Application of Ultra-High-Performance Liquid Chromatography Coupled with LTQ-Orbitrap Mass Spectrometry for the Qualitative and Quantitative Analysis of Polygonum multiflorum Thumb. and Its Processed Products
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of the LC-MS Conditions
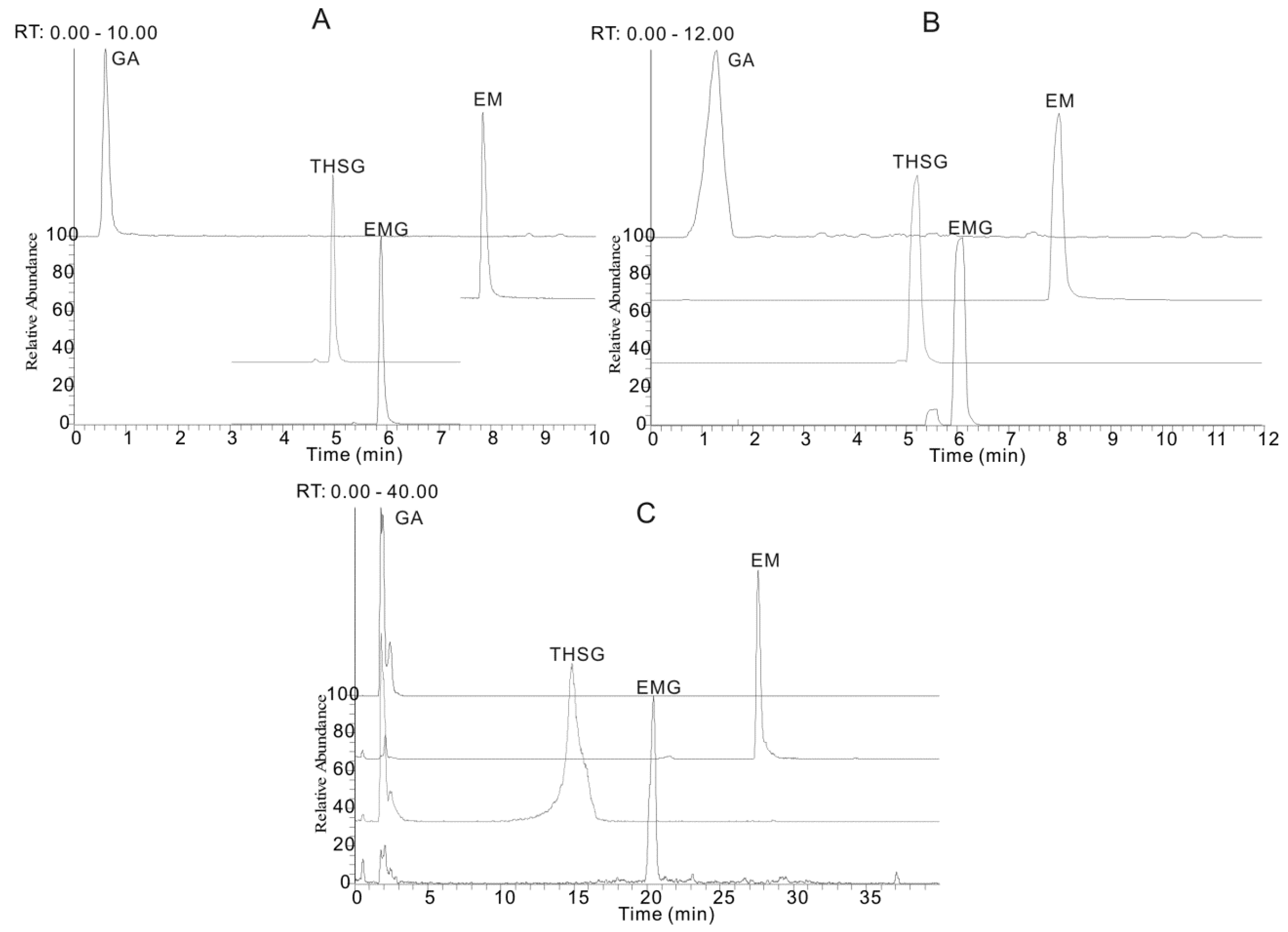

2.2. Sample Extraction Optimization
| Compound | Methanol Concentration (%) | Solid-Liquid Ratio | Extraction Time (min) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 50 | 70 | 100 | 1:50 | 1:125 | 1:250 | 15 | 30 | 60 | |
| Gallic acid | 0.46 | 0.52 | 0.67 | 0.69 | 0.88 | 0.89 | 0.26 | 0.77 | 0.73 |
| Emodin | 1.25 | 1.42 | 1.55 | 1.23 | 1.75 | 1.77 | 0.37 | 1.88 | 1.68 |
| trans-2,3,5,4′-Tetrahydroxy-stilbene-2-O-β-d-glucopyranoside | 1.26 | 1.32 | 1.61 | 1.18 | 1.93 | 1.69 | 0.79 | 1.78 | 1.86 |
| Emodin-8-O-β-d-glucopyranoside | 0.86 | 1.46 | 1.44 | 0.43 | 1.65 | 1.44 | 0.73 | 1.42 | 1.22 |
2.3. Tentative Identification of the Major Compounds
| No. | tR (min) | Precursor ions [M − H] | Formula | Mass Error (ppm) | MSn | Identification |
|---|---|---|---|---|---|---|
| 1 | 0.89 | 341.1078 | C12H21O11 | 0.18 | MS2[377]: 341 (100), 215 (15) | Sucrose * |
| 377.0843 [M + Cl] | MS3: 179 (100), 161 (23), 143 (22), 113 (17) | |||||
| 2 | 1.12 | 179.0556 225.0609 [M + HCOO] | C6H11O6 | 3.38 | MS2: 161 (100), 143 (90), 119 (44), 113 (40), 89 (40) | Glucose * |
| 3 | 2.17 | 169.0136 | C7H5O5 | 2.66 | MS2: 125 (100) | gallic acid * |
| MS3: 81 | ||||||
| 4 | 4.24 | 577.1330 | C30H25O12 | −1.8 | MS2: 425 (100), 407 (48), 457 (20), 471 (17), 289 (10) | procyanidin B |
| MS3: 407 (100) | ||||||
| 5 | 4.34 | 289.0708 | C15H13O6 | 0.47 | MS2: 245 (100), 205 (42), 179 (19) | epicatechin/catechin |
| MS3: 203 (100), 227 (23), 187 (22), 161 (20) | ||||||
| 6 # | 4.48 | 531.1488 | C26H27O12 | −2.2 | MS2: 369 (100), 351 (29), 405 (21), 243 (18) | unknown |
| MS3: 351 (100) | ||||||
| 7 # | 4.63 | 549.1594 | C26H29O13 | −0.97 | MS2: 387 (100), 459 (73), 531 (22), 297 (16) | unknown |
| 8 | 4.82 | 577.1330 | C30H25O12 | −1.53 | MS2: 425 (100), 407 (48), 457 (20), 471 (17), 289 (10) | procyanidin B |
| MS3: 407 (100) | ||||||
| 9 # | 4.98 | 421.1123 | C20H21O10 | −1.38 | MS2: 259 (100) | 6-methoxyl-2-acetyl-3-methyljuglone-8-O-glu |
| MS3: 259 (100), 331 (50), 128 (20) | ||||||
| 10 | 5.02 | 613.1751 [M + HCOO] | C27H33O16 | −1.21 | MS2: 405 (100), 567 (36) | tetrahydroxystilbene-O-di-glu |
| MS3: 243 (100) | ||||||
| 11 | 5.37 | 405.1177 | C20H21O9 | −0.74 | MS2: 243 (100) | THSG * |
| 811.2428 [2M − H] | MS3: 225 (100), 149 (79), 137 (73), 215 (70), 173 (36) | |||||
| 12 | 5.41 | 557.1286 | C27H25O13 | −0.66 | MS2: 313 (100), 243 (30), 405 (20), 169 (5) | tetrahydroxystilbene-O-(galloyl)-glu |
| MS3: 169 (100), 125 (20), 151 (20), 295 (17) | ||||||
| 13 | 5.71 | 557.1285 | C27H25O13 | −0.82 | MS2: 313 (100), 243 (80), 405 (70), 169 (10) | tetrahydroxystilbene-O-(galloyl)-glu |
| MS3: 169 (100), 125 (20), 151 (20), 295 (17) | ||||||
| 14 | 5.72 | 431.0970 | C21H19O10 | −0.63 | MS2: 269 (100) | emodin-1-O-glu |
| MS3: 225 (100), 241 (21), 181 (4) | ||||||
| 15 | 5.75 | 567.1488 | C29H27O12 | −1.59 | MS2: 243 (100) | tetrahydroxystilbene-O-(caffeoyl)-glu |
| MS3: 225 (100), 215 (72), 149 (67) | ||||||
| 16 | 5.90 | 551.1543 | C29H27O11 | −0.89 | MS2: 405 (100), 243 (31) | tetrahydroxystilbene-2-O-(coumaroyl)-glu |
| 17 | 5.92 | 447.0919 | C21H19O11 | −0.64 | MS2: 303 (100), 285 (100) | citreorosein-O-glu |
| MS3: 285 (100), 177 (11), 125 (8) | ||||||
| 18 | 6.02 | 407.1334 | C20H23O9 | −0.64 | MS2: 245 (100) | torachrysone-O-glu |
| MS3: 230 (100) | ||||||
| 19 | 6.11 | 431.0973 | C21H19O10 | 0.11 | MS2: 269 (100) | emodin-8-O-glu * |
| MS3: 225 (100), 241 (21), 197 (5) | ||||||
| 20 | 6.26 | 517.0978 | C24H21O13 | 0.26 | MS2: 473 (100), 431 (10) | emodin-O-(malonyl)-glu |
| MS3: 269 (100), 311 (12), 225 (5) | ||||||
| 21 | 6.38 | 445.1127 | C22H21O10 | −0.22 | MS2: 283 (100), 445 (42) | physcion-8-O-glu |
| 491.1182 [M + HCOO] | MS3: 240 (100), 268 (36) | |||||
| 22 | 6.42 | 313.0345 | C16H9O7 | 0.96 | MS2: 269 | carboxyl emodin |
| MS3: 225, 241, 197 | ||||||
| 23 | 7.21 | 269.0447 | C15H9O5 | 0.33 | MS2: 225, 241 | Emodin * |
| 24 | 7.63 | 269.0446 | C15H9O5 | 0.17 | MS2: 225, 241, 254 | aloe-emodin |
| 25 | 8.30 | 283.0605 | C16H11O5 | 1.41 | MS2: 240 | physcion |
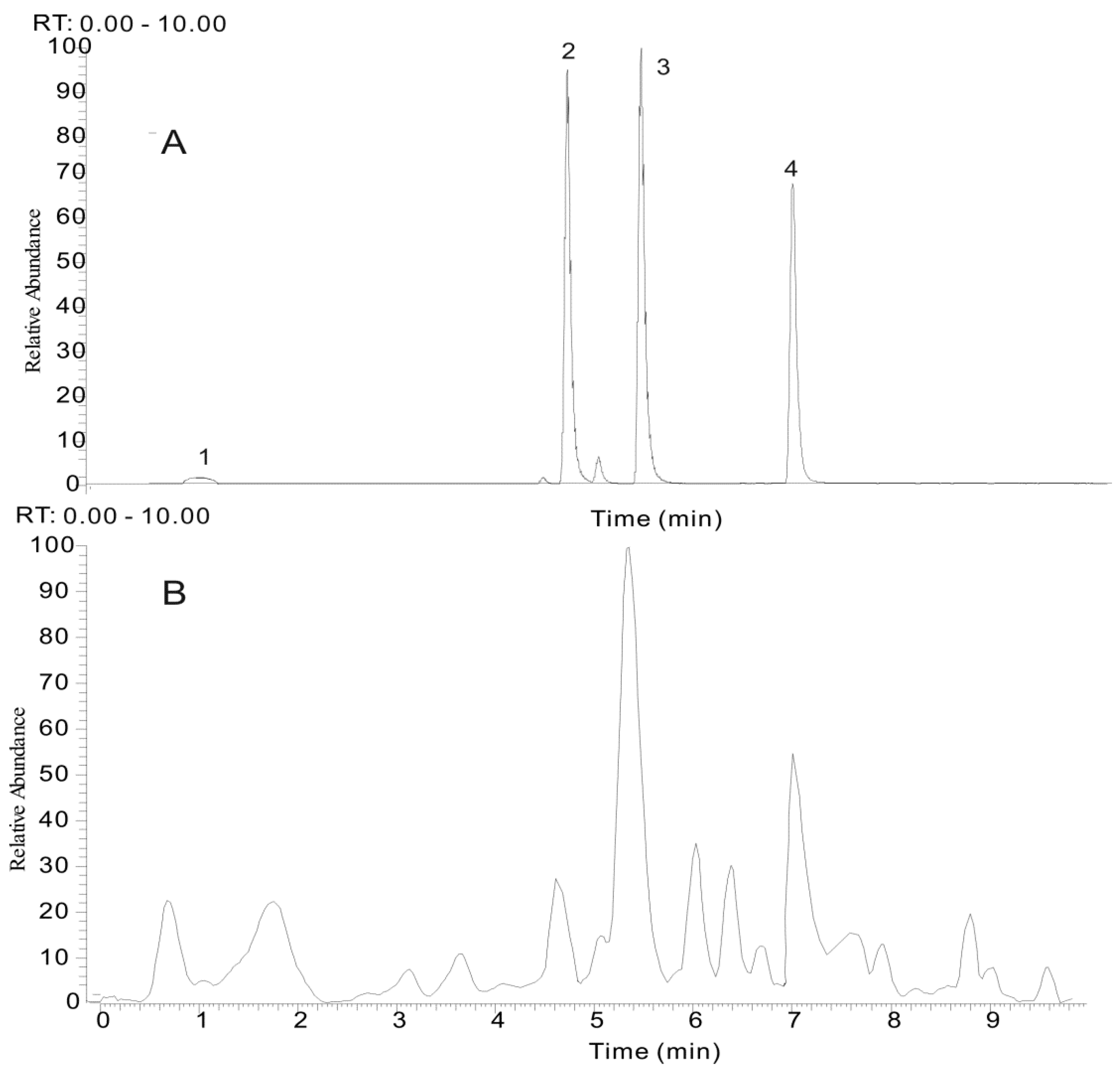
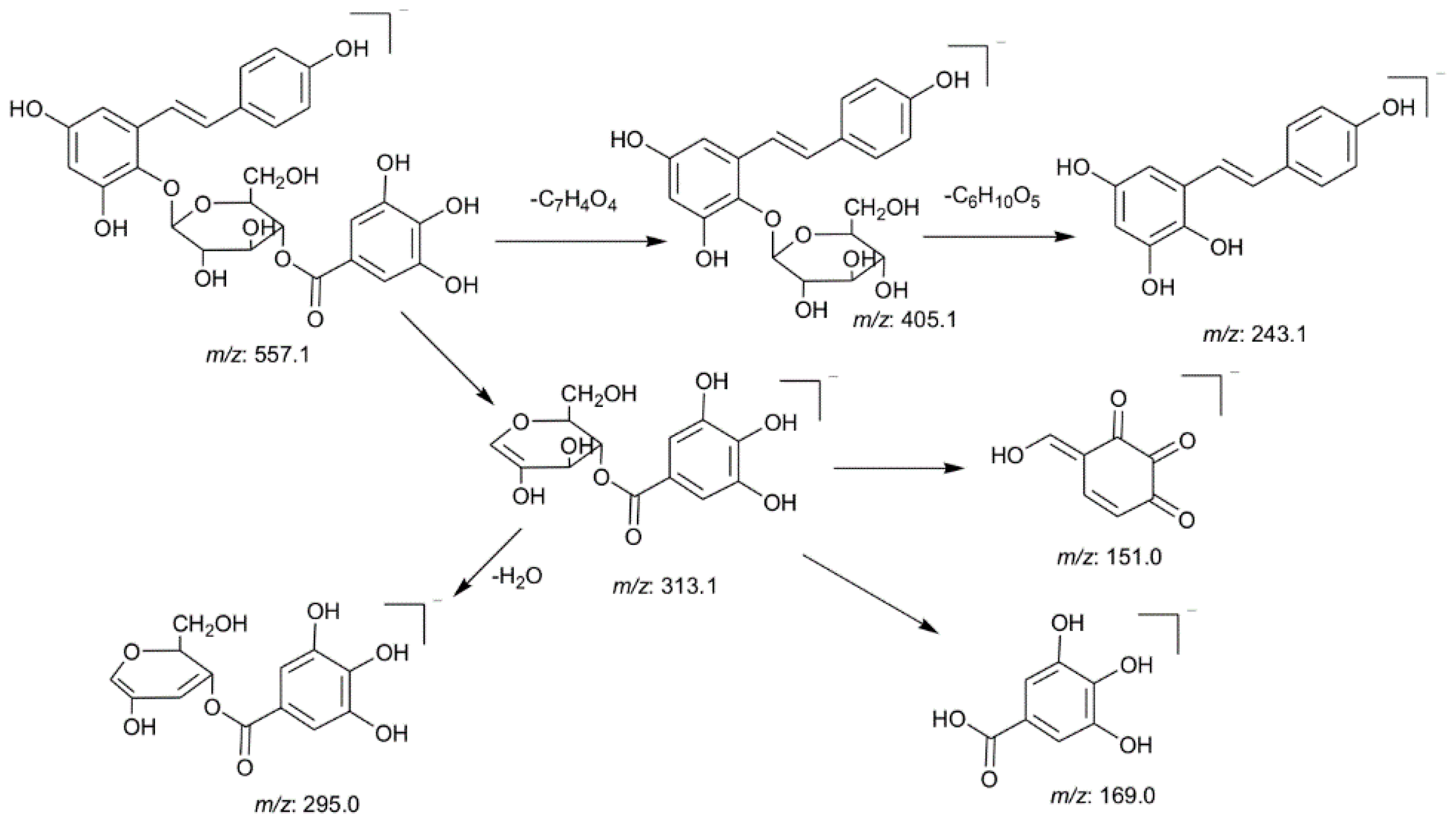
2.3.1. Identification of the Main Stilbene Glycosides
2.3.2. Identification of the Main Anthraquinones
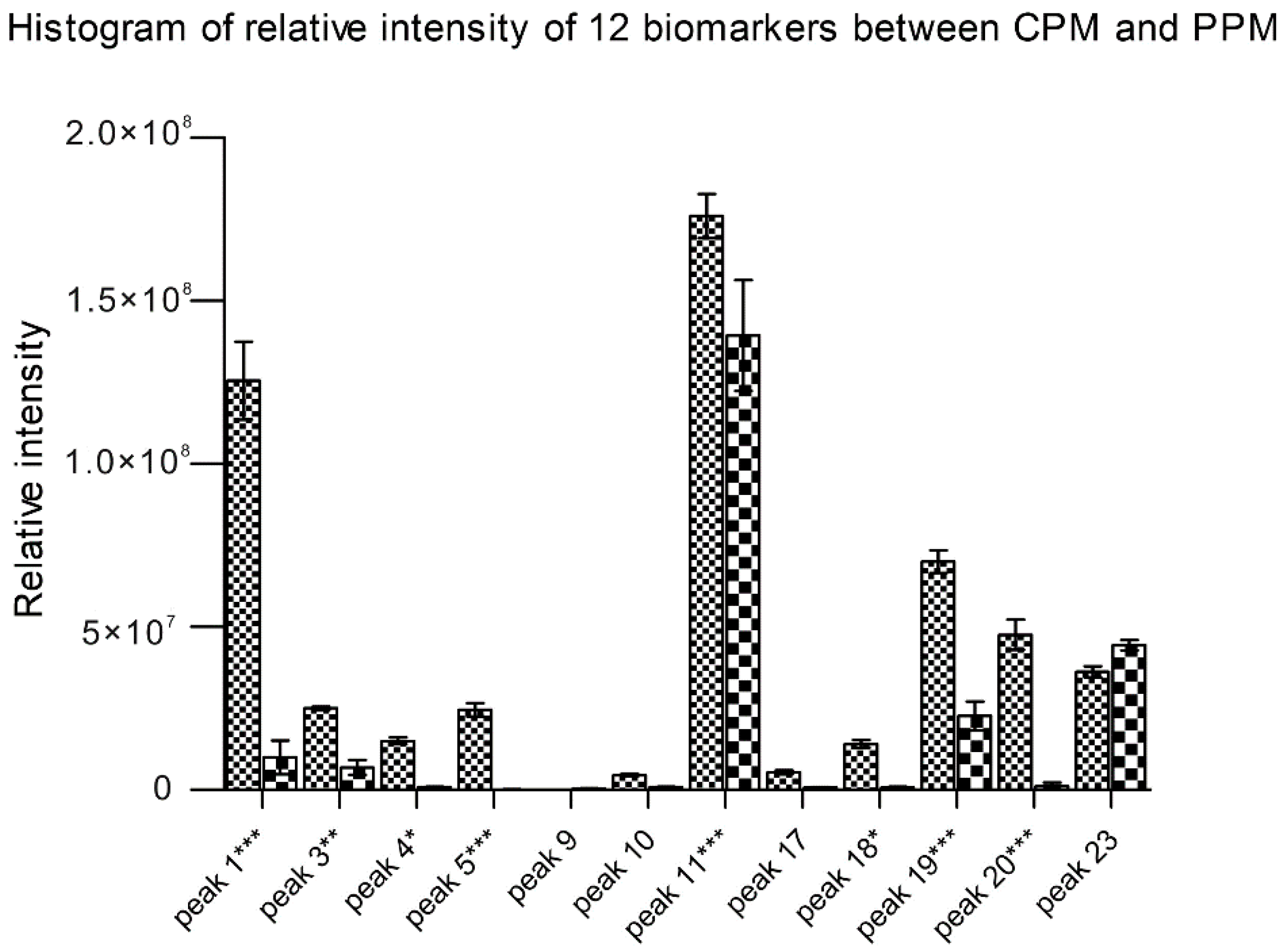
2.4. Changes of the Relative Intensity of the Main Chemical Components
2.5. Quantification of Four Major Phenolic Components of the UHPLC-MS/MS System
2.5.1. Calibration Curves, LOD and LOQ
| Analytes | Linear Regression Data Regression Equation | Test Range (μg/mL) | r2 | LOD a (ng/mL) | LOQ b (ng/mL) |
|---|---|---|---|---|---|
| Gallic acid | y = 3598.5x + 205.82 | 0.03–4.00 | 0.9997 | 0.03 | 0.10 |
| Emodin | y = 25639x + 2283.1 | 0.01–1.40 | 0.9916 | 0.01 | 0.02 |
| trans-2,3,5,4′-Tetrahydroxy-stilbene-2-O-β-d-gluco-pyranoside | y = 652.2x − 5013.2 | 1.15–105.00 | 0.9997 | 0.01 | 0.02 |
| Emodin-8-O-β-d-gluco-pyranoside | y = 7690.7x + 14652 | 0.29–114.00 | 0.9998 | 0.01 | 0.06 |
2.5.2. Precision
| Compound | Intra-Day (n = 6) | Inter-Day (n = 3) | ||
|---|---|---|---|---|
| Mean (μg/mL) | RSD a (%) | Mean (μg/mL) | RSD (%) | |
| Gallic acid | 0.01 | 1.27 | 0.07 | 3.27 |
| 0.25 | 1.15 | 0.25 | 1.62 | |
| 1.00 | 0.92 | 0.97 | 2.74 | |
| Emodin | 0.00 | 1.90 | 0.04 | 2.14 |
| 0.34 | 1.67 | 0.34 | 1.87 | |
| 2.56 | 1.48 | 2.50 | 2.39 | |
| trans-2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glucopyranoside | 2.17 | 1.45 | 2.08 | 1.68 |
| 10.10 | 1.23 | 10.12 | 2.71 | |
| 107.67 | 0.77 | 106.33 | 1.65 | |
| Emodin-8-O-β-d-glucopyranoside | 0.37 | 1.14 | 0.37 | 2.45 |
| 1.52 | 1.49 | 1.50 | 1.49 | |
| 67.88 | 1.30 | 68.13 | 2.57 | |
2.5.3. Repeatability, Stability, Specificity and Selectivity
| Compound | Repeatability (n = 6) | Stability (n = 6) | ||
|---|---|---|---|---|
| Mean (μg/mL) | RSD a (%) | Mean (μg/mL) | RSD (%) | |
| Gallic acid | 0.33 | 3.81 | 0.34 | 2.01 |
| Emodin | 1.04 | 2.53 | 1.02 | 1.17 |
| trans-2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glucopyranoside | 27.34 | 1.85 | 27.83 | 1.49 |
| Emodin-8-O-β-d-glucopyranoside | 0.83 | 2.98 | 0.80 | 1.37 |
2.5.4. Accuracy
| Compound | Amount (μg/mL) | Recovery a (%) | RSD b (%) | |
|---|---|---|---|---|
| Spiked | Found | |||
| Gallic acid | 0.26 | 0.26 ± 0.00 | 98.39 | 1.19 |
| 0.33 | 0.32 ± 0.01 | 98.87 | 1.81 | |
| 0.39 | 0.39 ± 0.00 | 98.54 | 0.04 | |
| Emodin | 0.90 | 0.91 ± 0.00 | 100.75 | 0.06 |
| 1.12 | 1.13 ± 0.02 | 100.53 | 1.43 | |
| 1.35 | 1.33 ± 0.01 | 98.73 | 1.41 | |
| trans-2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glucopyranoside | 23.46 | 22.83 ± 0.14 | 97.34 | 0.63 |
| 29.32 | 29.43 ± 0.37 | 100.37 | 1.26 | |
| 35.19 | 34.54 ± 0.40 | 98.17 | 1.18 | |
| Emodin-8-O-β-d-glucopyranoside | 7.35 | 7.17 ± 0.06 | 97.56 | 0.77 |
| 9.19 | 9.16 ± 0.13 | 99.75 | 1.46 | |
| 11.03 | 11.07 ± 0.05 | 100.40 | 0.43 | |
2.5.5. Sample Analysis
| No. | Content (mg/g) (n = 3) | |||
|---|---|---|---|---|
| Gallic Acid (GA) | Emodin (EM) | 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glucoside (THSG) | Emodin-8-O-β-d-glucoside (EMG) | |
| A1 | 0.63 ± 0.01 | 1.01 ± 0.02 | 34.43 ± 0.59 | 21.11 ± 0.34 |
| A2 | 0.44 ± 0.05 | 0.23 ± 0.00 | 4.53 ± 0.15 | 1.32 ± 0.05 |
| A3 | 0.32 ± 0.01 | 0.18 ± 0.00 | 24.54 ± 0.93 | 7.29 ± 0.20 |
| A4 | 0.30 ± 0.04 | 0.52 ± 0.06 | 16.27 ± 0.57 | 7.98 ± 0.17 |
| A5 | 0.50 ± 0.01 | 0.82 ± 0.01 | 24.01 ± 0.76 | 14.44 ± 0.65 |
| A6 | 0.31 ± 0.00 | 2.46 ± 0.04 | 26.57 ± 0.46 | 15.77 ± 0.25 |
| A7 | 0.58 ± 0.01 | 0.32 ± 0.00 | 20.55 ± 0.67 | 3.85 ± 0.08 |
| A8 | 0.43 ± 0.01 | 0.10 ± 0.00 | 24.97 ± 0.58 | 7.83 ± 0.25 |
| A9 | 0.60 ± 0.01 | 2.38 ± 0.05 | 19.71 ± 0.23 | 15.87 ± 0.15 |
| A10 | 0.23 ± 0.00 | 3.27 ± 0.04 | 23.51 ± 0.55 | 14.59 ± 0.31 |
| B1 | 0.02 ± 0.00 | 0.49 ± 0.01 | 2.54 ± 0.04 | 2.12 ± 0.03 |
| B2 | 0.09 ± 0.00 | 0.36 ± 0.00 | 1.85 ± 0.03 | 0.35 ± 0.00 |
| B3 | 0.28 ± 0.00 | 0.45 ± 0.01 | 2.63 ± 0.04 | 3.11 ± 0.04 |
| B4 | 0.15 ± 0.01 | 0.50 ± 0.01 | 1.97 ± 0.05 | 2.59 ± 0.04 |
| B5 | 0.90 ± 0.01 | 0.85 ± 0.03 | 10.42 ± 0.37 | 2.51 ± 0.04 |
| C1 | 0.31 ± 0.01 | 0.56 ± 0.02 | 2.05 ± 0.03 | 3.49 ± 0.04 |
| C2 | 0.02 ± 0.01 | 1.13 ± 0.02 | 5.94 ± 0.10 | 3.92 ± 0.04 |
| C3 | 1.31 ± 0.04 | 0.32 ± 0.01 | 2.55 ± 0.06 | 0.47 ± 0.01 |
| C4 | 0.47 ± 0.01 | 1.31 ± 0.04 | 11.71 ± 0.41 | 4.92 ± 0.09 |
| C5 | 0.39 ± 0.01 | 0.67 ± 0.02 | 8.21 ± 0.10 | 2.62 ± 0.09 |
3. Experimental
3.1. Reagents and Chemicals
3.2. Chromatography and MS Conditions
3.3. Sample Preparation
3.4. Preparation of Standard Solutions
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, H.Y.; Song, L.X.; Feng, S.B.; Liu, Y.C.; Zuo, G.; Lai, F.L.; He, G.Y.; Chen, M.J.; Huang, D. Characterization of proanthocyanidins in stems of Polygonum multiflorum Thunb. as strong starch hydrolase inhibitors. Molecules 2014, 18, 2255–2265. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.N.; Li, W.; Kim, J.H. Chemical constituents from the root of Polygonum multiflorum and their soluble epoxide hydrolase inhibitory activity. Arch. Pharm. Res. 2015, 38, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Li, L.F.; Ni, B.R.; Lin, H.M.; Zhang, M. Traditional usages, botany, phytochemistry and toxicology of Polygonum multiflorum Thunb.: A review. J. Ethnopharmacol. 2015, 159, 158–183. [Google Scholar]
- Choi, S.G.; Kim, J.; Sung, N.D.; Son, K.H.; Cheon, H.G.; Kim, K.R.; Kwon, B.M. Anthraquinones, Cdc25B phosphatase inhibitors, isolated from the roots of Polygonum multiflorum Thunb. Nat. Prod. Res. 2007, 6, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.Y.; Su, Y.F.; Chen, L. Polygonumosides A−D, Stilbene Derivatives from Processed Roots of Polygonum multiflorum. J. Nat. Prod. 2014, 77, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Shim, D.W.; Shin, W.Y. Anti-inflammatory effect of emodin via attenuation of NLRP3 inflammasome activation. Int. J. Mol. Sci. 2015, 4, 8102–8109. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, G.; Anto, R.J.; Srinivas, P.; Vidhyalakshmi, S. Emodin induces apoptosis of human cervical cancer cells through poly (ADP-ribose) polymerase cleavage and activation of caspase-9. Eur. J. Pharmacol. 2003, 473, 117–125. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Praveen, N.; Kim, E.H.; Kim, S.H.; Chung, I.M. Production of anthraquinones, phenolic compounds and biological activities from hairy root cultures of Polygonum multiflorum Thunb. Protoplasma 2014, 251, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.C.; Wang, M.F.; Chen, Y.C.; Yang, D.Y.; Lee, M.S.; Cheng, F.C. Long-term administration of Polygonum multiflorum Thunb. reduces cerebral ischemia-induced infarct volume in gerbils. Am. J. Chin. Med. 2003, 31, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Shen, J.F.; Xu, J.Y.; Xiao, J.H.; Wang, J.L. Inhibitory effects of 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside on experimental inflammation and cyclo-oxygenase 2 activity. J. Asian Nat. Prod. Res. 2007, 9, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Zhao, L.B.; Han, T.Z.; Wang, J.L. Protective effects of 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside, an active component of Polygonum multiflorum Thunb. on experimental colitis in mice. Eur. J. Pharmacol. 2008, 578, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Xiao, J.H.; Chang, M.J.; Wang, J.L. Beneficial effects of THSG on acetic acid-induced experimental Colitis: Involvement of upregulation of PPAR-γ and inhibition of the NF-κB Inflammatory Pathway. Molecules 2011, 16, 8552–8568. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.S.; Gu, X.H.; Tang, J.; Ho, C.T.; Tang, J. Stilbene glycoside from the roots of Polygonum multiflorum Thunb. and their in vitro antioxidant activities. J. Food Lipids 2006, 13, 131–144. [Google Scholar]
- Lv, L.S.; Gu, X.H.; Tang, J.; Ho, C.T. Antioxidant activity of stilbene glycoside from Polygonum multiflorum Thunb in vivo. Food Chem. 2007, 104, 1678–1681. [Google Scholar] [CrossRef]
- Liu, Q.L.; Xiao, J.H.; Ma, R.; Ban, Y.; Wang, J.L. Effect of 2,3,5,4′-tetrahydroxystilbene -2-O-β-d-glucoside on lipoprotein oxidation and proliferation of coronary arterial smooth cells. J. Asian Nat. Prod. Res. 2007, 9, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.C.; Huang, G.H.; Ho, Y.L.; Hsieh, P.C.; Chung, H.P.; Chou, F.I.; Chang, Y.S. Influence of gamma irradiation on microbial load and antioxidative characteristics of Polygoni multiflori. Radix. Process. Biochem. 2011, 46, 777–782. [Google Scholar] [CrossRef]
- Xie, W.D.; Zhao, Y.N.; Du, L.J. Emerging approaches of traditional Chinese medicine formulas for the treatment of hyperlipidemia. J. Ethnopharmacol. 2012, 140, 345–367. [Google Scholar] [CrossRef] [PubMed]
- Cheung, F.W.; Leung, A.W.; Liu, W.K.; Che, C.T. Tyrosinase inhibitory activity of a glucosylated hydroxystilbene in mouse melan—A melanocytes. J. Nat. Prod. 2014, 77, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Um, M.Y.; Choi, W.H.; Aan, J.Y.; Kim, S.R.; Ha, T.Y. Protective effect of Polygonum multiflorum Thunb. on amyloid β-peptide 25–35 induced cognitive deficits in mice. J. Ethnopharmacol. 2006, 104, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Tang, Y.; Feng, B.; Ye, C.; Fang, L.; Zhang, L.; Li, L. Changes in hippocampal synapses and learning-memory abilities in age-increasing rats and effects of tetrahydroxystilbene glucoside in aged rats. Neuroscience 2007, 149, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.X.; Fan, Y.J.; Luo, A.S. In vitro free radicals scavenging activities of polysaccharide from Polygonum multiflorum Thunb. J. Med. Plants Res. 2011, 5, 966–972. [Google Scholar]
- Guan, S.Y.; Su, W.W.; Wang, N.; Li, P.B.; Wang, Y.G. A potent tyrosinase activator from Radix Polygoni multiflori and its melanogenesis stimulatory effect in B16 melanoma cells. Phytother. Res. 2008, 22, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Q.; Xu, J.M.; Long, M.H.; Tu, Z.M.; Yang, G.X.; He, G.Y. 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glucoside (THSG) induces melanogenesis in B16 cells by MAP kinase activation and tyrosinase upregulation. Life Sci. 2009, 85, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Zhang, N.N.; Park, D.K. Topical application of Polygonum multiflorum extract induces hair growth of resting hair follicles through upregulating Shh and β-catenin expression in C57BL/6 mice. J. Ethnopharmacol. 2011, 135, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, S.J.; Cui, T.B.; Liu, Z.Y.; Zhao, W. 2,3,5,4′-Tetrahydroxystilbene- 2-O-β-d-glycoside biosynthesis by suspension cells cultures of Polygonum multiflorum Thunb. and production enhancement by methyl jasmonate and salicylic acid. Molecules 2012, 17, 2240–2247. [Google Scholar]
- Han, L.F.; Wu, B.; Pan, G.X.; Wang, Y.F.; Song, X.B.; Gao, X.M. UPLC-PDA analysis for simultaneous quantification of four active compounds in crude and processed rhizome of Polygonum multiflorum Thunb. Chromatographia 2009, 70, 657–659. [Google Scholar] [CrossRef]
- Yan, H.J.; Fang, Z.J.; Fu, J.; Yu, S.X. The correlation between bioactive components of Fallopia multiflora root and environmental factors. Am. J. Chin. Med. 2010, 38, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, W.S.; Sun, L.N. LC-VWD-MS determination of three anthraquinones and one stilbene in the quality control of crude and prepared roots of Polygonum multiflorum Thunb. Chromatographia 2008, 67, 869–874. [Google Scholar] [CrossRef]
- Lin, Z.L.; Liu, Y.Y.; Wang, C.; Guo, N.; Song, Z.Q.; Wang, C.; Xin, L.; Lu, A.P. Comparative analyses of chromatographic fingerprints of the roots of Polygonum multiflorum Thunb. and their processed products using RRLC/DAD/ESI-MSn. Planta. Med. 2011, 77, 1855–1860. [Google Scholar]
- Yi, T.; Leung, K.S.Y.; Lu, G.H.; Zhang, H.; Chan, K. Identification and determination of the major constituents in traditional Chinese medicinal plant Polygonum multiflorum Thunb. by HPLC coupled with PAD and ESI/MS. Phytochem. Anal. 2007, 18, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, J.; Huang, Z.H.; Qiu, X.H. Identification of new dianthrone glycosides from Polygonum multiflorum Thunb. using high-performance liquid chromatography coupled with LTQ-Orbitrap mass spectrometry detection: A strategy for the rapid detection of new low abundant metabolites from traditional Chinese medicines. Anal. Methods 2012, 4, 1806–1812. [Google Scholar]
- Qiu, X.H.; Zhang, J.; Huang, Z.H.; Zhu, D.Y.; Xu, W. Profiling of phenolic constituents in Polygonum multiflorum Thunb. by combination of ultra-high-pressure liquid chromatography with linear ion trap-Orbitrap mass spectrometry. J. Chromatogr. A 2013, 1292, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Song, Z.Q.; Wang, C. Content variances of catechin and gallic acid in Polygonum multiflorum after steaming. Chin. Tradit. Pat. Med. 2009, 31, 1392–1394. [Google Scholar]
- Liang, Z.T.; Chen, H.B.; Yu, Z.L. Comparison of raw and processed Radix Polygoni multiflori (Heshouwu) by HPLC-MS. Chin. Med. 2010, 5, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds gallic acid (GA), trans-2,3,5,4′-tetra-hydroxystilbene-2-O-β-d-glucopyranoside (THSG), emodin-8-O-β-d-glucopyranoside (EMG), emodin (EM) and 20 batches CPM and PPM (crude P. multiflorum and processed P. multiflorum) are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.-H.; Zhang, J.; Qiu, X.-H.; Bai, J.-Q.; Gao, Y.-H.; Xu, W. Application of Ultra-High-Performance Liquid Chromatography Coupled with LTQ-Orbitrap Mass Spectrometry for the Qualitative and Quantitative Analysis of Polygonum multiflorum Thumb. and Its Processed Products. Molecules 2016, 21, 40. https://doi.org/10.3390/molecules21010040
Wang T-H, Zhang J, Qiu X-H, Bai J-Q, Gao Y-H, Xu W. Application of Ultra-High-Performance Liquid Chromatography Coupled with LTQ-Orbitrap Mass Spectrometry for the Qualitative and Quantitative Analysis of Polygonum multiflorum Thumb. and Its Processed Products. Molecules. 2016; 21(1):40. https://doi.org/10.3390/molecules21010040
Chicago/Turabian StyleWang, Teng-Hua, Jing Zhang, Xiao-Hui Qiu, Jun-Qi Bai, You-Heng Gao, and Wen Xu. 2016. "Application of Ultra-High-Performance Liquid Chromatography Coupled with LTQ-Orbitrap Mass Spectrometry for the Qualitative and Quantitative Analysis of Polygonum multiflorum Thumb. and Its Processed Products" Molecules 21, no. 1: 40. https://doi.org/10.3390/molecules21010040





