DNA Binding, Photonuclease Activity and Human Serum Albumin Interaction of a Water-Soluble Freebase Carboxyl Corrole
Abstract
:1. Introduction

2. Results and Discussion
2.1. DNA Binding Properties
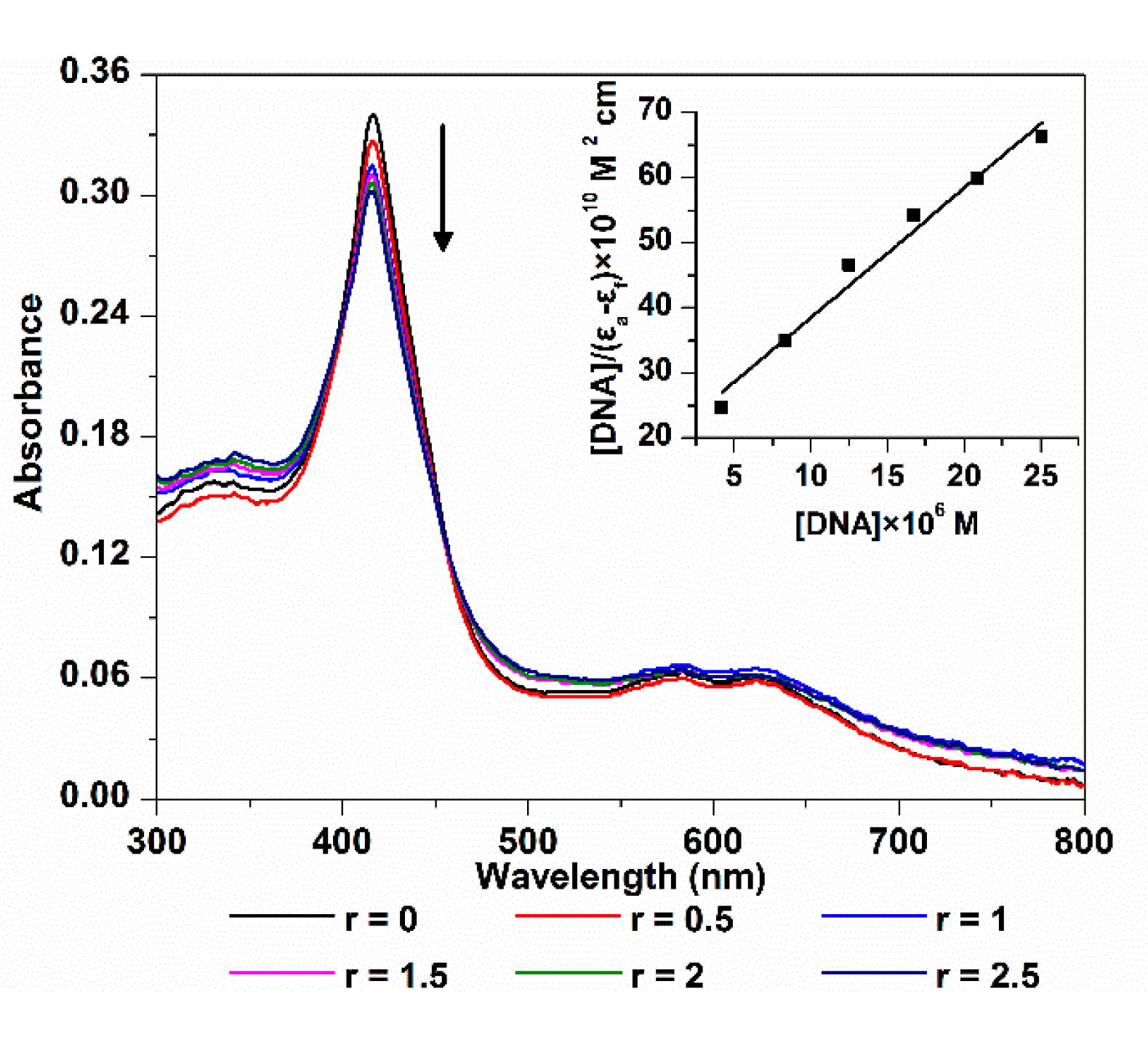
2.1.1. Electronic Spectroscopy
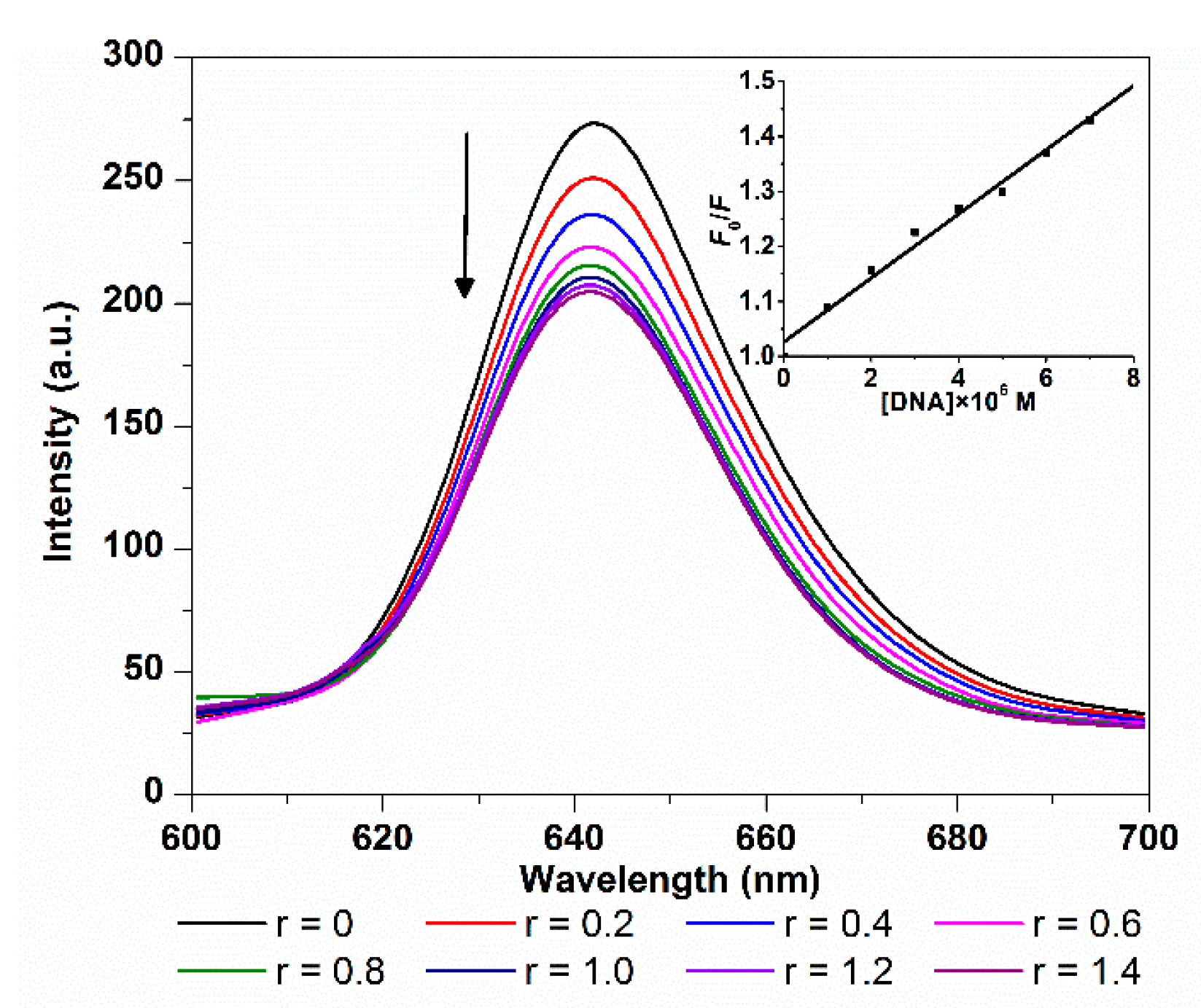
2.1.2. Fluorescence Spectroscopy
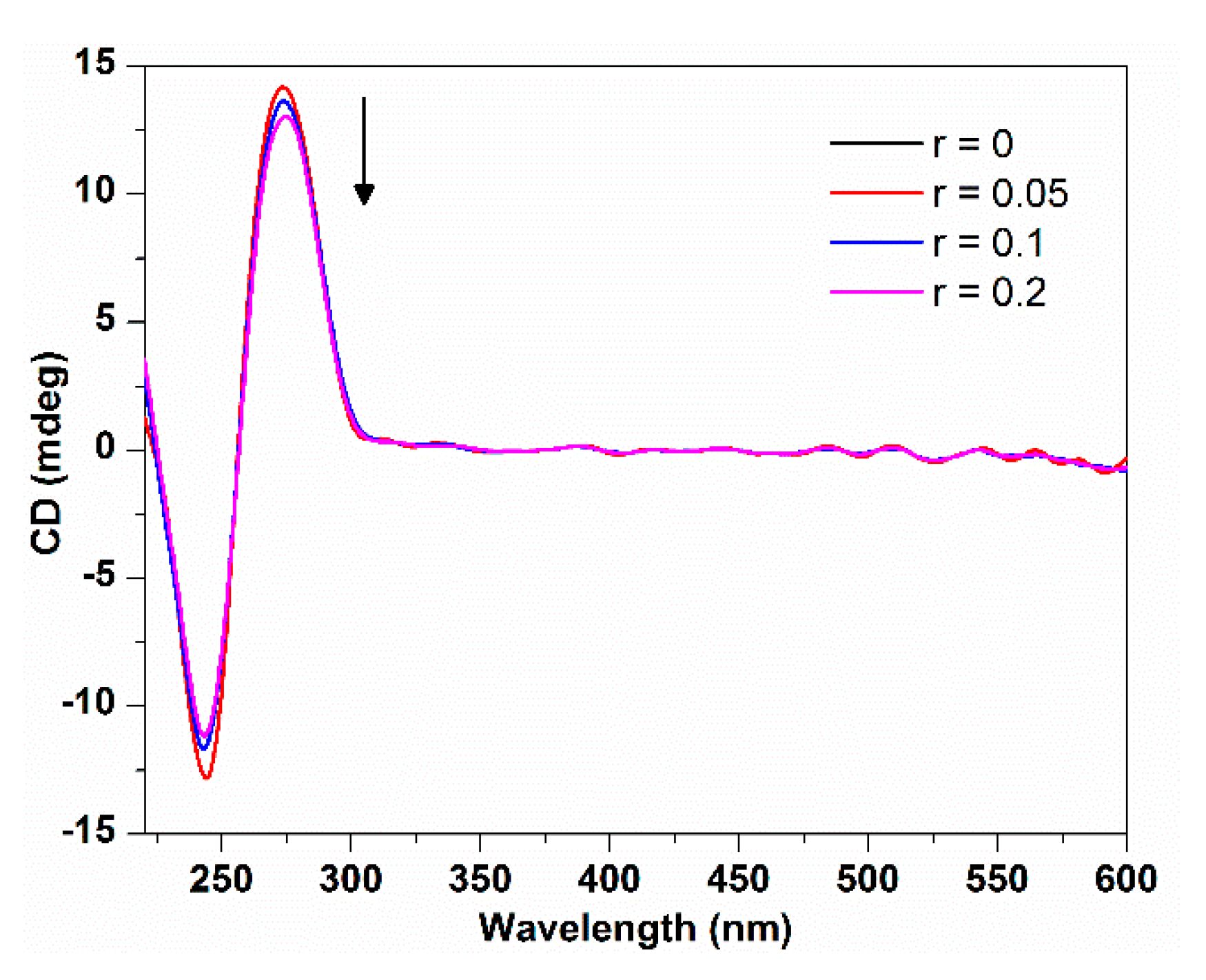
2.1.3. Circle Dichroism Spectroscopy
2.1.4. Viscosity Measurements

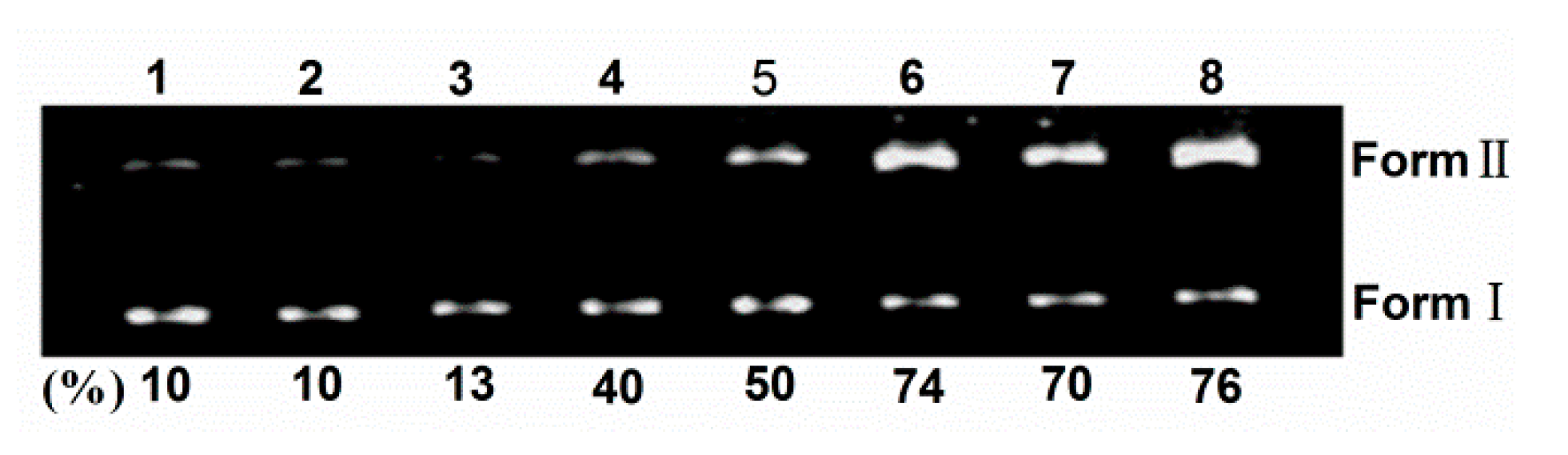
2.2. DNA Photocleavage
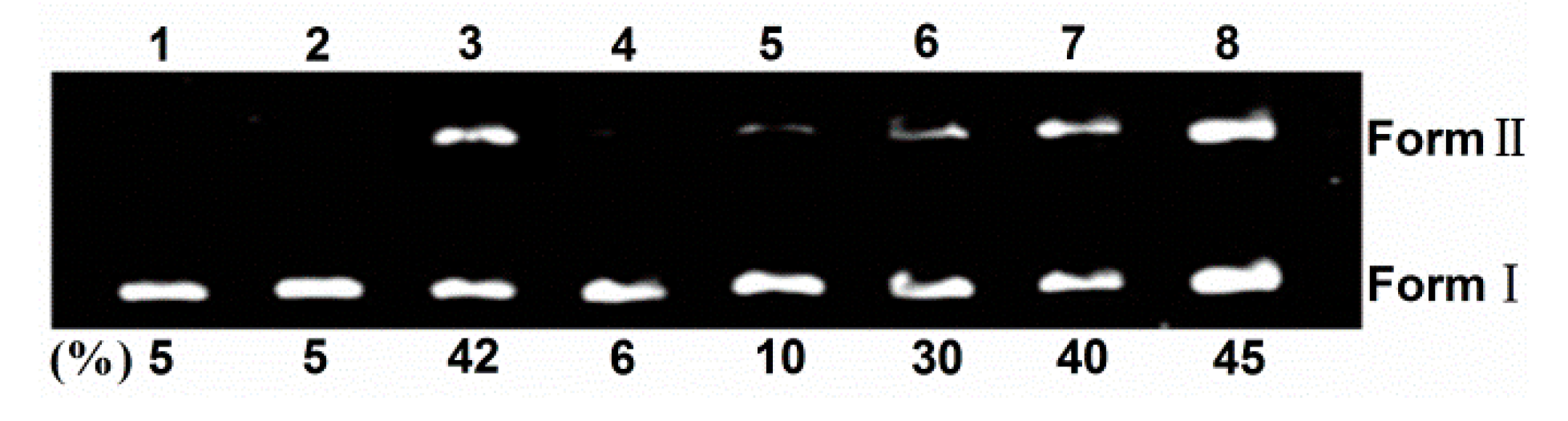
2.3. HSA Binding Behavior
2.3.1. HSA–TCPC Fluorescence Characteristics
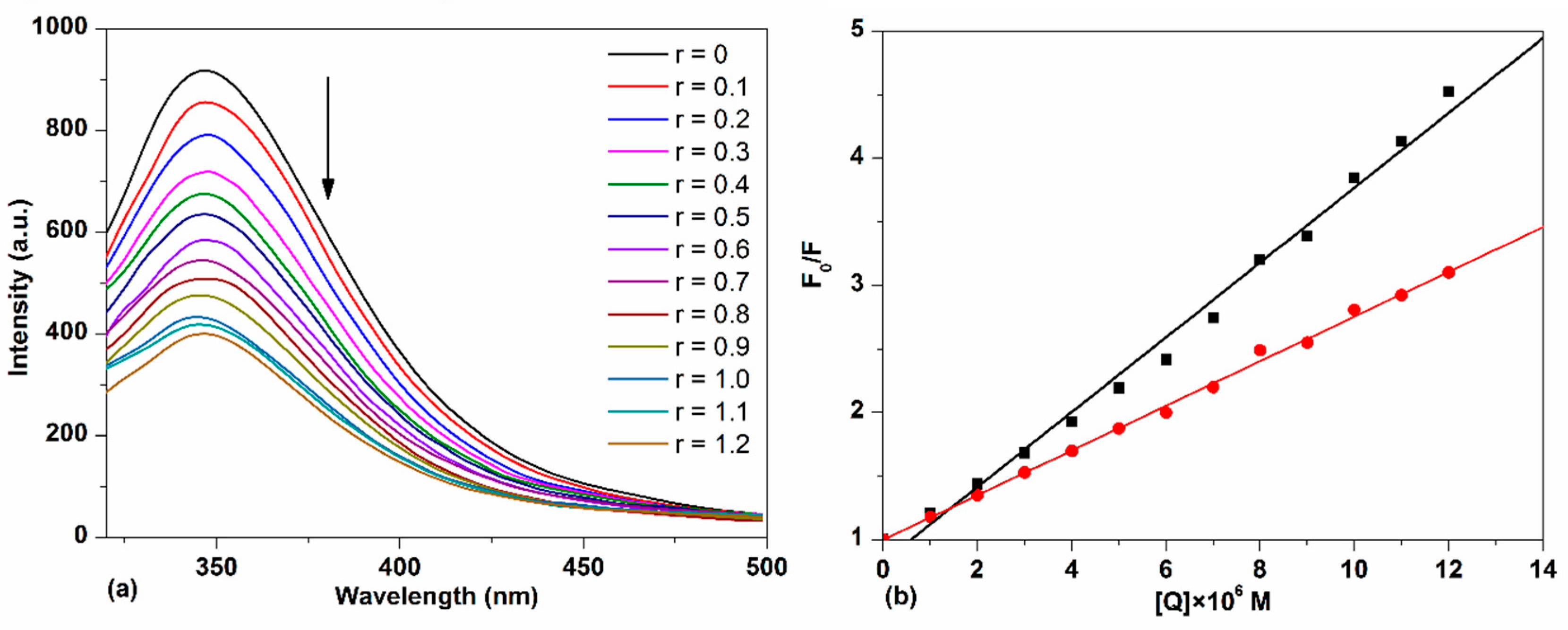
| Site Marker | KSV × 10−5 (M−1) | Kq × 10−13 (M−1·s−1) | KA × 10−5 (M−1) | n |
|---|---|---|---|---|
| blank | 1.75 | 1.75 | 2.24 | 1.14 |
| ibuprofen | 1.23 | 1.23 | 1.62 | 1.23 |
| warfarin | 0.98 | 0.98 | 1.21 | 1.36 |
2.3.2. Site-Selective Binding of TCPC on HSA
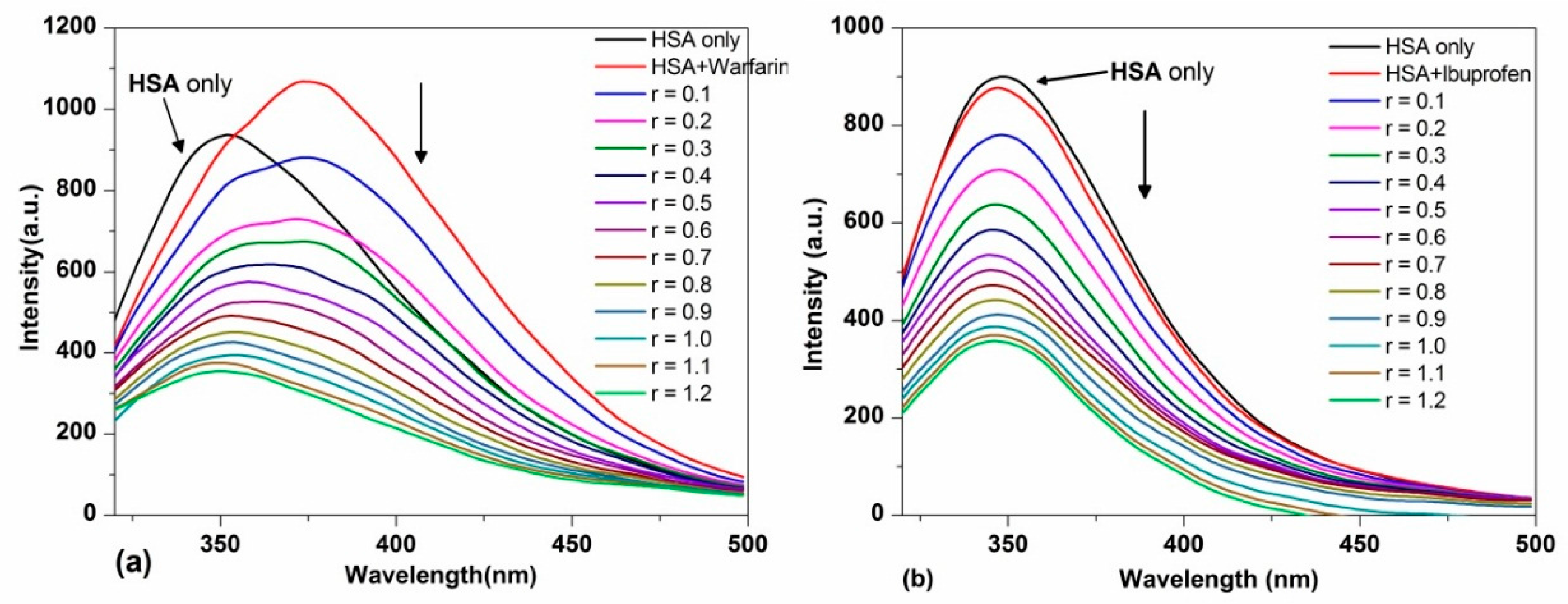
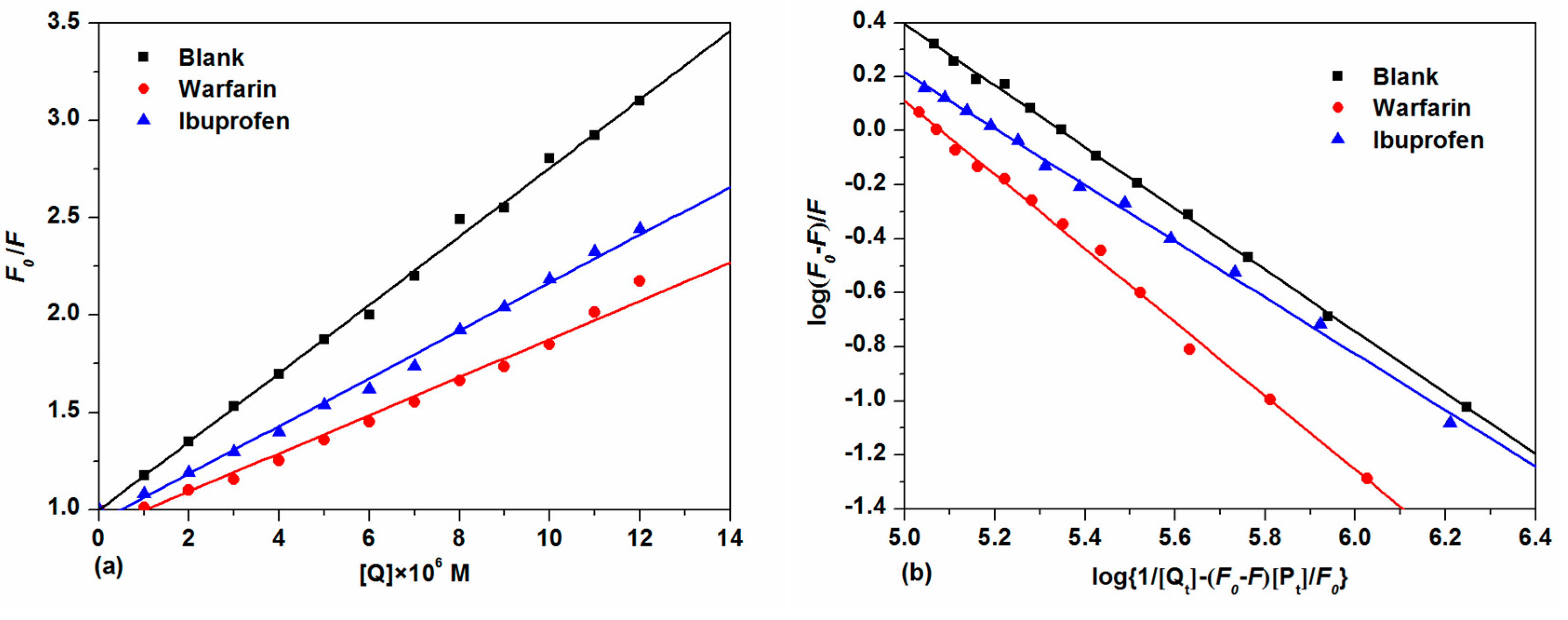
2.3.3. Energy Transfer between TCPC and HSA
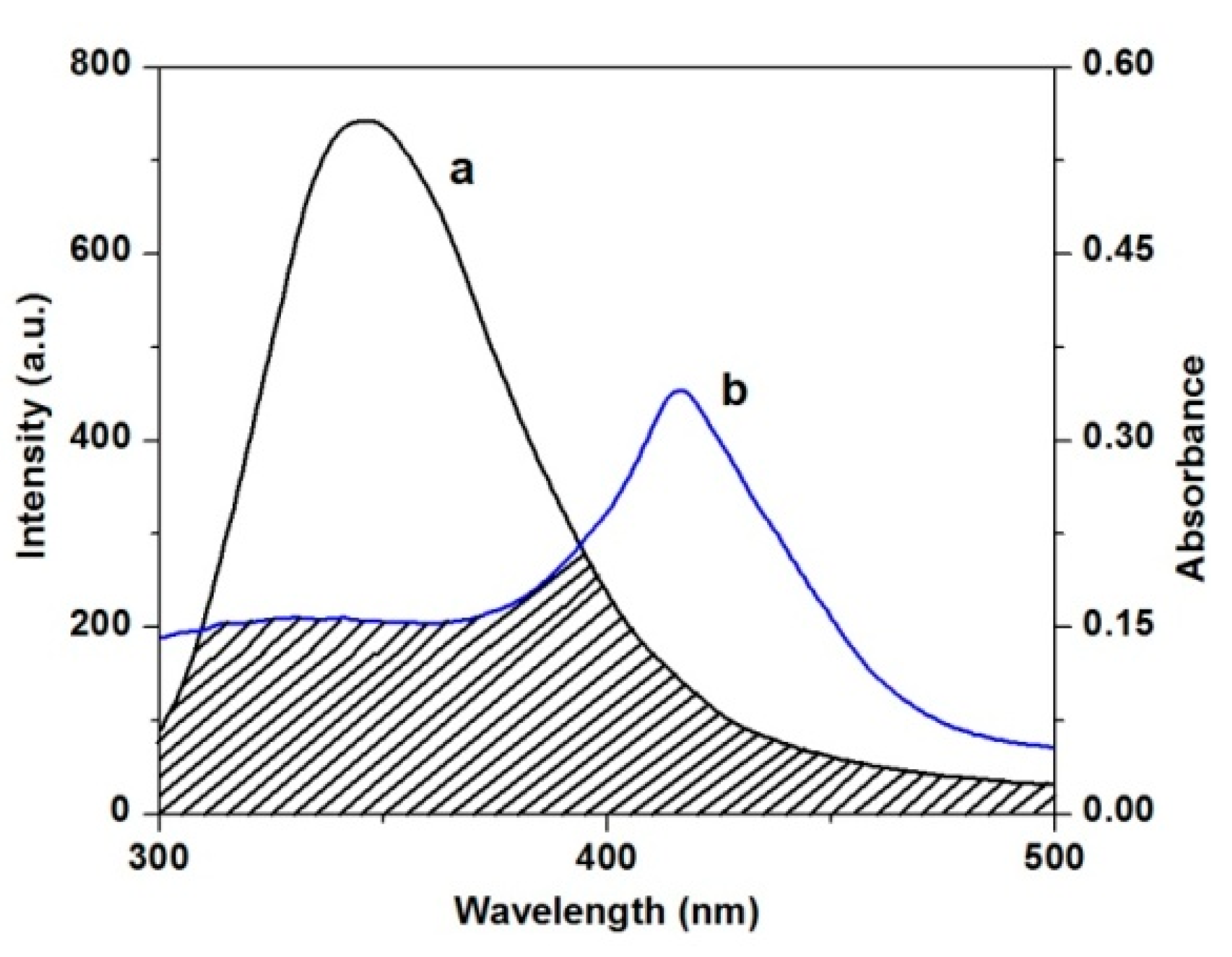
2.3.4. HSA Conformation
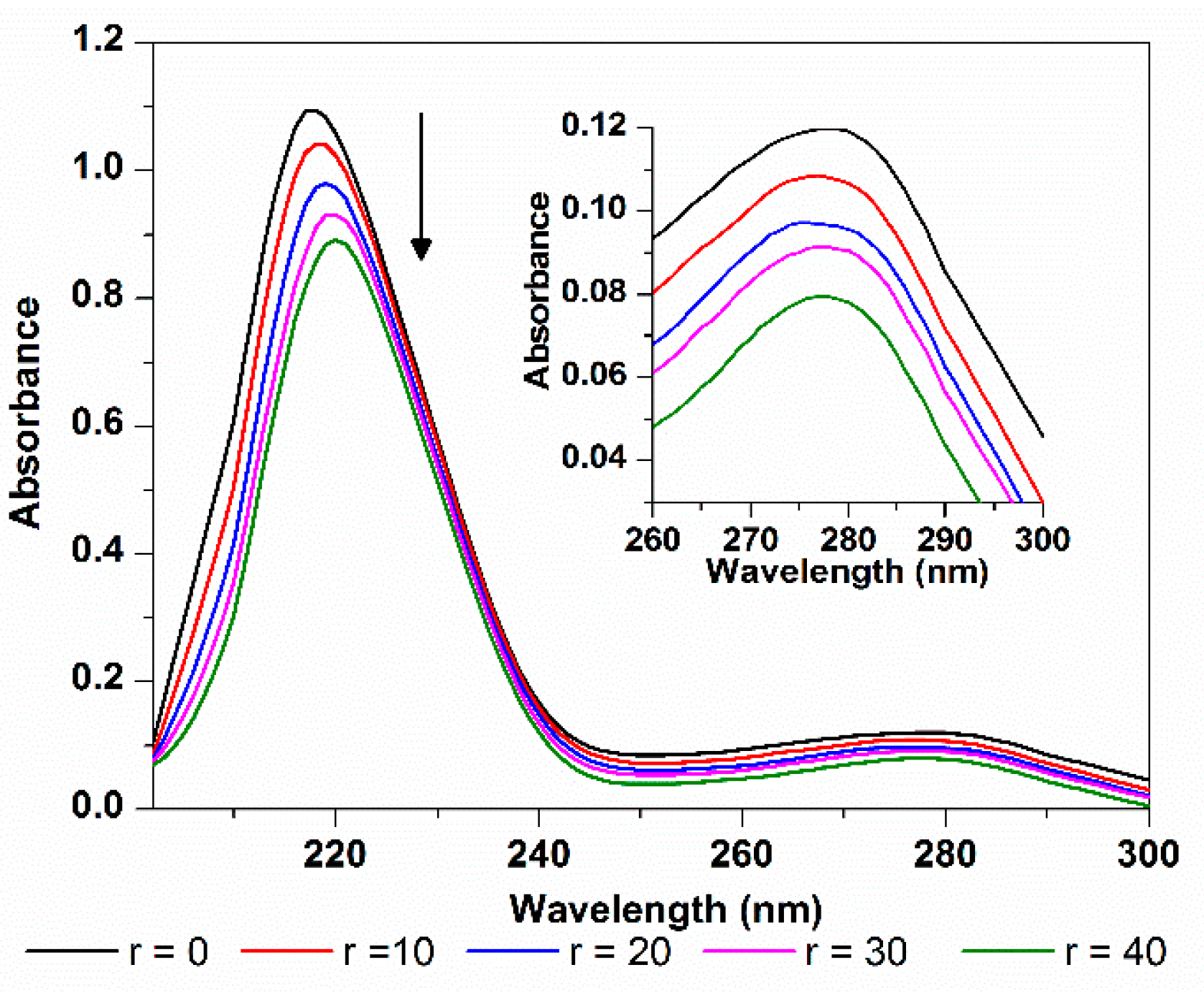
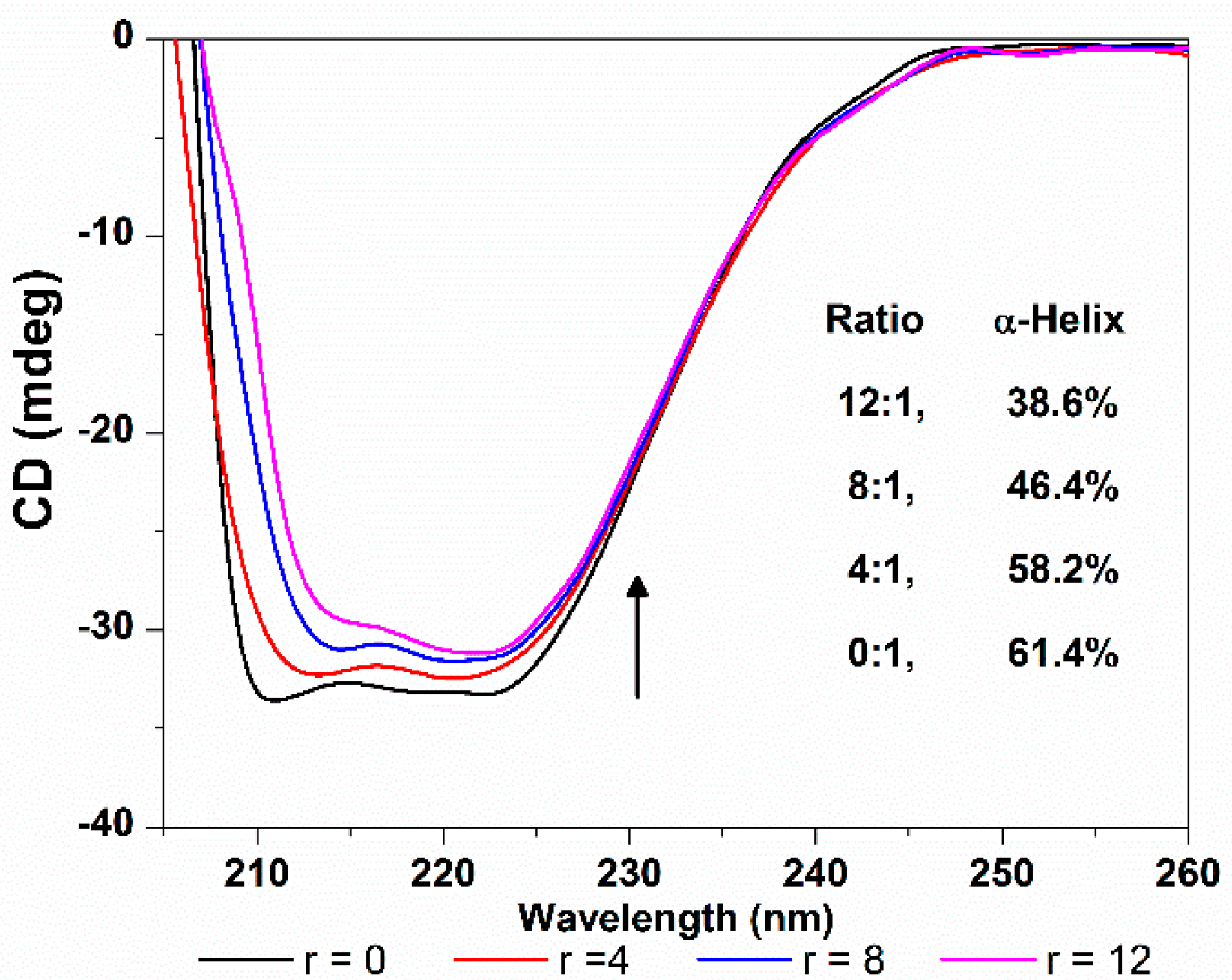
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of Carboxyl Corrole
3.3. DNA Binding Experiments
3.4. DNA Cleavage Experiments
3.5. HSA Binding Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, H.Y.; Mahmood, M.H.R.; Qiu, S.X.; Chang, C.K. Recent developments in manganese corrole chemistry. Coord. Chem. Rev. 2013, 257, 1306–1333. [Google Scholar]
- D’Urso, A.; Nardis, S.; Pomarico, G.; Fragala, M.E.; Paolesse, R.; Purrello, R. Interaction of tricationic corroles with single/double helix of homopolymeric nucleic acids and DNA. J. Am. Chem. Soc. 2013, 135, 8632–8638. [Google Scholar] [CrossRef] [PubMed]
- Le, D.D.; di Antonio, M.; Chan, L.K.M.; Balasubramanian, S. G-quadruplex ligands exhibit differential G-tetrad selectivity. Chem. Commun. 2015, 51, 8048–8050. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.Q.; Zhang, D.; Weng, X.C.; Zhang, M.; Ma, H.; Ma, Y.Z.; Zhou, X. Cationic Metal-Corrole Complexes: Design, Synthesis, and Properties of Guanine-Quadruplex Stabilizers. Chem. Eur. J. 2008, 14, 9431–9441. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.Q.; Huang, J.; Ren, L.G.; Weng, X.C.; Zhou, Y.Y.; Du, Y.H.; Wu, X.J.; Zhou, X.; Yang, G.F. Cationic corrole derivatives: a new family of G-quadruplex inducing and stabilizing ligands. Chem. Commun. 2007, 31, 3264–3266. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liu, H.Y.; Shi, L.; Wang, X.L.; Ying, X.; Zhang, L.; Ji, L.N.; Zang, L.Q.; Chang, C.K. DNA cleavage mediated by water-soluble manganese corrole. Chin. Chem. Lett. 2011, 22, 101–104. [Google Scholar] [CrossRef]
- Huang, J.T.; Wang, X.L.; Zhang, Y.; Mahmood, M.H.R.; Huang, Y.Y.; Ying, X.; Ji, L.N.; Liu, H.Y. DNA binding and nuclease activity of a water-soluble sulfonated manganese(III) corrole. Transit. Metal. Chem. 2013, 38, 283–289. [Google Scholar] [CrossRef]
- Huang, J.T.; Zhang, Y.; Wang, X.L.; Ji, L.N.; Liu, H.Y. DNA Binding and Photonuclease Activity of Sulfonated Corrole and Its Gallium(III) Complex. Chin. J. Inorg. Chem. 2013, 29, 1649–1656. [Google Scholar]
- Mahammed, A.; Gross, Z. Albumin-conjugated corrole metal complexes: Extremely simple yet very efficient biomimetic oxidation systems. J. Am. Chem. Soc. 2005, 127, 2883–2887. [Google Scholar] [CrossRef] [PubMed]
- Agadjanian, H.; Weaver, J.J.; Mahammed, A.; Rentsendorj, A.; Bass, S.; Kim, J.; Dmochowski, I.J.; Margalit, R.; Gray, H.B.; Gross, Z.; et al. Specific delivery of corroles to cells via noncovalent conjugates with viral proteins. Pharm. Res. 2006, 23, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Agadjanian, H.; Ma, J.; Rentsendorj, A.; Valluripalli, V.; Hwang, J.Y.; Mahammed, A.; Farkas, D.L.; Gray, H.B.; Gross, Z.; Medina-Kauwe, L.K. Tumor detection and elimination by a targeted gallium corrole. Proc. Natl. Acad. Sci. USA 2009, 106, 6105–6110. [Google Scholar] [CrossRef] [PubMed]
- Mahammed, A.; Gray, H.B.; Weaver, J.J.; Sorasaenee, K.; Gross, Z. Amphiphilic corroles bind tightly to human serum albumin. Bioconjugate Chem. 2004, 15, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Q.; Wen, J.Y.; Wang, X.L.; Mahmood, M.H.R.; Ji, L.N.; Liu, H.Y. DNA Binding and Oxidative Cleavage by a Water-soluble Carboxyl Manganese(III) Corrole. Chin. J. Chem. 2013, 31, 1321–1328. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Wen, J.Y.; Wang, X.L.; Wang, H.; Ji, L.N.; Liu, H.Y. Oxidative DNA Cleavage Mediated by Water-soluble Carboxyl Iron(III) Corrole. Chem. J. Chin. Univ. 2013, 34, 2462–2469. [Google Scholar]
- Pasternack, R.F.; Gibbs, E.J.; Villafranca, J.J. Interactions of porphyrins with nucleic acids. Biochemistry 1983, 22, 5409–5417. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Mandal, G.; Ganguly, T. Detailed spectroscopic investigations to reveal the nature of interaction of anionic porphyrin with calf thymus DNA. J. Photochem. Photobiol. B 2010, 101, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.F.; Chen, X.J.; Shen, J.L.; Liang, X.L. Synthesis, DNA-binding and photocleavage studies of Ru(II) complexes of phenyl-(4,5,9,14-tetraaza-benzo[b]triphenylen-1,1-yl)-methanone. J. Chem. Sci. 2009, 121, 397–405. [Google Scholar] [CrossRef]
- Kang, J.W.; Wu, H.X.; Lu, X.Q.; Wang, Y.S.; Zhou, L. Study on the interaction of new water-soluble porphyrin with DNA. Spectrochim. Acta A 2005, 61, 2041–2047. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.X.; Yan, F.F.; Zhang, Y.X.; Ye, L. Spectroscopic investigation of the interaction between rifabutin and bovine serum albumin. J. Photochem. Photobiol. A 2007, 192, 23–28. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, J.Y.; Mahmood, M.H.R.; Wang, X.L.; Lv, B.B.; Ying, X.; Wang, H.; Ji, L.N.; Liu, H.Y. DNA/HSA interaction and nuclease activity of an iron(III) amphiphilic sulfonated corrole. Luminescence 2015, 30, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, A.; Nair, B.U. Unprecedented dual binding behaviour of acridine group of dye: A combined experimental and theoretical investigation for the development of anticancer chemotherapeutic agents. Biochim. Biophys. Acta. 2006, 1760, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Gershman, Z.; Goldberg, I.; Zeev, G. DNA binding and catalytic properties of positively charged Corroles. Angew. Chem. Int. Ed. 2007, 46, 4320–4324. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Yamakawa, N.; Uno, T. Potent DNA photocleavage by zinc(II) complexes of cationic bis-porphyrins linked with aliphatic diamine. Bioorg. Med. Chem. 2002, 10, 1953–1960. [Google Scholar] [CrossRef]
- Shi, L.; Liu, H.Y.; Si, L.P.; Peng, K.M.; You, L.L.; Wang, H.; Zhang, L.; Ji, L.N.; Chang, C.K.; Jiang, H.F. The heavy atom effect on photocleavage of DNA by mono-hydroxyl halogenated corroles. Chin. Chem. Lett. 2010, 21, 373–375. [Google Scholar] [CrossRef]
- Tabassum, S.; Al-Asbahy, W.M.; Afzal, M.; Arjmand, F.; Hasan Khan, R. Interaction and photo-induced cleavage studies of a copper based chemotherapeutic drug with human serum albumin: Spectroscopic and molecular docking study. Mol. BioSyst. 2012, 8, 2424–2433. [Google Scholar] [CrossRef] [PubMed]
- Sułkowska, A. Interaction of drugs with bovine and human serum albumin. J. Mol. Struct. 2002, 614, 227–232. [Google Scholar] [CrossRef]
- VanScyoc, W.S.; Sorensen, B.R.; Sorensen, E.; Laws, W.R.; Alexander Ross, J.B.; Shea, M.A. Calcium Binding to Calmodulin Mutants Monitored by Domain-Specific Intrinsic Phenylalanine and Tyrosine Fluorescence. Biophys. J. 2002, 83, 2767–2780. [Google Scholar] [CrossRef]
- Gauthler, T.D.; Shane, E.C.; Guerln, W.F.; Seltz, W.R.; Grant, C.L. Fluorescence quenching method for determining equilibrium constants for polycyclic aromatic hydrocarbons binding to dissolved humic materials. Environ. Sci. Technol. 1986, 20, 1162–1166. [Google Scholar] [CrossRef]
- Soares, S.; Mateus, N.; Freitas, V.D. Interaction of Different Polyphenols with Bovine Serum Albumin (BSA) and Human Salivary α-Amylase (HSA) by Fluorescence Quenching. J. Agric. Food. Chem. 2007, 55, 6726–6735. [Google Scholar] [CrossRef] [PubMed]
- Shahabadi, N.; Khorshidi, A.; Moghadam, N.H. Study on the interaction of the epilepsy drug, zonisamide with human serum albumin (HSA) by spectroscopic and molecular docking techniques. Spectrochim. Acta A 2013, 114, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, G.; Birkett, D.J.; Wade, D.N. Further characterization of specific drug binding sites on human serum albumin. Mol. Pharmacol. 1976, 12, 1052–1061. [Google Scholar] [PubMed]
- Wanwimolruk, S.; Birkett, D.J.; Brooks, P.M. Structural requirements for drug binding to site II on human serum albumin. Mol. Pharmacol. 1983, 24, 458–463. [Google Scholar] [PubMed]
- Li, X.G.; Chen, D.J.; Wang, G.K.; Lu, Y. Study of interaction between human serum albumin and three antioxidants: Ascorbic acid, α-tocopherol, and proanthocyanidins. Eur. J. Med. Chem. 2013, 70, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Mallick, A.; Haldar, B.; Chakrabarty, A.; Adhyay, N.C. Fluorescence resonance energy transfer from tryptophan in human serum albumin to a bioactive indoloquinolizine system. J.Chem. Sci. 2007, 119, 77–82. [Google Scholar] [CrossRef]
- Cheng, Z.J. Interaction of tetramethylpyrazine with two serum albumins by a hybrid spectroscopic method. Spectrochim. Acta A 2012, 93, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.Y.; Zhang, L.N.; Ji, Z.J.; Wan, X.F. Spectroscopic investigation of the interaction between thiourea-zinc complex and serum albumin. J. Lumin. 2010, 130, 1280–1284. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Wang, X.Y.; Wang, J.; Zhao, Y.M.; He, W.J.; Guo, Z.J. Noncovalent Interactions between a Trinuclear Monofunctional Platinum Complex and Human Serum Albumin. Inorg. Chem. 2011, 50, 12661–12668. [Google Scholar] [CrossRef] [PubMed]
- Sudhamalla, B.; Gokara, M.; Ahalawat, N.; Amooru, D.G.; Subramanyam, R. Molecular dynamics simulation and binding studies of beta-sitosterol with human serum albumin and its biological relevance. J. Phys. Chem. B 2010, 114, 9054–9062. [Google Scholar] [CrossRef] [PubMed]
- Han, X.L.; Mei, P.; Liu, Y.; Xiao, Q.; Jiang, F.L.; Li, R. Binding interaction of quinclorac with bovine serum albumin: a biophysical study. Spectrochim. Acta A 2009, 74, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Marmur, J.; Doty, P. Thermal renaturation of deoxyribonucleic acids. J. Mol. Biol. 1961, 3, 585–594. [Google Scholar] [CrossRef]
- Reichmann, M.E.; Rice, S.A.; Thomas, C.A.; Doty, P. A Further Examination of the Molecular Weight and Size of Desoxypentose Nucleic Acid. J. Am. Chem. Soc. 1954, 76, 3047–3053. [Google Scholar] [CrossRef]
- Samari, F.; Hemmateenejad, B.; Shamsipur, M.; Rashidi, M.; Samouei, H. Affinity of Two Novel Five-Coordinated Anticancer Pt(II) Complexes to Human and Bovine Serum Albumins: A Spectroscopic Approach. Inorg. Chem. 2012, 51, 3454–3464. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples and extracts are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, N.; Zhao, D.-Q.; Li, H.; Jiang, N.; Wen, J.-Y.; Liu, H.-Y. DNA Binding, Photonuclease Activity and Human Serum Albumin Interaction of a Water-Soluble Freebase Carboxyl Corrole. Molecules 2016, 21, 54. https://doi.org/10.3390/molecules21010054
Na N, Zhao D-Q, Li H, Jiang N, Wen J-Y, Liu H-Y. DNA Binding, Photonuclease Activity and Human Serum Albumin Interaction of a Water-Soluble Freebase Carboxyl Corrole. Molecules. 2016; 21(1):54. https://doi.org/10.3390/molecules21010054
Chicago/Turabian StyleNa, Ning, Da-Qiang Zhao, Heng Li, Nan Jiang, Jin-Yan Wen, and Hai-Yang Liu. 2016. "DNA Binding, Photonuclease Activity and Human Serum Albumin Interaction of a Water-Soluble Freebase Carboxyl Corrole" Molecules 21, no. 1: 54. https://doi.org/10.3390/molecules21010054






