Low Temperature Soda-Oxygen Pulping of Bagasse
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Depithed Bagasse
| Species | Holecellulose (%) | Cellulose a (%) | Klason Lignin (%) | Pentosans (%) | 1% NaOH Extractive (%) | Cellulose: Lignin |
|---|---|---|---|---|---|---|
| Bagasse (Xinping) | 77.1 | 48.8 | 20.2 | 24.3 | 28.5 | 2.4 |
| Bagasse (refs) | ND b | 46.2–54.8 | 17.6–22.9 | 23.0–27.6 | 27.7–41.0 | 2.0–3.1 |
| Wheat straw | ND b | 38.2 | 15.1 | ND b | ND b | 2.5 |
| Rice straw | 58.9 | 34.4 | 22.9 | ND b | ND b | 1.5 |
| Carpolobia lutea | ND b | 44.1 | 26.0 | ND b | 21.0 | 1.7 |
2.2. Soda-Oxygen Pulping of Bagasse at Lower Temperatures
2.2.1. Effect of Temperature
| Tmax (°C) | Pulp Properties | Black Liquor | |||||
|---|---|---|---|---|---|---|---|
| Screened Yield (%) | Rejects (%) | Kappa Number | Viscosity (dm3/kg) | Brightness (% ISO) | pH Value | Residual NaOH (g/L) | |
| 125 | 59.14 | 0.35 | 8.9 | 721 | 60.8 | 9.42 | 0.24 |
| 120 | 59.11 | 0.47 | 9.9 | 734 | 64.2 | 9.90 | 0.30 |
| 115 | 59.14 | 0.44 | 11.2 | 739 | 65.2 | 10.48 | 0.66 |
| 110 | 60.42 | 0.37 | 12.4 | 745 | 64.6 | 11.76 | 1.66 |
| 105 | 60.89 | 0.53 | 14.3 | 763 | 62.7 | 12.22 | 2.32 |
| 100 | 60.73 | 1.82 | 18.0 | 809 | 56.3 | 12.55 | 4.32 |
| 95 | 58.96 | 3.96 | 18.3 | 815 | 55.6 | 12.65 | 5.83 |
| Pulping Method and Pulps | Screened Yield (%) | Rejects (%) | Kappa Number | Viscosity (dm3/kg) | Brightness (% ISO) | |
|---|---|---|---|---|---|---|
| Soda | Brownstock | 50.1 | 3.6 | 21.5 | 900 | 35.6 |
| Oxygen Delignified | 48.4 | - | 11.8 | 820 | 45.0 | |
| Soda-AQ | Brownstock | 49.3 | 1.2 | 13.3 | 860 | 37.2 |
| Oxygen Delignified | 48.1 | - | 6.8 | 790 | 49.9 | |
2.2.2. Effects of Cooking Time
| Cooking Time (min) | Pulp Properties | Black Liquor | |||||
|---|---|---|---|---|---|---|---|
| Screened Yield (%) | Rejects (%) | Kappa Number | Viscosity (dm3/kg) | Brightness (%ISO) | pH Value | Residual NaOH (g/L) | |
| 90 | 60.73 | 1.82 | 18.0 | 809 | 56.3 | 12.55 | 4.32 |
| 120 | 61.07 | 1.18 | 16.4 | 806 | 61.3 | 12.48 | 3.96 |
| 180 | 60.93 | 0.51 | 14.0 | 766 | 63.7 | 11.94 | 2.19 |
2.2.3. Effect of Alkali Charge
| Dosage of NaOH (%) | Pulp Properties | Black Liquor | |||||
|---|---|---|---|---|---|---|---|
| Screened Yield (%) | Rejects (%) | Kappa Number | Viscosity (dm3/kg) | Brightness (%ISO) | pH Value | Residual NaOH (g/L) | |
| 22 | 60.85 | 0.97 | 14.7 | 786 | 63.2 | 11.57 | 1.73 |
| 23 | 60.93 | 0.51 | 14.0 | 766 | 63.7 | 11.94 | 2.19 |
| 24 | 60.25 | 0.33 | 13.9 | 743 | 63.9 | 12.16 | 3.01 |
2.2.4. Effect of Initial Pressure of Oxygen

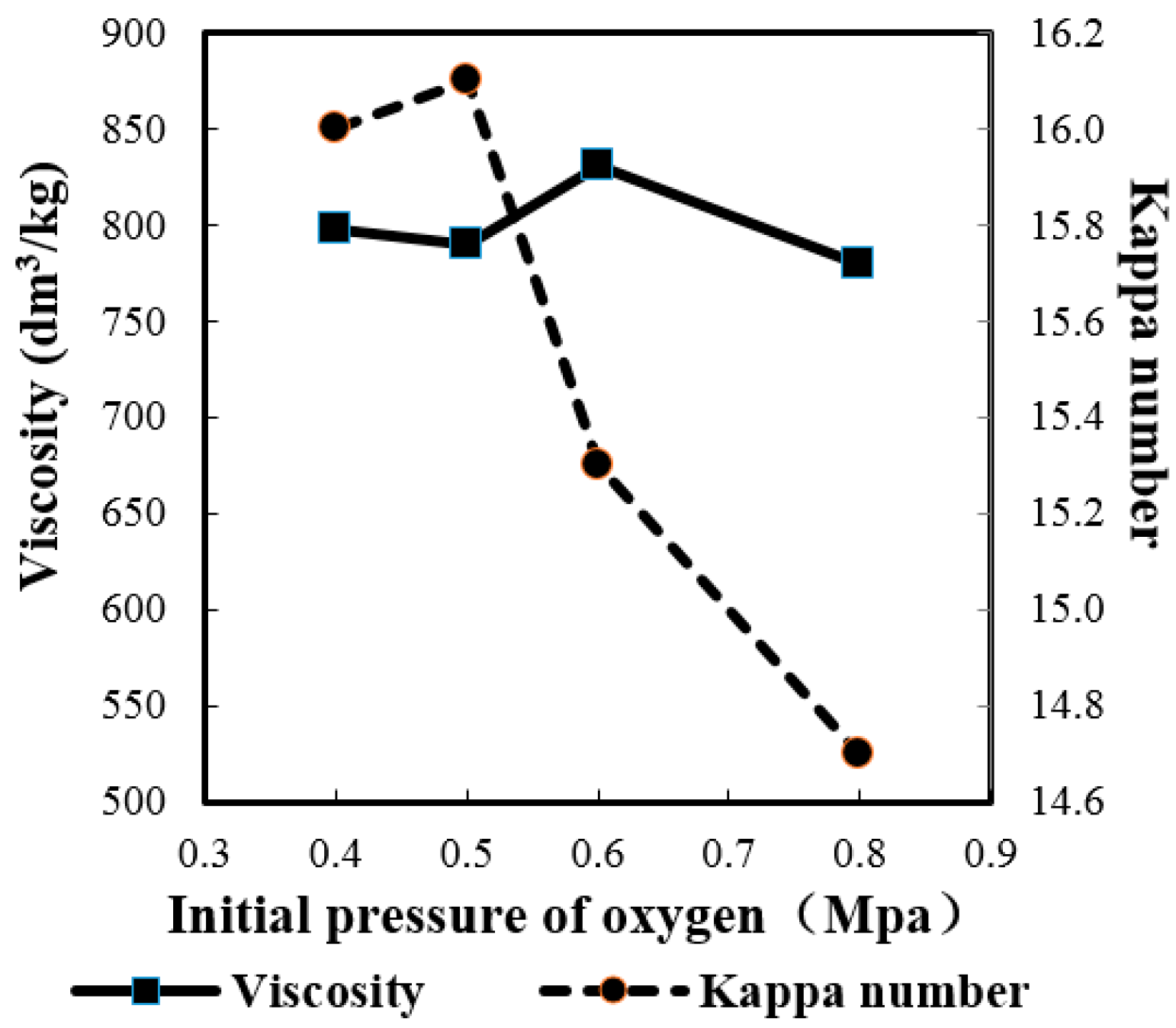
2.2.5. Effect of MgSO4 Charge
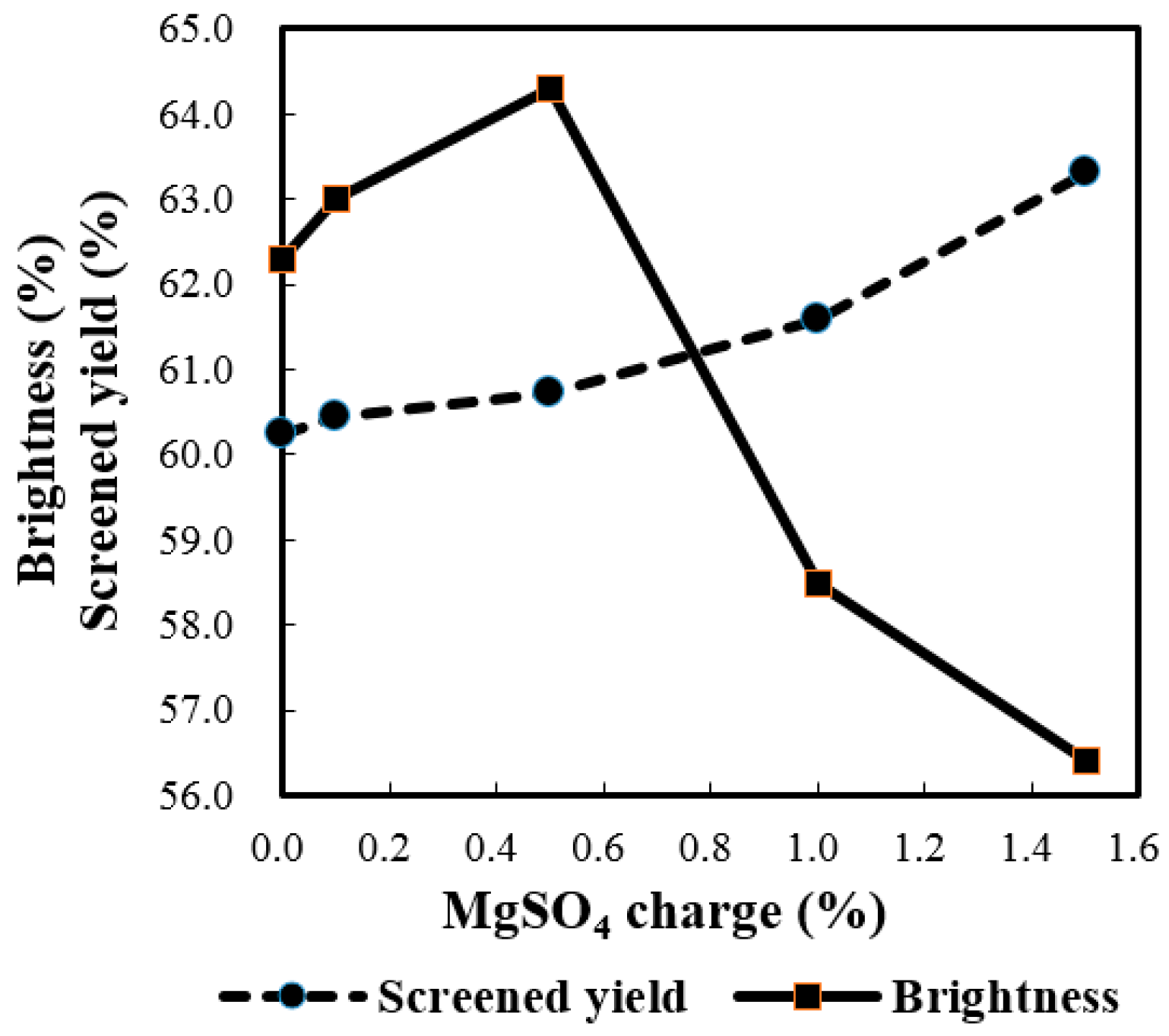
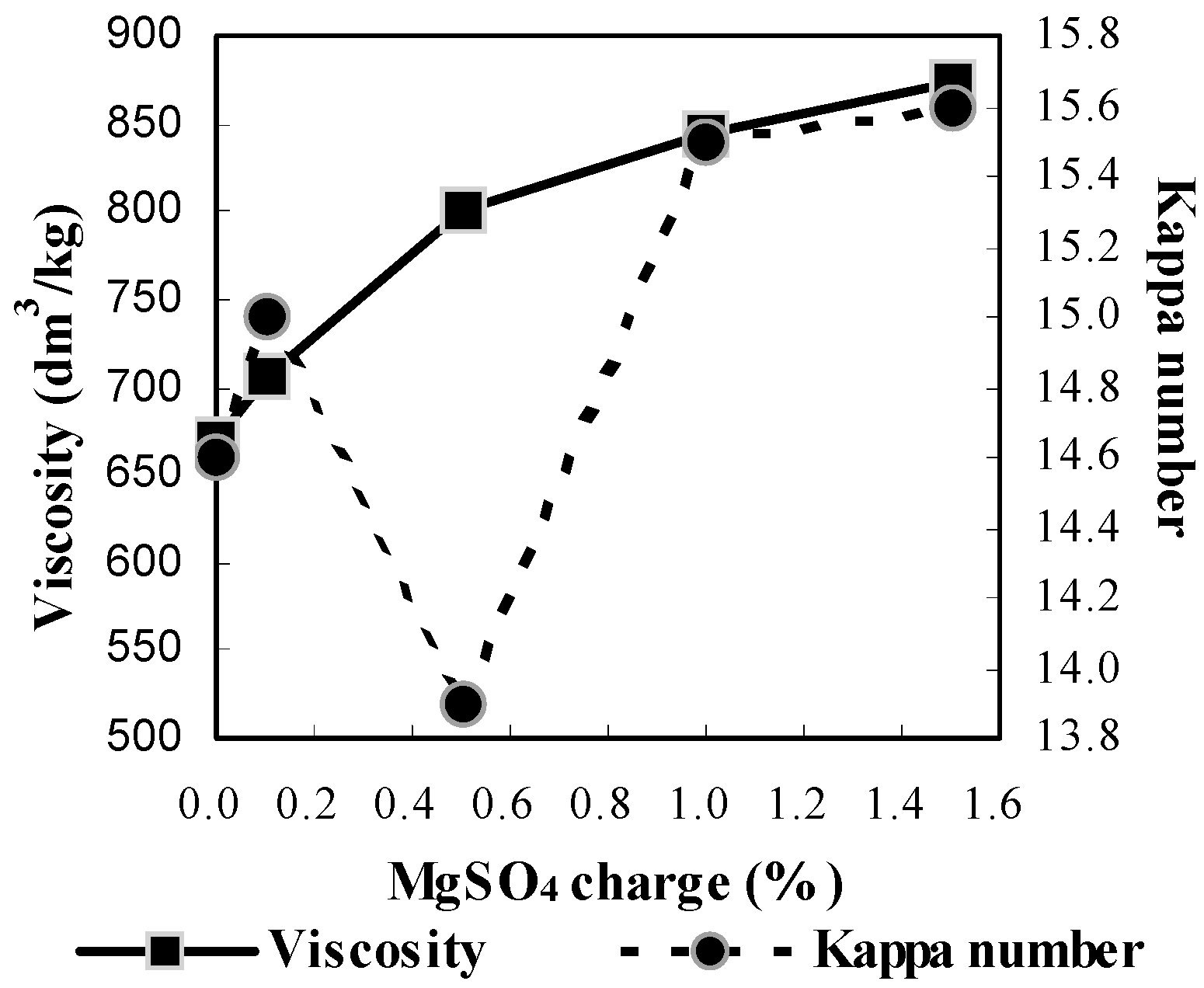
2.2.6. Effect of Bagasse Consistency


3. Experimental Section
3.1. Materials
3.2. Chemical Composition Analyses
3.3. Pulping Process
3.4. Analysis of Pulp Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Best Available Techniques (BAT) Reference Document for the Production of Pulp, Paper and Board, 2013. Available online: http://eippcb.jrc.ec.europa.eu/reference/BREF/PP_BREF_FD_07_2013.pdf (accessed on 21 October 2015).
- Sarwar Jahan, M.; Khalidul Islam, M.; Nasima Chowdhury, D.A.; Iqbal Moeiz, S.M.; Arman, U. Pulping and papermaking properties of pati (Typha). Ind. Crops Prod. 2007, 26, 259–264. [Google Scholar] [CrossRef]
- Jimenez, L.; Ramos, E.; de la Torre, M.J.; Perez, I.; Ferrer, J.L. Bleaching of soda pulp of fibres of Musa textilis nee (abaca) with peracetic acid. Bioresour. Technol. 2008, 99, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Khristova, P.; Kordsachia, O.; Patt, R.; Karar, I.; Khider, T. Environmentally friendly pulping and bleaching of bagasse. Ind. Crops Prod. 2006, 23, 131–139. [Google Scholar] [CrossRef]
- Pasquini, D.; Pimenta, M.T.B.; Ferreira, L.H.; Curvelo, A.A.S. Sugar cane bagasse pulping using supercritical CO2 associated with co-solvent 1-butanol/water. J. Supercrit. Fluids 2005, 34, 125–131. [Google Scholar] [CrossRef]
- Abou-Yousef, H.; El-Sakhawy, M.; Kamel, S. Multi-stage bagasse pulping by using alkali/Caro’s acid treatment. Ind. Crops Prod. 2005, 21, 337–341. [Google Scholar] [CrossRef]
- Ogunsile, B.O.; Quintana, G.C. Modeling of Soda-Ethanol Pulps from Carpolobia lutea. Bioresources 2010, 5, 2417–2430. [Google Scholar]
- Chen, K.-L.; Tosaka, K.; Hayashi, J. Alkali-oxygen pulping of rice straw. Two-stage pulping by alkali soaking and oxygen cooking. Tappi J. 1994, 77, 109–114. [Google Scholar]
- Vilay, V.; Mariatti, M.; Mat Taib, R.; Todo, M. Effect of fiber surface treatment and fiber loading on the properties of bagasse fiber-reinforced unsaturated polyester composites. Compos. Sci. Technol. 2008, 68, 631–638. [Google Scholar] [CrossRef]
- Tutus, A.; Eroglu, H. A practical solution to the silica problem in straw pulping. Appita J. 2003, 56, 111–115. [Google Scholar]
- Ashford, N.A. Government Strategies and Policies for Cleaner Production. UNEP, 1994. Available online: http://hdl.handle.net/1721.1/1560 (accessed on 30 October 2015).
- Dumitrescu, I.; Mocioiu, A.M.; Visileanu, E. Cleaner production in Romanian textile industry: A case study. Int. J. Environ. Stud. 2008, 65, 549–562. [Google Scholar] [CrossRef]
- Khristova, P.; Gabir, S.; Bentcheva, S.; Dafalla, S. Soda-anthraquinone pulping of sunflower stalks. Ind. Crops Prod. 1998, 9, 9–17. [Google Scholar] [CrossRef]
- Harper, S.H.T.; Lynch, J.M. The Chemical-Components and Decomposition of Wheat Straw Leaves, Internodes and Nodes. J. Sci. Food Agric. 1981, 32, 1057–1062. [Google Scholar] [CrossRef]
- Lou, R.; Wu, S.B.; Lv, G.-J.; Guo, D.-L. Pyrolytic Products from Rice Straw and Enzymatic/Mild Acidolysis Lignin (Emal). Bioresources 2010, 5, 2184–2194. [Google Scholar]
- Huang, G.-L.; Shi, J.X.; Langrish, T.A.G. Environmentally friendly bagasse pulping with NH4OH-KOH-AQ. J. Clean. Prod. 2008, 16, 1287–1293. [Google Scholar] [CrossRef]
- Cao, Z.-H.; Wang, Y.-M.; Guo, H.-X. Research Progress on Oxygen-Alkali Pulping. Hebei Chem. Eng. Ind. 2007, 30, 7–9. [Google Scholar]
- Raguauskas, A.J.; Lucia, A.L.; Jameel, H. High Selectivity Oxygen Delignification. In Final Technical Report; Atlanta, GA, Institute of Paper Science and Technology, Georgia Institute of Technology: Atlanta, GA, USA. Available online: http://www.osti.gov/scitech/biblio/859934/ (accessed on 18 November 2015).
- Mohta, D.; Upadhyaya, J.S.; Kapoor, S.K.; Ray, A.K.; Roy, D.N. Oxygen delignification of soda and soda-aq bagasse pulps. Tappi J. 1998, 81, 184–187. [Google Scholar]
- Violette, S.M. Oxygen Delignification Kinetics and Selectivity Improvement. Ph.D. Thesis, The University of Maine, Orono, ME, USA, 2003. [Google Scholar]
- Zhang, X.-J.; Chen, K.-L. Study on the Condition of Bagasse Oxygen-Alkali Pulping. Pap. Pap. Mak. 2007, 26, 29–31. [Google Scholar]
- Hamzeh, Y.; Abyaz, A.; Niaraki, M.-O.-S.-M.; Abdulkhani, A. Application of surfactants as pulping additives in soda pulping of bagasse. Bioresources 2009, 4, 1267–1275. [Google Scholar]
- De Carvalho, D.-M.; Perez, A.; Garcia, J.-C.; Colodette, J.-L.; Lopez, F.; Diaz, M.-J. Ethanol-Soda Pulping of Sugarcane Bagasse and Straw. Cellul. Chem. Technol. 2014, 48, 355–364. [Google Scholar]
- Chen, J.X. High Efficiency and Clean Pulping and Bleaching New Technique; China Light Industry Press: Beijing, China, 1996. [Google Scholar]
- Liden, J.; Ohman, L.O. Redox stabilization of iron and manganese in the +II oxidation state by magnesium precipitates and some anionic polymers. Implications for the use of oxygen-based bleaching chemicals. J. Pulp Pap. Sci. 1997, 23, J193–J199. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, F.; Chen, K.-L.; Lu, F. Low Temperature Soda-Oxygen Pulping of Bagasse. Molecules 2016, 21, 85. https://doi.org/10.3390/molecules21010085
Yue F, Chen K-L, Lu F. Low Temperature Soda-Oxygen Pulping of Bagasse. Molecules. 2016; 21(1):85. https://doi.org/10.3390/molecules21010085
Chicago/Turabian StyleYue, Fengxia, Ke-Li Chen, and Fachuang Lu. 2016. "Low Temperature Soda-Oxygen Pulping of Bagasse" Molecules 21, no. 1: 85. https://doi.org/10.3390/molecules21010085





