CH3 | C-15, C-16 |
| 18 | 1.58, s | 21.0, CH3 | C-3, C-4, C-5, C-19 |
| 19 | 1.53, s | 24.3, CH3 | C-3, C-4, C-5, C-18 |
| 20 | 1.44, s | 20.0, CH3 | C-1, C-5, C-9, C-10 |
| 21-OCH3 | 3.87, s | 61.7,OCH3 | C-12 |
| Compounds | HL-60 | SMMC-7721 | A-549 | MCF-7 |
|---|---|---|---|---|
| 1 | 12.5 ± 1.2 | 13.6 ± 0.8 | 7.4 ± 1.1 | 27.7 ± 2.2 |
| 2 | 18.6 ± 1.8 | 15.9 ±1.6 | 10.2 ± 1.2 | 15.4 ± 2.7 |
| 3 | 23.5 ± 2.0 | 33.0 ± 2.4 | 10.4 ± 1.3 | 19.2 ± 1.8 |
| 4 | 9.9 ± 0.9 | 6.7 ± 1.0 | 8.7 ± 0.8 | 10.0 ± 0.8 |
| 5 | 15.5 ± 1.9 | 15.7 ± 1.6 | 11.8 ± 2.4 | 22.4 ± 2.9 |
| 6 | 4.8 ± 0.5 | 3.8 ± 0.9 | 2.7 ± 0.7 | 5.0 ± 1.0 |
| 7 | 15.7 ± 1.8 | 5.8 ± 0.8 | 7.9 ± 0.7 | 19.2 ± 1.4 |
| 8 | 1.8 ± 0.3 | 4.9 ± 0.7 | 2.5 ± 0.7 | 3.1 ± 0.5 |
| Cisplatin | 4.2 ± 0.5 | 5.9 ± 0.9 | 9.8 ± 1.1 | 11.3 ± 1.0 |
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Anti-Proliferative Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gazim, Z.C.; Rodrigues, F.; Amorin, A.C.; de Rezende, C.M.; Sokovic, M.; Tesevic, V.; Vuckovic, I.; Krstic, G.; Cortez, L.E.; Colauto, N.B.; et al. New natural diterpene-type abietane from Tetradenia riparia essential oil with cytotoxic and antioxidant activities. Molecules 2013, 19, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.R.; Feng, D.Q.; Xu, Y.K. A new triterpenoid bearing octacosanoate from the stems and roots of Clerodendrum philippinum var. simplex (Verbenaceae). Nat. Prod. Res. 2015, 29, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Bharitkar, Y.P.; Hazra, A.; Shah, S.; Saha, S.; Matoori, A.K.; Mondal, N.B. New flavonoid glycosides and other chemical constituents from Clerodendrum phlomidis leaves: Isolation and characterisation. Nat. Prod. Res. 2015, 29, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Kim, J.R.; Han, J.M.; Jang, K.C.; Sok, D.E.; Jeong, T.S. Antioxidant activities of abietane diterpenoids isolated from Torreya nucifera leaves. J. Agric. Food Chem. 2006, 54, 5369–5374. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, H.J.; Li, P.F.; Yang, Y.B.; Wu, L.H.; Chou, G.X.; Wang, Z.T. Diterpenoids and phenylethanoid glycosides from the roots of Clerodendrum bungei and their inhibitory effects against angiotensin converting enzyme and alpha-glucosidase. Phytochemistry 2014, 103, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Panthong, A.; Kanjanapothi, D.; Taesotikul, T.; Wongcome, T.; Reutrakul, V. Anti-inflammatory and antipyretic properties of Clerodendrum petasites S. Moore. J. Ethnopharmacol. 2003, 85, 151–156. [Google Scholar] [CrossRef]
- Patel, J.J.; Acharya, S.R.; Acharya, N.S. Clerodendrum serratum (L.) Moon—A review on traditional uses, phytochemistry and pharmacological activities. J. Ethnopharmacol. 2014, 154, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Waliullah, T.M.; Yeasmin, A.M.; Alam, A.; Islam, W.; Hassan, P. In vitro antimicrobial study for biological evaluation of Clerodendrum infortunatum Linn. Recent Pat. Anti-Infect. Drug Discov. 2015, 10, 98–104. [Google Scholar] [CrossRef]
- Xu, R.L.; Wang, R.; Ding, L.; Shi, Y.P. New cytotoxic steroids from the leaves of Clerodendrum trichotomum. Steroids 2013, 78, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.D.; Li, X.; Zhao, J.H.; Liu, Y.H.; Row, K.H. Abietane diterpenoids from Isodon inflexus. Phytochemistry 2012, 81, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Hasuda, T.; Hitotsuyanagi, Y.; Aoyagi, Y.; Fujikawa, N.; Onozaki, A.; Watanabe, A.; Kinoshita, T.; Takeya, K. Abietane diterpenoids and a sesquiterpene pyridine alkaloid from Euonymus lutchuensis. J. Nat. Prod. 2013, 76, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Fang, J.M.; Cheng, Y.S. Diterpenes from Taxus mairei. Phytochemistry 1998, 49, 2037–2043. [Google Scholar] [CrossRef]
- Rodriguez, B. Structural and spectral assignment by two-dimensional NMR of two new derivatives of the abietane diterpenoid taxodione. Magn. Reson. Chem. 2005, 43, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Fronza, M.; Murillo, R.; Slusarczyk, S.; Adams, M.; Hamburger, M.; Heinzmann, B.; Laufer, S.; Merfort, I. In vitro cytotoxic activity of abietane diterpenes from Peltodon longipes as well as Salvia miltiorrhiza and Salvia sahendica. Bioorg. Med. Chem. 2011, 19, 4876–4881. [Google Scholar] [CrossRef] [PubMed]
- Min, Z.D.; Jiang, H.; Liang, J.Y. Studies on the taxane diterpenes of the heartwood from Taxus mairei. Yao Xue Xue Bao 1989, 24, 673–677. [Google Scholar] [PubMed]
- Yao, S.; Tang, C.P.; Ke, C.Q.; Ye, Y. Abietane diterpenoids from the bark of Cryptomeria fortunei. J. Nat. Prod. 2008, 71, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Ni, W.; Yan, H.; Li, G.T.; Zhong, H.M.; Li, Y.; Liu, H.Y. Daphnane-type diterpenoid glucosides and further constituents of Euphorbia pilosa. Chem. Biodivers. 2014, 11, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhang, T.; Zhang, X.; Zhang, J.; Zhao, C. An alkaloid and a steroid from the endophytic fungus Aspergillus fumigatus. Molecules 2015, 20, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Caamal-Fuentes, E.E.; Peraza-Sanchez, S.R.; Torres-Tapia, L.W.; Moo-Puc, R.E. Isolation and identification of cytotoxic compounds from Aeschynomene fascicularis, a Mayan medicinal plant. Molecules 2015, 20, 13563–13574. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Wang, S.; Jia, O.; Zhu, Q.; Shi, L. Bioactive Diterpenoids from Clerodendrum kiangsiense. Molecules 2016, 21, 86. https://doi.org/10.3390/molecules21010086
Xu M, Wang S, Jia O, Zhu Q, Shi L. Bioactive Diterpenoids from Clerodendrum kiangsiense. Molecules. 2016; 21(1):86. https://doi.org/10.3390/molecules21010086
Chicago/Turabian StyleXu, Mingfeng, Shengjia Wang, Ouya Jia, Qin Zhu, and Lu’e Shi. 2016. "Bioactive Diterpenoids from Clerodendrum kiangsiense" Molecules 21, no. 1: 86. https://doi.org/10.3390/molecules21010086