Asteltoxins with Antiviral Activities from the Marine Sponge-Derived Fungus Aspergillus sp. SCSIO XWS02F40
Abstract
:1. Introduction
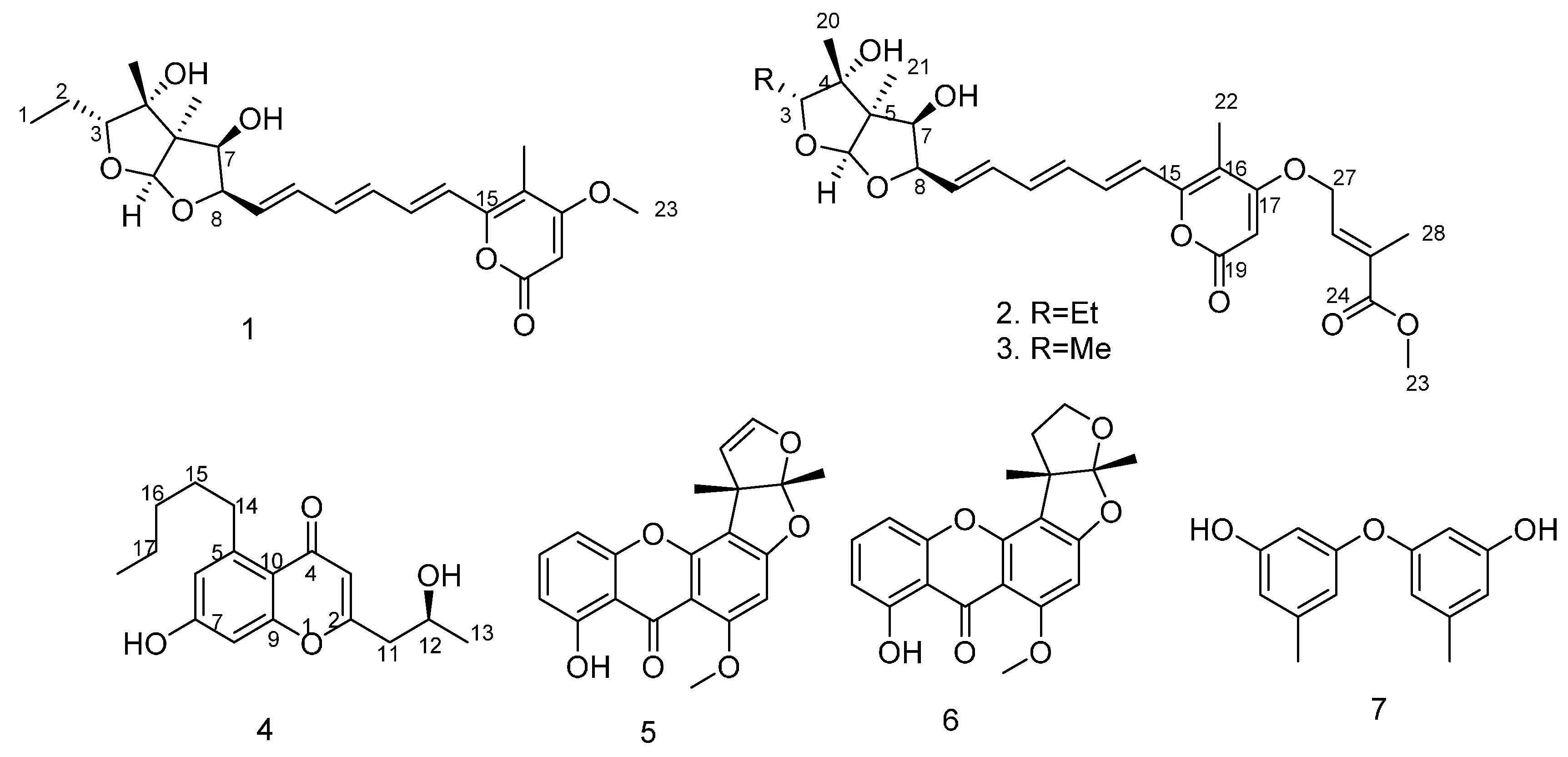
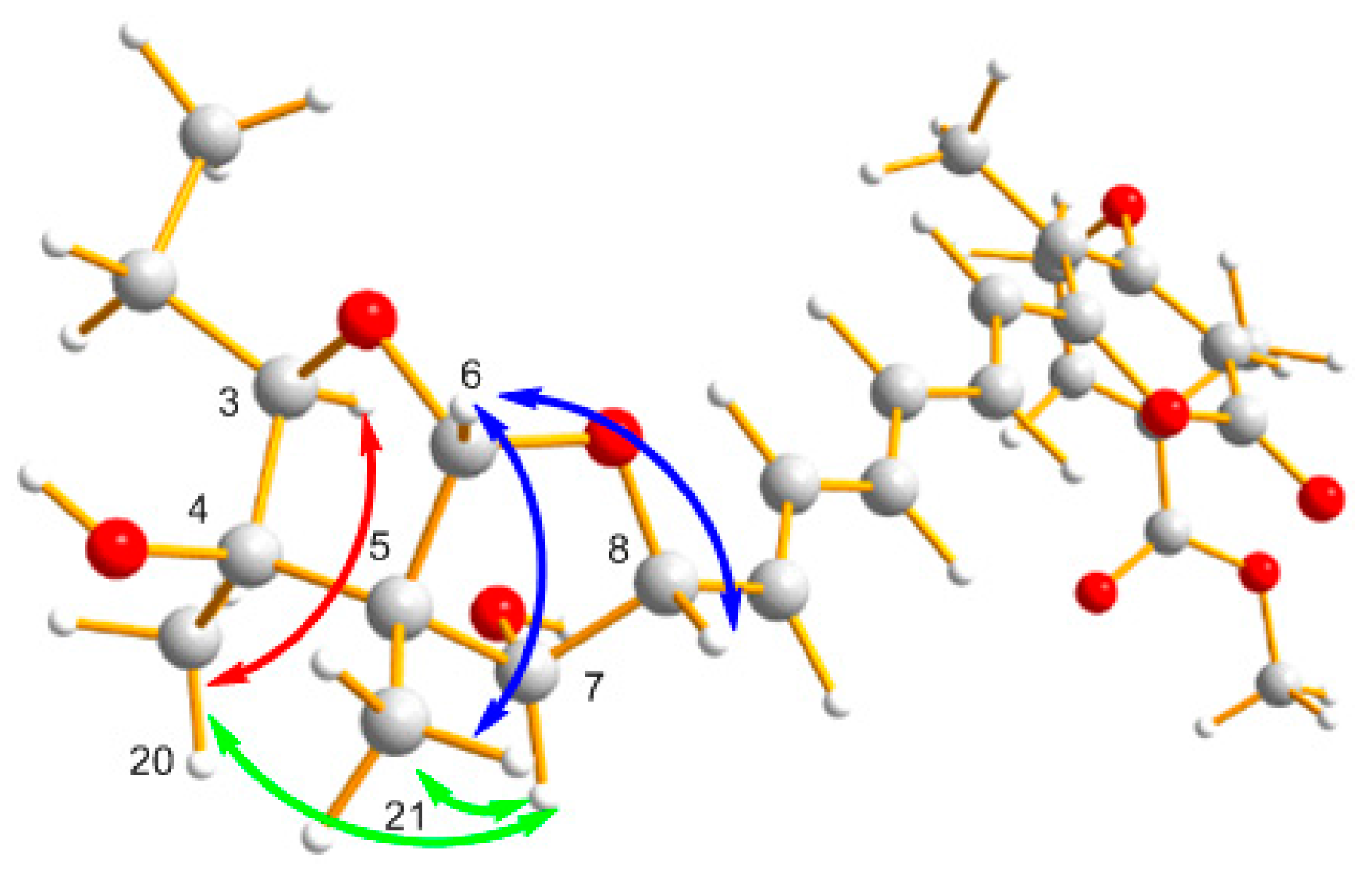
2. Results and Discussion
2.1. Structural Elucidation
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δc | δH | δc | δH | δc | δH | |
| 1 | 11.5, CH3 | 1.05, t (7.5) | 11.4, CH3 | 1.03, t (7.5) | 12.9, CH3 | 1.17, d (6.5) |
| 2 | 21.8, CH2 | 1.56, m | 21.8, CH2 | 1.56, m | ||
| 3 | 89.9, CH | 4.31, dd (5.0, 8.0) | 89.9, CH | 4.31, dd, (5.0, 8.0) | 83.8, CH | 4.57, q (6.0) |
| 4 | 81.2, C | 81.1, C | 81.2, C | |||
| 5 | 62.4 C | 62.3, C | 62.0, C | |||
| 6 | 111.9, CH | 5.29, s | 111.9, CH | 5.27, s | 119.9, CH | 5.26, s |
| 7 | 78.8, CH | 3.73, d (2.0) | 78.9, CH | 3.73, d (2.0) | 78.7, CH | 3.72, br s |
| 8 | 83.2, CH | 4.74, br.s | 83.3, CH | 4.70, m | 83.1, CH | 4.73, br s |
| 9 | 129.4, CH | 5.86, dd (5.0, 15.0) | 129.9, CH | 5.88, dd (5.5, 15.5) | 129.3, CH | 5.84, dd (5.0, 15.5) |
| 10 | 134.2, CH | 6.63, dd (11.5, 15.0) | 133.9, CH | 6.59, dd (11.0, 15.0) | 134.2, CH | 6.64, dd (11.0, 15.0) |
| 11 | 136.5, CH | 6.49, dd (11.0, 15.0) | 136.8, CH | 6.46, dd (11.0, 15.0) | 136.6, CH | 6.49, dd (11.0, 15.0) |
| 12 | 133.0, CH | 6.40, dd (11.0, 15.0) | 132.8, CH | 6.37, dd (11.0, 15.0) | 133.0, CH | 6.40, dd (11.0, 15.0) |
| 13 | 135.5, CH | 7.16, dd (11.0, 15.0) | 135.8, CH | 7.12, dd (11.5, 15.0) | 135.8, CH | 7.16, dd (11.0, 15.0) |
| 14 | 120.4, CH | 6.40, d (15.0) | 120.1, CH | 6.36, dd (11.0, 15.0) | 120.3, CH | 6.37, dd (11.0, 15.0) |
| 15 | 154.3, C | 154.7, C | 154.7, C | |||
| 16 | 108.6, C | 108.4, C | 108.5, C | |||
| 17 | 170.7, C | 169.5, C | 169.4, C | |||
| 18 | 89.2, CH | 5.51, s | 89.9, CH | 5.45, s | 90.1, CH | 5.44, s |
| 19 | 163.9, C | 163.7, C | 163.6, C | |||
| 20 | 18.1, CH3 | 1.38, s | 18.1, CH3 | 1.37, s | 17.6, CH3 | 1.34, s |
| 21 | 16.2, CH3 | 1.18, s | 16.2, CH3 | 1.17, s | 16.5, CH3 | 1.20, s |
| 22 | 9.13, CH3 | 1.97, s | 9.18, CH3 | 1.97, s | 9.23, CH3 | 1.98, s |
| 23 | 56.4, CH3 | 3.83, s | 52.4, CH3 | 3.76, s | 52.5, CH3 | 3.76, s |
| 24 | 167.5, C | 167.5, C | ||||
| 25 | 131.4, C | 131.4, C | ||||
| 26 | 134.0, CH | 6.82, td (1.5, 5.5) | 133.9, CH | 6.82, td (1.5, 5.5) | ||
| 27 | 65.9, CH2 | 4.69, d (5.5) | 65.9, CH2 | 4.68, d (5.5) | ||
| 28 | 13.4, CH3 | 1.90, s | 13.4, CH3 | 1.90, s | ||


| Position | δH mult (J in Hz) | δC | COSY | HMBC |
|---|---|---|---|---|
| 1 | ||||
| 2 | 164.5, C | |||
| 3 | 5.95, s | 111.6, CH | H-2 | |
| 4 | 177.8, C | |||
| 5 | 146.2, C | |||
| 6 | 6.59, d (2.0) | 116.1, CH | C-7, 8, 10, 14 | |
| 7 | 161.5, C | |||
| 8 | 6.62, d (2.0) | 100.7, CH | C-4, 6, 7, 9, 10 | |
| 9 | 159.5, C | |||
| 10 | 113.6, C | |||
| 11 | 2.57, m | 42.8, CH2 | C-2, 3, 12, 13 | |
| 12 | 4.03, m | 64.1, CH | H-11, H-13 | C-2, 11 |
| 13 | 1.15, d (6.5) | 23.5, CH3 | C-11, 12 | |
| 14 | 3.08, td (7.5, 4.5) | 34.2, CH2 | H-15 | C-5, 6, 10, 15 |
| 15 | 1.48, m | 30.8, CH2 | C-5, 14, 16, 17 | |
| 16 | 1.29, m | 31.3, CH2 | ||
| 17 | 1.29, m | 22.0, CH2 | H-18 | C-16, 18 |
| 18 | 0.86, t (7.0) | 14.0, CH3 | C-16, 17 |
2.2. Biological Activities
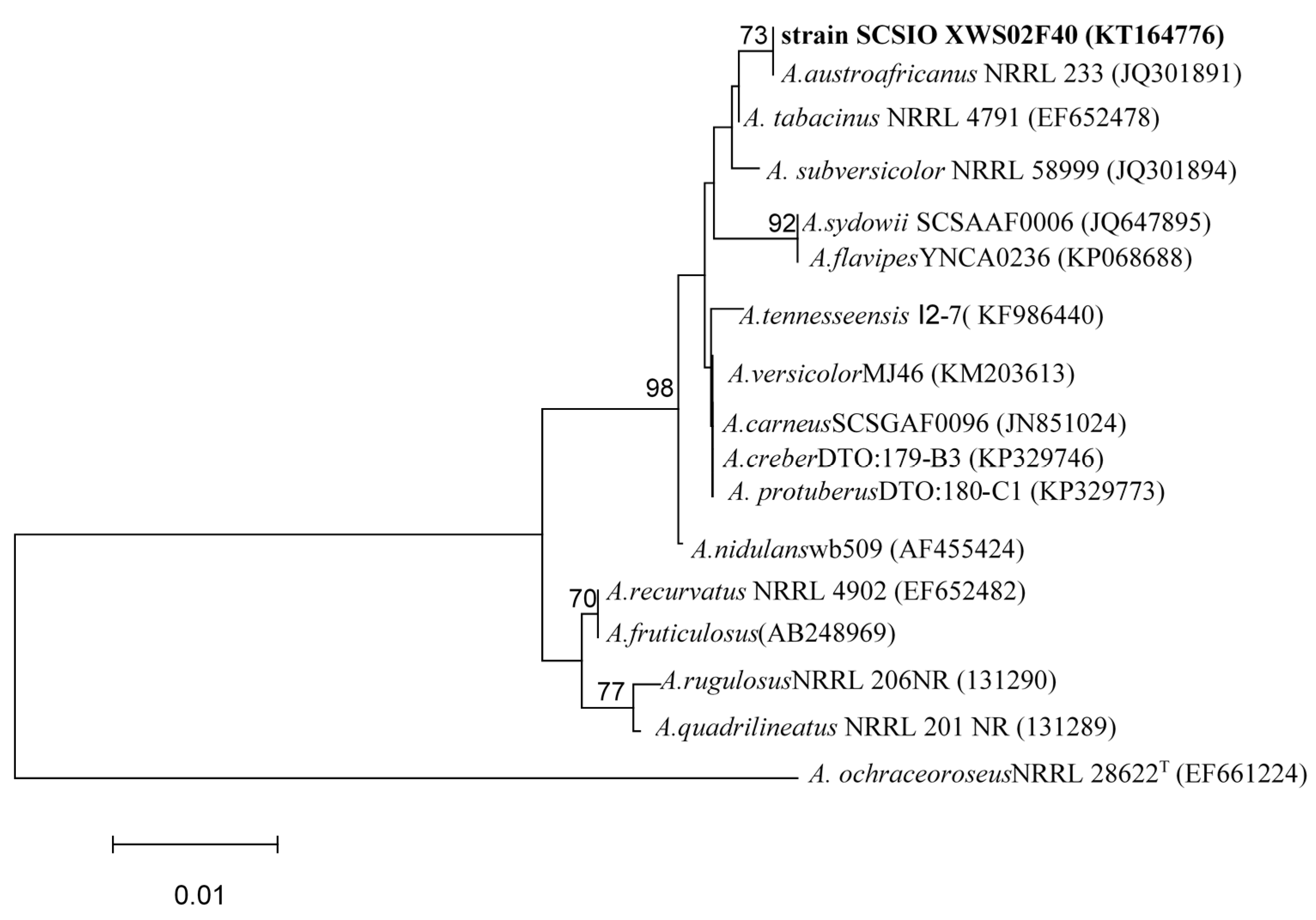
2.3. Characterization and Identification of Isolated Strain SCSIO XWS02F40
3. Experimental Section
3.1. General Experimental Procedures
3.2. Fungal Materials
3.3. Cultural and Morphological Properties of Strain SCSIO XWS02F40
3.4. ITS Region Sequence and Phylogenetic Analysis
3.5. Nucleotide Sequence Accession Number
3.6. Fermentation and Extraction
3.7. Bioassay Protocols
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Herrington, C.S.; Coates, P.J.; Duprex, W.P. Viruses and disease: Emerging concepts for prevention, diagnosis and treatment. J. Pathol. 2015, 235, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Hampson, A.W.; Mackenzie, J.S. The influenza viruses. Med. J. Aust. 2006, 185, S39–S43. [Google Scholar] [PubMed]
- Nelson, M.I.; Stratton, J.; Killian, M.L.; Janas-Martindale, A.; Vincent, A.L. Continual reintroduction of human pandemic h1n1 influenza a viruses into swine in the united states, 2009 to 2014. J. Virol. 2015, 89, 6218–6226. [Google Scholar] [CrossRef] [PubMed]
- Barr, I.G.; Hurt, A.C.; Deed, N.; Iannello, P.; Tomasov, C.; Komadina, N. The emergence of adamantane resistance in influenza A(H1) viruses in australia and regionally in 2006. Antivir. Res. 2007, 75, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Baltina, L.A.; Zarubaev, V.V.; Baltina, L.A.; Orshanskaya, I.A.; Fairushina, A.I.; Kiselev, O.I.; Yunusov, M.S. Glycyrrhizic acid derivatives as influenza A/H1N1 virus inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 1742–1746. [Google Scholar] [CrossRef] [PubMed]
- Kulshreshtha, G.; Burlot, A.S.; Marty, C.; Critchley, A.; Hafting, J.; Bedoux, G.; Bourgougnon, N.; Prithiviraj, B. Enzyme-assisted extraction of bioactive material from chondrus crispus and codium fragile and its effect on herpes simplex virus (HSV-1). Mar. Drugs. 2015, 13, 558–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuhara-Bell, J.; Lu, Y.A. Marine compounds and their antiviral activities. Antivir. Res. 2010, 86, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef] [PubMed]
- Eom, K.D.; Raman, J.V.; Kim, H.; Cha, J.K. Total synthesis of (+)-asteltoxin. J. Am. Chem. Soc. 2003, 125, 5415–5421. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.J.; Li, C.W.; Cui, C.B. Seven new and two known lipopeptides as well as five known polyketides: The activated production of silent metabolites in a marine-derived fungus by chemical mutagenesis strategy using diethyl sulphate. Mar. Drugs 2014, 12, 1815–1838. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Lin, Y.C. Three xanthones from a marine-derived mangrove endophytic fungus. Chem. Nat. Compd. 2007, 43, 132–135. [Google Scholar] [CrossRef]
- Yabe, K.; Matsushima, K.; Koyama, T.; Hamasaki, T. Purification and characterization of O-methyltransferase i involved in conversion of demethylsterigmatocystin to sterigmatocystin and of dihydrodemethy lsterigmatocystin to dihydrosterigmatocystin during aflatoxin biosynthesis. Appl. Environ. Microb. 1998, 64, 166–171. [Google Scholar]
- Tian, Y.; Qin, X.; Lin, X.; Kaliyaperumal, K.; Zhou, X.; Liu, J.; Ju, Z.; Tu, Z.; Liu, Y. Sydoxanthone C and acremolin B produced by deep-sea-derived fungus Aspergillus sp. SCSIO Ind09f01. J. Antibiot. 2015, 68, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chang, W.Q.; Zhang, M.; Li, X.B.; Jiao, Y.; Lou, H.X. Synergistic and drug-resistant reversing effects of diorcinol d combined with fluconazole against Candida albicans. FEMS Yeast Res. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.H.; Xu, T.T.; Yu, H.B.; Chen, G.D.; Huang, X.J.; Yang, F.; Li, Y.S.; Han, B.N.; Liu, X.Y.; Lin, H.W. Dysideanones A–C, unusual sesquiterpene quinones from the south china sea sponge dysidea avara. J. Nat. Prod. 2014, 77, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Mulzer, J.; Mohr, J.T. Stereoselective synthesis of the bis-tetrahydrofuran fragment (C-1-C-9) of asteltoxin. J. Org. Chem. 1994, 59, 1160–1165. [Google Scholar] [CrossRef]
- Schreiber, S.L.; Satake, K. Total synthesis of (+/−)-asteltoxin. J. Am. Chem. Soc. 1984, 106, 4186–4188. [Google Scholar] [CrossRef]
- Adachi, H.; Doi, H.; Kasahara, Y.; Sawa, R.; Nakajima, K.; Kubota, Y.; Hosokawa, N.; Tateishi, K.; Nomoto, A. Asteltoxins from the entomopathogenic fungus pochonia bulbillosa 8-H-28. J. Nat. Prod. 2015, 78, 1730–1734. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Q.; Zhang, X.; Han, Q.J.; Li, X.B.; Li, G.; Li, R.J.; Jiao, Y.; Zhou, J.C.; Lou, H.X. Xanthone derivatives from Aspergillus sydowii, an endophytic fungus from the liverwort scapania ciliata S. Lac and their immunosuppressive activities. Phytochem. Lett. 2013, 6, 318–321. [Google Scholar] [CrossRef]
- Song, Y.X.; Liu, G.F.; Li, J.; Huang, H.B.; Zhang, X.; Zhang, H.; Ju, J.H. Cytotoxic and antibacterial angucycline- and prodigiosin- analogues from the deep-sea derived Streptomyces sp. SCSIO 11594. Mar. Drugs 2015, 13, 1304–1316. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kjer, J.; Sendker, J.; Wray, V.; Guan, H.S.; Edrada, R.; Lin, W.H.; Wu, J.; Proksch, P. Chromones from the endophytic fungus pestalotiopsis sp. isolated from the chinese mangrove plant rhizophora mucronata. J. Nat. Prod. 2009, 72, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Cao, Y.; Tian, L.; Lin, W.; Zhang, K. A new polyunsaturated acid from the marine-derived streptomyces violans (No. HTTA-F04129). Chem. Nat. Compd. 2015, 51, 198–198. [Google Scholar] [CrossRef]
- Fang, W.; Lin, X.P.; Zhou, X.F.; Wan, J.T.; Lu, X.; Yang, B.; Ai, W.; Lin, J.; Zhang, T.Y.; Tu, Z.C.; et al. Cytotoxic and antiviral nitrobenzoyl sesquiterpenoids from the marine-derived fungus Aspergillus ochraceus Jcma1f17. Med. Chem. Comm. 2014, 5, 701–705. [Google Scholar] [CrossRef]
- Pfeiffer, E.; Fleck, S.C.; Metzler, M. Catechol formation: A novel pathway in the metabolism of sterigmatocystin and 11-methoxysterigmatocystin. Chem. Res. Toxicol. 2014, 27, 2093–2099. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Z.; Sun, M.W.; Hao, H.L.; Lu, C.H. Avertoxins A–D, prenylasteltoxin derivatives from Aspergillus versicolor Y10, an endophytic fungus of huperziaserrata. J. Nat. Prod. 2015. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–7 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.-Q.; Lin, X.-P.; Wang, Z.; Zhou, X.-F.; Qin, X.-C.; Kaliyaperumal, K.; Zhang, T.-Y.; Tu, Z.-C.; Liu, Y. Asteltoxins with Antiviral Activities from the Marine Sponge-Derived Fungus Aspergillus sp. SCSIO XWS02F40. Molecules 2016, 21, 34. https://doi.org/10.3390/molecules21010034
Tian Y-Q, Lin X-P, Wang Z, Zhou X-F, Qin X-C, Kaliyaperumal K, Zhang T-Y, Tu Z-C, Liu Y. Asteltoxins with Antiviral Activities from the Marine Sponge-Derived Fungus Aspergillus sp. SCSIO XWS02F40. Molecules. 2016; 21(1):34. https://doi.org/10.3390/molecules21010034
Chicago/Turabian StyleTian, Yong-Qi, Xiu-Ping Lin, Zhen Wang, Xue-Feng Zhou, Xiao-Chu Qin, Kumaravel Kaliyaperumal, Tian-Yu Zhang, Zheng-Chao Tu, and Yonghong Liu. 2016. "Asteltoxins with Antiviral Activities from the Marine Sponge-Derived Fungus Aspergillus sp. SCSIO XWS02F40" Molecules 21, no. 1: 34. https://doi.org/10.3390/molecules21010034






