Mechanism of NO Photocatalytic Oxidation on g-C3N4 Was Changed by Pd-QDs Modification
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of Photocatalysts
2.2. Characterization
2.3. Photocatalytic Activity Test
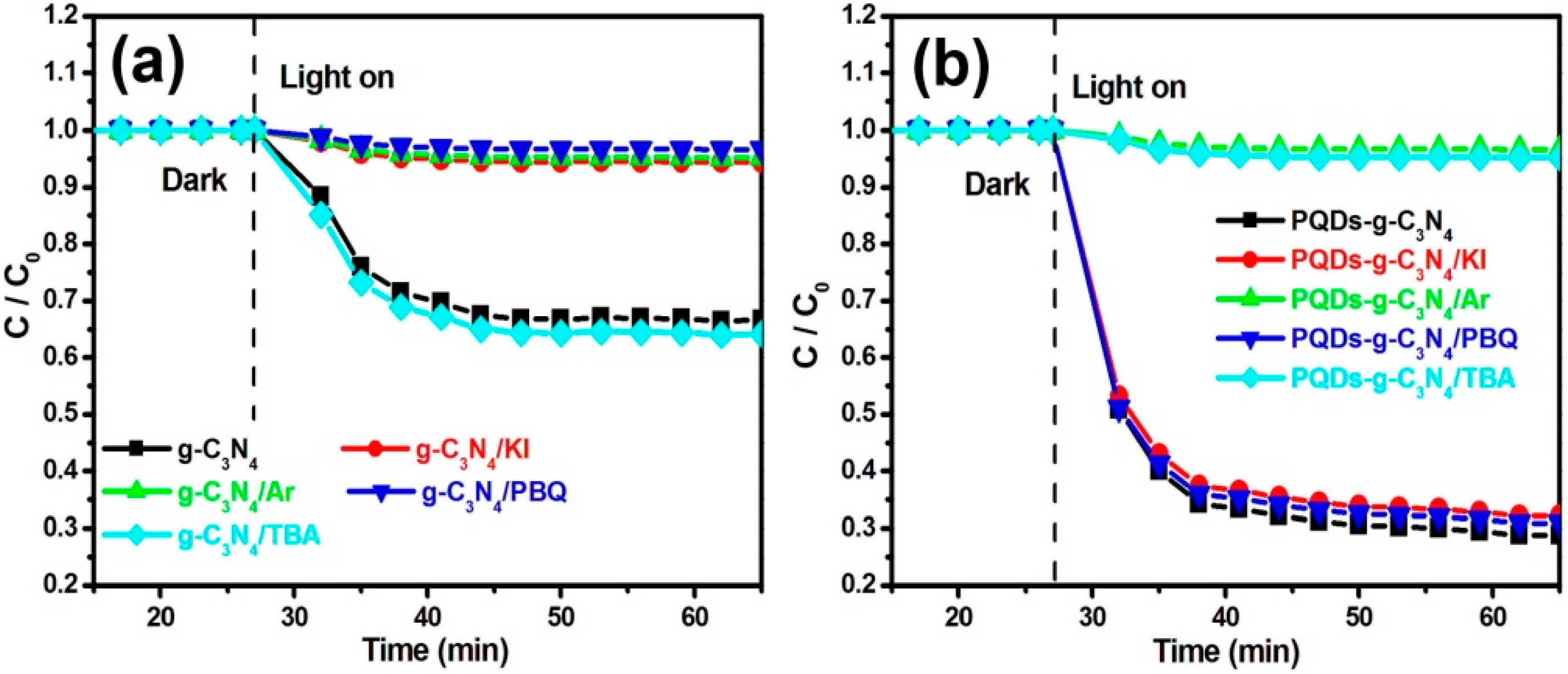
2.4. Trapping Experiment
3. Results and Discussion
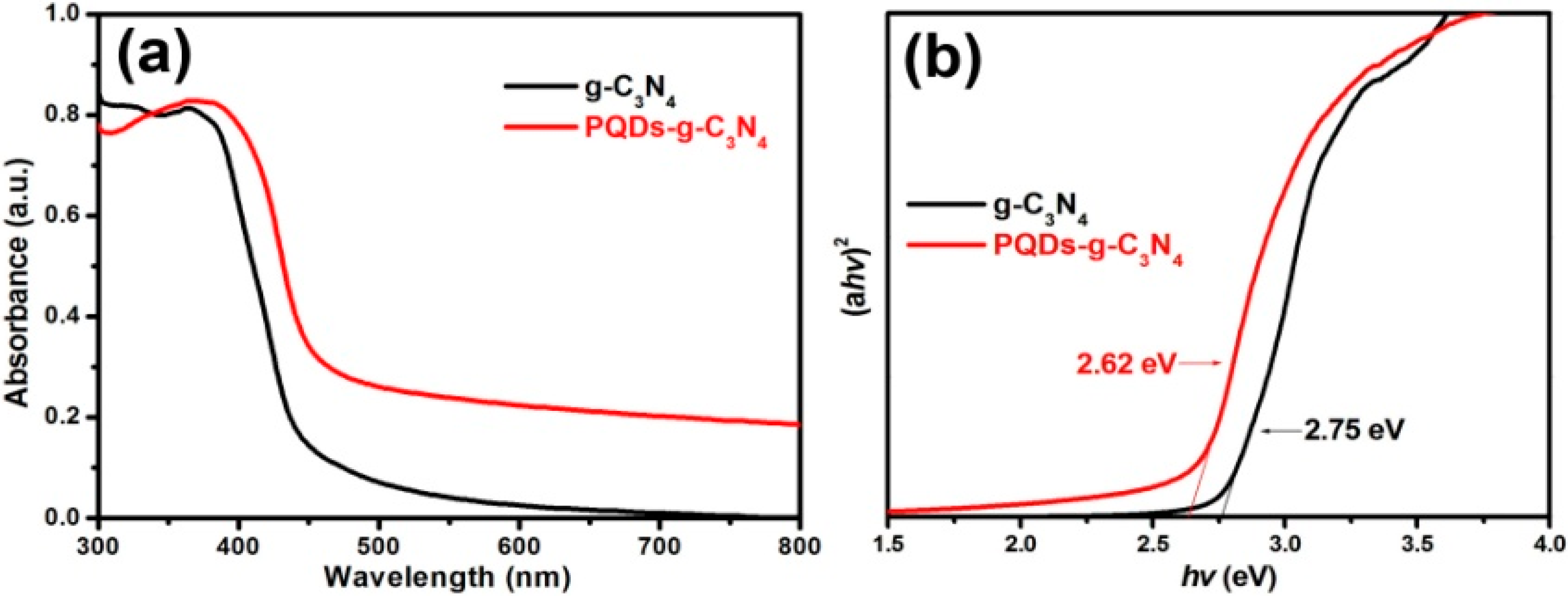
3.1. Structural Characterization of Result Samples
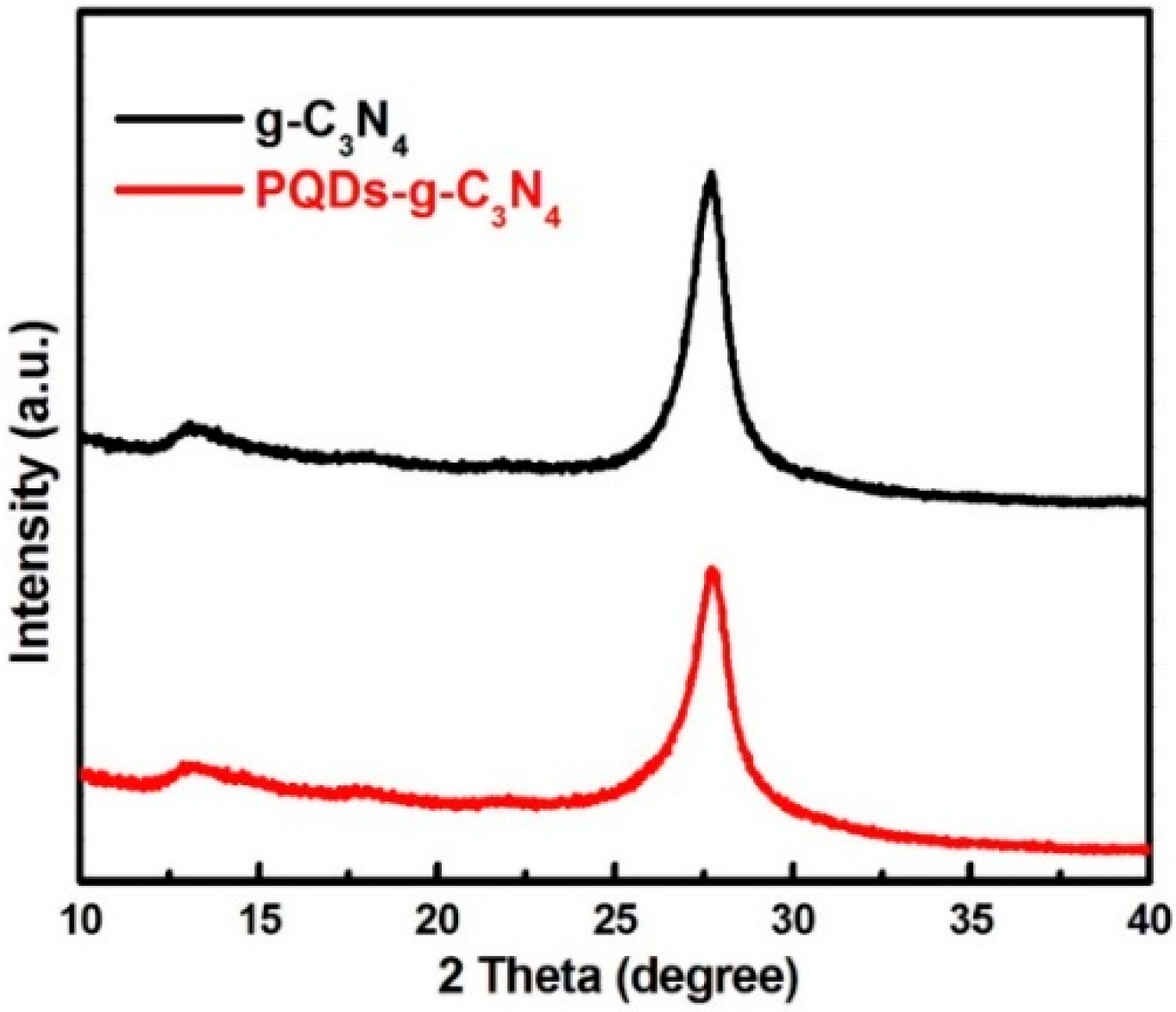
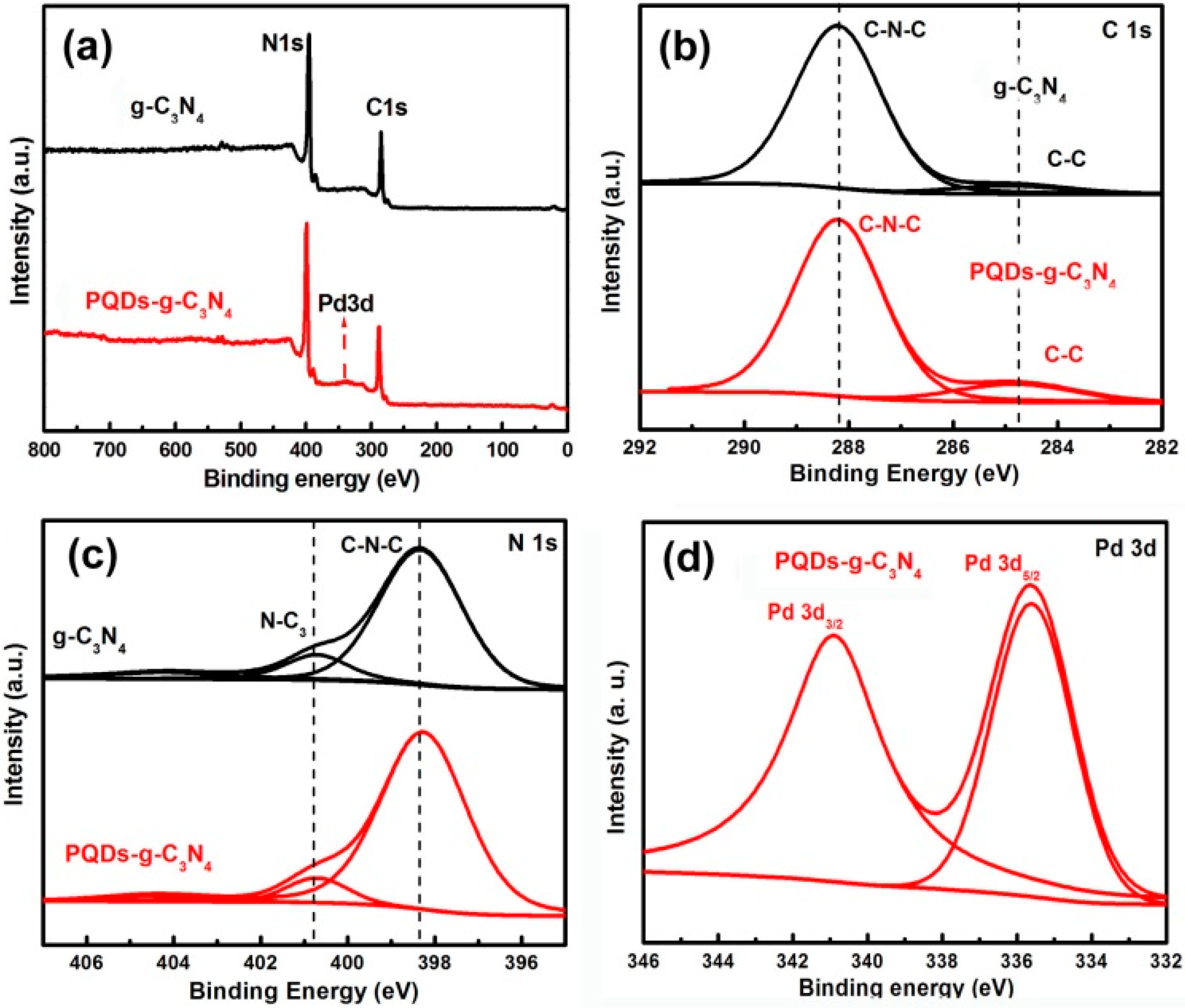
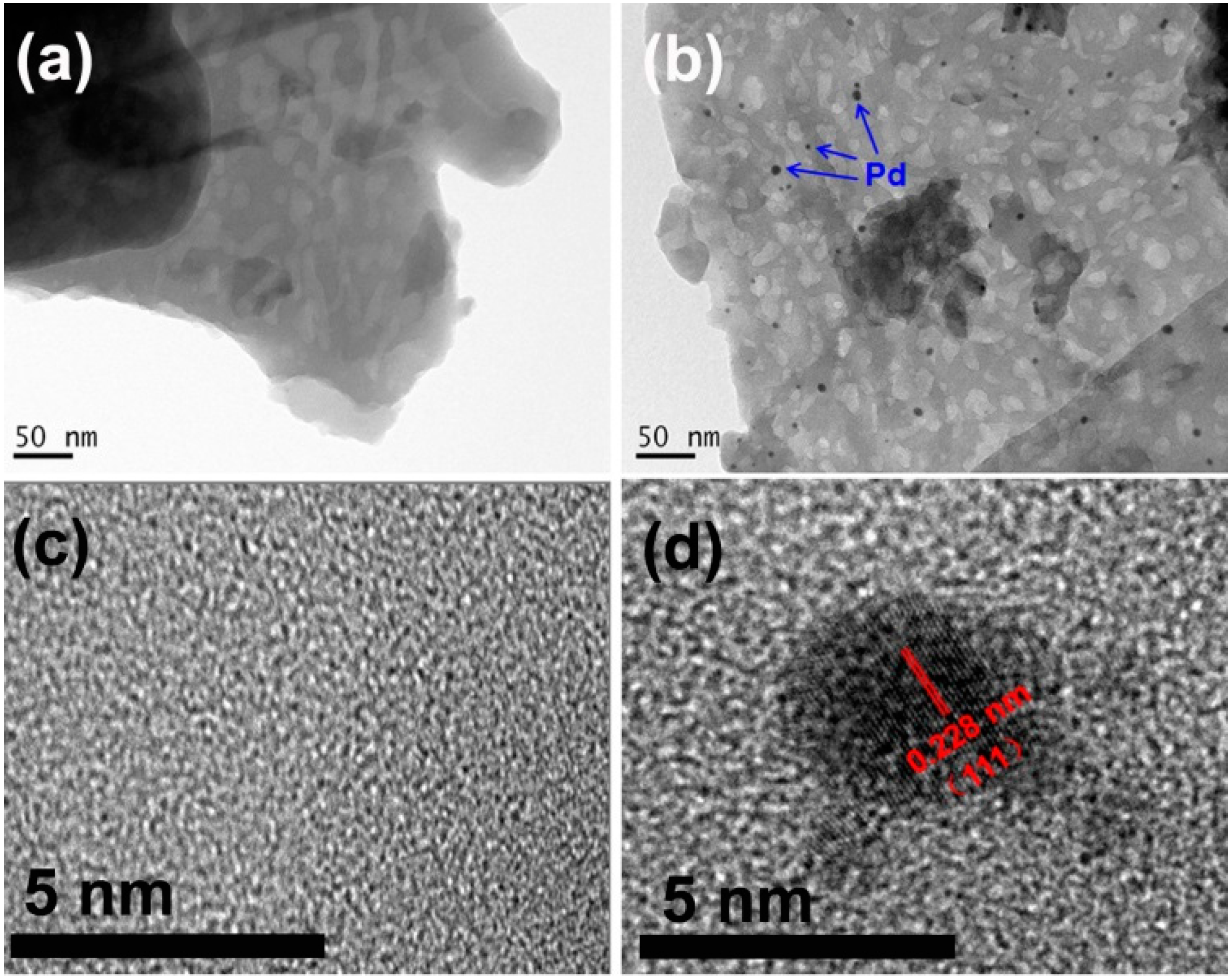
3.2. Photocatalytic Activities
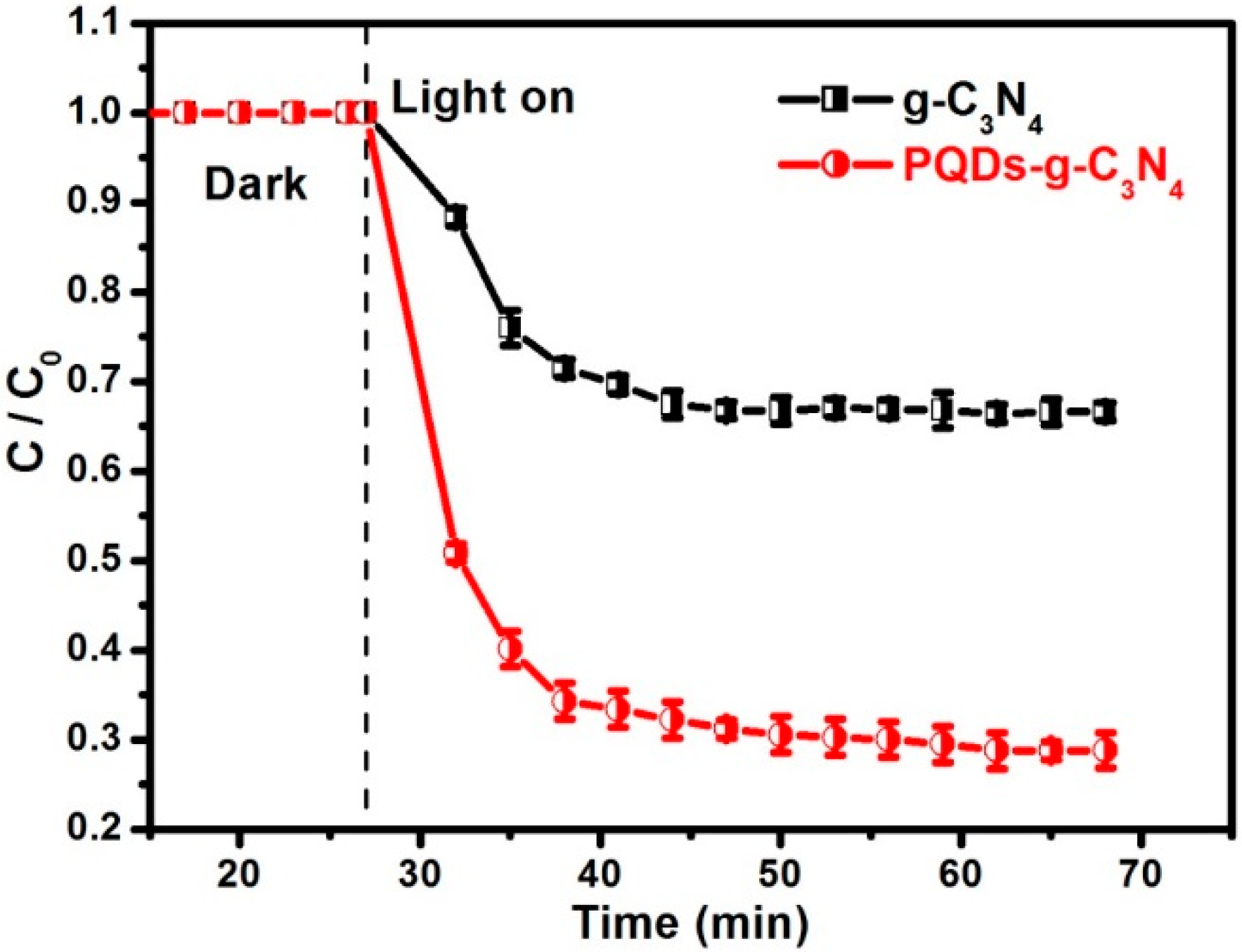
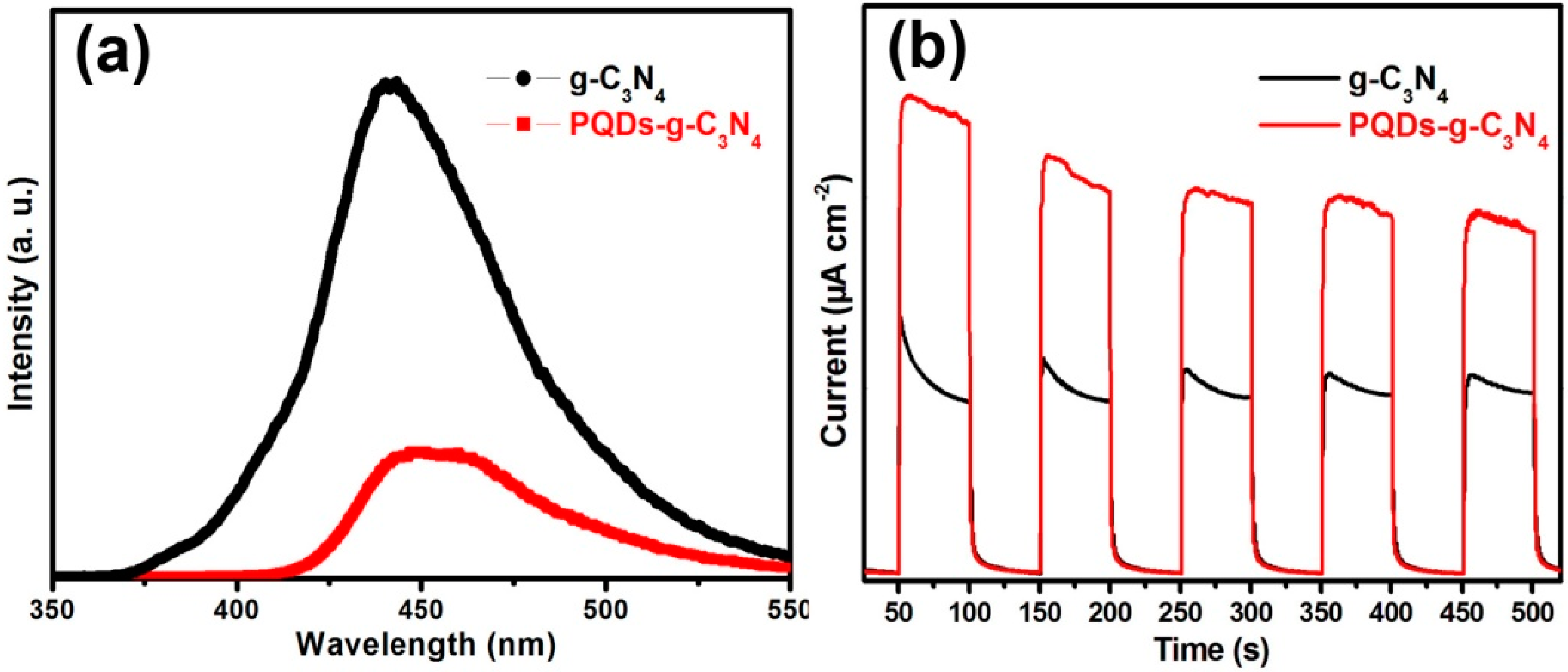
3.3. Mechanism of Activity Enhancement

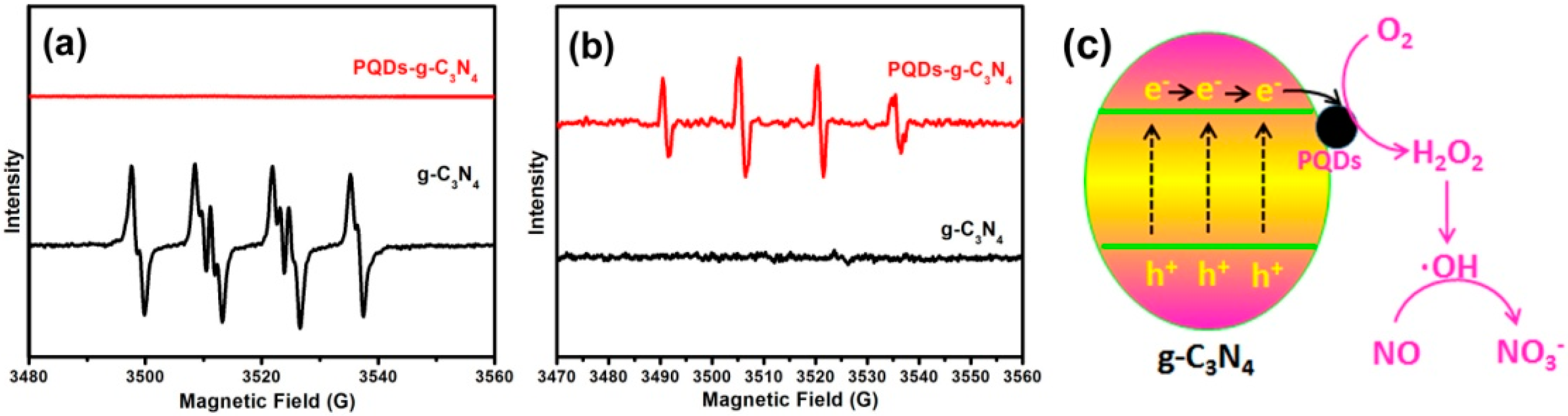
3.4. The Mechanism of NO Removal
| Photocatalyst + solar light → h+ + e− |
| e− + O2 → ·O2− |
| ·O2− + 2H+ + e−→ H2O2 |
| H2O2 + e− → 2·OH |
| h+ + H2O → ·OH + H+ |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Carraway, E.R.; Hoffman, A.J.; Hoffmann, M.R. Photocatalytic oxidation of organic acids on quantum-sized semiconductor colloids. Environ. Sci. Technol. 1994, 28, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Marta, I.L. Heterogeneous photocatalysis transition metal ions in photocatalytic systems. Appl. Catal. B 1999, 23, 89–114. [Google Scholar]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Yamasita, D.; Takata, T.; Hara, M.; Kondo, J.N.; Domen, K. Recent progress of visible-light-driven heterogeneous photocatalysts for overall water splitting. Solid State Ion. 2004, 172, 591–595. [Google Scholar] [CrossRef]
- Ettedgui, J.; Diskin-Posner, Y.; Weiner, L.; Neumann, R. Photoreduction of carbon dioxide to carbon monoxide with hydrogen catalyzed by a Rhenium(I) phenanthroline-polyoxometalate hybrid complex. J. Am. Chem. Soc. 2011, 133, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, W.; Jiang, D.; Gao, E.; Sun, S. Photoreduction of CO on BiOCl nanoplates with the assistance of photoinduced oxygen vacancies. Nano Res. 2015, 3, 821–831. [Google Scholar] [CrossRef]
- Kim, Y.I.; Salim, S.; Huq, M.J.; Mallouk, T.E. Visible light photolysis of hydrogen iodide using sensitized layered semiconductor particles. J. Am. Chem. Soc. 1991, 113, 9561–9563. [Google Scholar] [CrossRef]
- Lee, Y.; Terashima, H.; Shimodaira, Y.; Teramura, K.; Hara, M.; Kobayashi, H.; Domen, K.; Yashima, M. Zinc germanium oxynitride as a photocatalyst for overall water splitting under visible light. J. Phys. Chem. C 2007, 111, 1042–1048. [Google Scholar] [CrossRef]
- Maeda, K.; Takata, T.; Hara, M.; Saito, N.; Inoue, Y.; Kobayashi, H.; Domen, K. GaN:ZnO solid solution as a photocatalyst for visible-light-driven overall water splitting. J. Am. Chem. Soc. 2005, 127, 8286–8287. [Google Scholar] [CrossRef] [PubMed]
- Hitoki, G.; Takata, T.; Kondo, J.N.; Hara, M.; Kobayashi, H.; Domen, K. An oxynitride, TaON, as an efficient water oxidation photocatalyst under visible light irradiation(λ <500 nm). Chem. Commun. 2002, 16, 1698–1699. [Google Scholar]
- Wang, X.W.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.F.; Zhang, J.S.; Fu, X.Z.; Antonietti, M.; Wang, X.C. Fe-g-C3N4-Catalyzed oxidation of benzene to phenol using hydrogen peroxide and visible light. J. Phys. Chem. A 2009, 131, 11658–11659. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.X.; Chen, X.F.; Antonietti, M.; Wang, X.C. Synthesis of transition metal-modified carbon nitride polymers for selective hydrocarbon oxidation. ChemSusChem 2010, 4, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation of rhodamine b and methyl orange over boron-doped g-C3N4 under visible light irradiation. Langmuir 2010, 26, 3894–3910. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.H.; Ai, Z.H.; Zhang, L.Z. Efficient anoxic pollutant removal with oxygen functionalized graphitic carbon nitride under visible light. RSC Adv. 2014, 4, 5553–5560. [Google Scholar] [CrossRef]
- Dong, G.H.; Ho, W.K.; Li, Y.H.; Zhang, L.Z. Facile synthesis of porous graphene-like carbon nitride (C6N9H3) with excellent photocatalytic activity for NO removal. Appl. Catal. B Environ. 2015, 174, 477–485. [Google Scholar] [CrossRef]
- Liu, G.; Niu, P.; Sun, C. Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4. J. Am. Chem. Soc. 2010, 132, 11642–11648. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.H.; Zhang, L.Z. Synthesis and enhanced Cr(VI) photoreduction property of formate anion containing graphitic carbon nitride. J. Phys. Chem. C 2013, 117, 4062–4068. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Xue, W.; Chen, H.; Lin, J.M. Peroxynitrous-acid-induced chemiluminescence of fluorescent carbon dots for nitrite sensing. Anal. Chem. 2011, 83, 8245–8251. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Xia, J.; Ji, M.; Li, H.; Hui, X.; Chen, R. The synergistic role of carbon quantum dots for the improved photocatalytic performances of Bi2MoO6. Nanoscale 2015, 7, 11433–11443. [Google Scholar] [CrossRef] [PubMed]
- Li, G.S.; Zhang, D.Q.; Yu, J.C. A new visible-light photocatalyst: CdS quantum dots embedded mesoporous TiO2. Environ. Sci. Technol. 2009, 43, 7079–7085. [Google Scholar] [CrossRef] [PubMed]
- Leutwyler, W.K.; Bürgi, S.L.; Burgl, H. Semiconductor clusters, nanocrystals, and quantum dots. Science 1996, 271, 933–937. [Google Scholar]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Graphene quantum dots: emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 2012, 48, 3686–3699. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, J. Graphene supported Co-g-C3N4 as a novel metal–macrocyclic electrocatalyst for the oxygen reduction reaction in fuel cells. Langmuir 2013, 29, 3821–3828. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Martha, S.; Parida, K. Facile synthesis of Au/g-C3N4 nanocomposites: an inorganic/organic hybrid plasmonic photocatalyst with enhanced hydrogen gas evolution under visible-light irradiation. ChemCatChem 2014, 6, 1453–1462. [Google Scholar] [CrossRef]
- Shalom, M.; Guttentag, M.; Fettkenhauer, C.; Inal, S.; Neher, D.; Llobet, A.; Antonietti, M. In situ formation of heterojunctions in modified graphitic carbon nitride: Synthesis and noble metal free photocatalysis. Chem. Mater. 2014, 26, 5812–5818. [Google Scholar] [CrossRef]
- Dong, G.H.; Zhang, L.Z. Porous structure dependent photoreactivity of graphitic carbon nitride under visible light. J. Mater. Chem. 2012, 22, 1160–1166. [Google Scholar] [CrossRef]
- Dong, G.H.; Ai, Z.H.; Zhang, L.Z. Total aerobic destruction of azo contaminants with nanoscale zero-valent copper at neutral pH: Promotion effect of in-situ generated carbon center radicals. Water Res. 2014, 66, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yang, L.; Dong, G.; Ho, W. Mechanism of NO Photocatalytic Oxidation on g-C3N4 Was Changed by Pd-QDs Modification. Molecules 2016, 21, 36. https://doi.org/10.3390/molecules21010036
Li Y, Yang L, Dong G, Ho W. Mechanism of NO Photocatalytic Oxidation on g-C3N4 Was Changed by Pd-QDs Modification. Molecules. 2016; 21(1):36. https://doi.org/10.3390/molecules21010036
Chicago/Turabian StyleLi, Yuhan, Liping Yang, Guohui Dong, and Wingkei Ho. 2016. "Mechanism of NO Photocatalytic Oxidation on g-C3N4 Was Changed by Pd-QDs Modification" Molecules 21, no. 1: 36. https://doi.org/10.3390/molecules21010036




