Synthesis of Chlorinated Tetracyclic Compounds and Testing for Their Potential Antidepressant Effect in Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
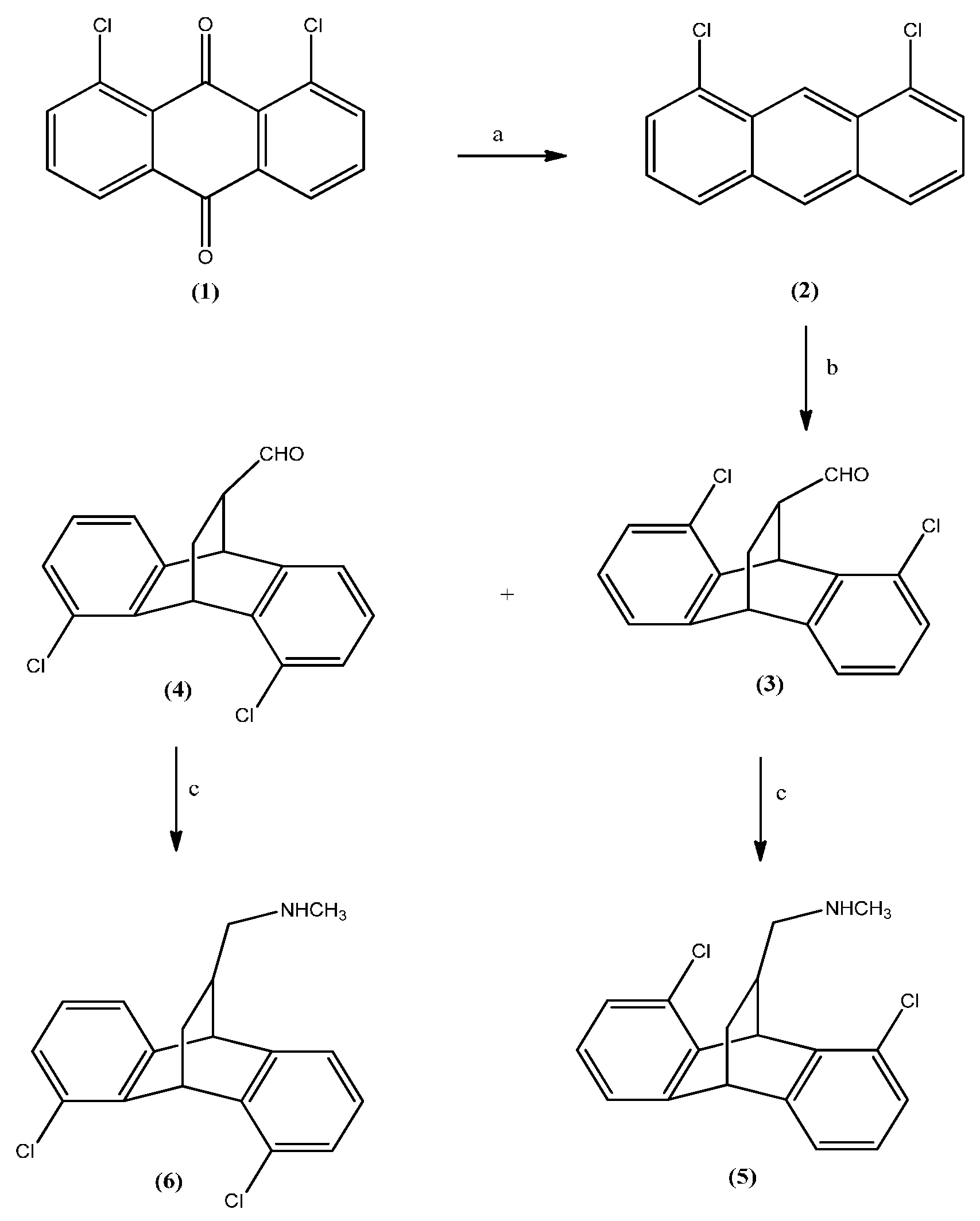
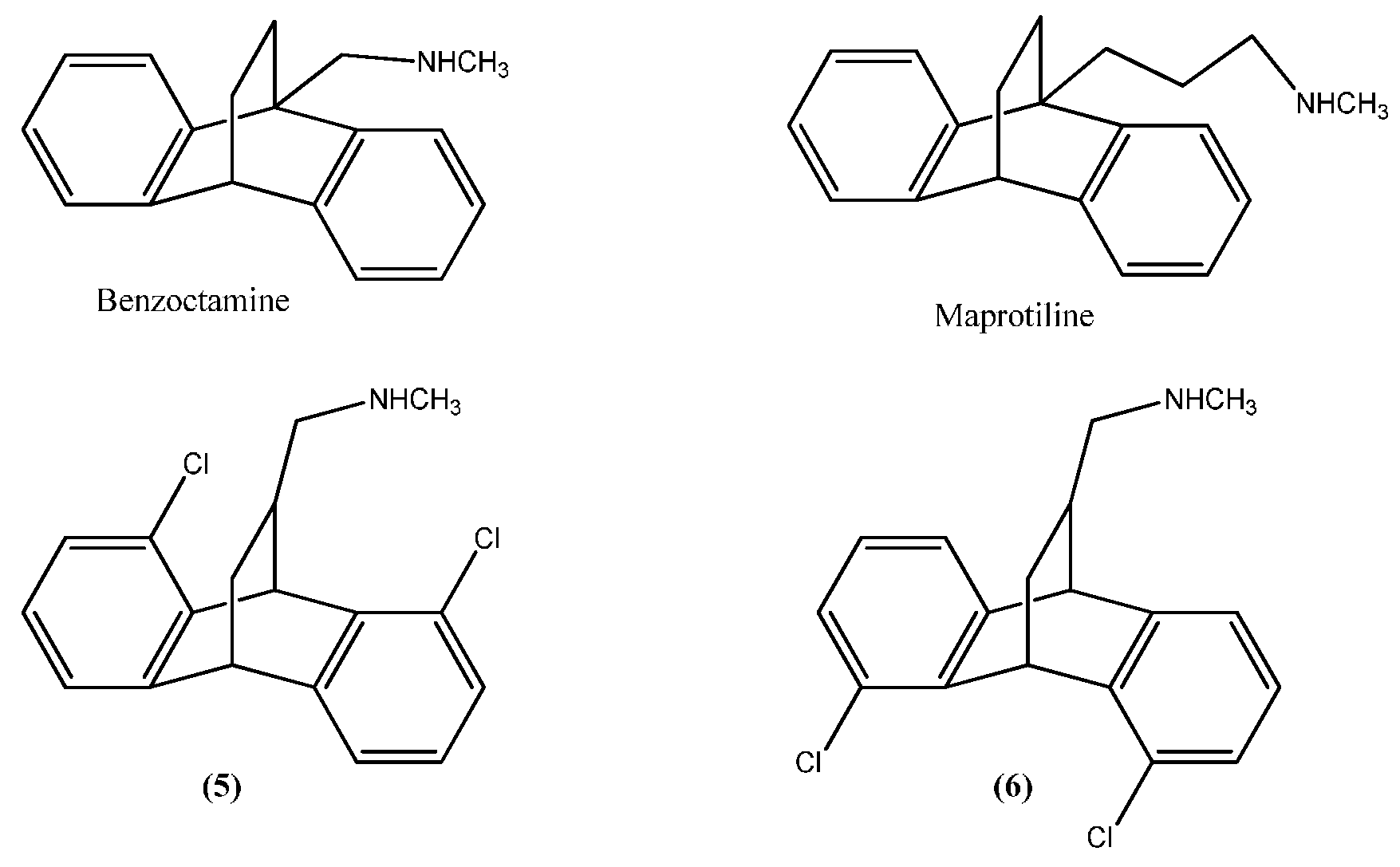
2.2. Assessment of Antidepressant Activity Using Forced Swimming Test
| Animal Group | Duration of Immobility in Seconds during the Last Four Minutes of the Six Minute Period | ||||
|---|---|---|---|---|---|
| 0 mg/kg | 10 mg/kg | 20 mg/kg | 40 mg/kg | 80 mg/kg | |
| Control vehicle | 225 ± 7 | ||||
| Maprotiline | 157 ± 5 | 156 ± 4 | 144 ± 5 | 134 ± 5 | |
| Treated with (3) | 240 | 240 | 160±11 b | 65 ± 4 a,b | |
| Treated with (4) | 240 | 240 | 240 | 240 | |
| Treated with (5) | 240 | 200 ± 12.3 | 140 ± 7.9 b | 36 ± 4.9 a,b | |
| Treated with (6) | 240 | 240 | 240 | 30 ± 5 a,b | |
| Compound | Percentage Decrease for Dosage of 40 mg/kg | Percentage Decrease for Dosage of 80 mg/kg |
|---|---|---|
| (3) | 28.9 | 71.1 |
| (5) | 37.8 | 84.0 |
| (6) | - | 86.7 |
3. Experimental
3.1. General Protocol
3.2. Synthesis
3.3. Assessment of Antidepressant Activity Using Forced Swimming Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- González, H.M.; Tarraf, W.; Whitfield, K.E.; Vega, W.A. The epidemiology of major depression and ethnicity in the united states. J. Psychiatr. Res. 2010, 44, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.T.; Michaud, C.M.; Murray, C.J.; Marks, J.S. Assessing the burden of disease in the united states using disability-adjusted life years. Am. J. Prev. Med. 2005, 28, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.A.; Fitterling, H.L. Physical exercise and depression. Mt. Sinai J. Med. 2009, 76, 204–214. [Google Scholar]
- Parisini, E.; Metrangolo, P.; Pilati, T.; Resnati, G.; Terraneo, G. Halogen bonding in halocarbon–protein complexes: A structural survey. Chem. Soc. Rev. 2011, 40, 2267–2278. [Google Scholar] [CrossRef] [PubMed]
- Erdelyi, M. Halogen bonding in solution. Chem. Soc. Rev. 2012, 41, 3547–3557. [Google Scholar] [CrossRef] [PubMed]
- Kolář, M.; Hobza, P.; Bronowska, A.K. Plugging the explicit σ-holes in molecular docking. Chem. Commun. 2013, 49, 981–983. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Z.; Yang, Z.; Chen, K.; Zhu, W. A knowledge-based halogen bonding scoring function for predicting protein-ligand interactions. J. Mol. Model. 2013, 19, 5015–5030. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Schmidt, P. Synthese und eigenschaften von 1-aminoalkyl-dibenzo[b,e]bicyclo[2.2.2]octadienen. Helv. Chim. Acta 1969, 52, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Karama, U.S.; Sultan, M.A.S.; EL-Tahir, K.E.H.; Almansour, A.I. Antidepressant Compounds. U.S. Patent 9125866 B1, 8 September 2015. [Google Scholar]
- House, H.O.; Ghali, N.I.; Haack, J.L.; van Derveer, D. Reactions of the 1,8-diphenylanthracene system. J. Org. Chem. 1980, 45, 1807–1817. [Google Scholar] [CrossRef]
- David, D.J.P.; Renard, C.E.; Jolliet, P.; Hascoët, M.; Bourin, M. Antidepressant-like effects in various mice strains in the forced swimming test. Psychopharmacology 2003, 166, 373–382. [Google Scholar] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karama, U.; Sultan, M.A.; Almansour, A.I.; El-Taher, K.E. Synthesis of Chlorinated Tetracyclic Compounds and Testing for Their Potential Antidepressant Effect in Mice. Molecules 2016, 21, 61. https://doi.org/10.3390/molecules21010061
Karama U, Sultan MA, Almansour AI, El-Taher KE. Synthesis of Chlorinated Tetracyclic Compounds and Testing for Their Potential Antidepressant Effect in Mice. Molecules. 2016; 21(1):61. https://doi.org/10.3390/molecules21010061
Chicago/Turabian StyleKarama, Usama, Mujeeb A. Sultan, Abdulrahman I. Almansour, and Kamal Eldin El-Taher. 2016. "Synthesis of Chlorinated Tetracyclic Compounds and Testing for Their Potential Antidepressant Effect in Mice" Molecules 21, no. 1: 61. https://doi.org/10.3390/molecules21010061





