1. Introduction
Marine macroalgae, or seaweeds (sometimes referred to as sea vegetables) play an integral role in the traditional diets of Pacific and Asian peoples, for reasons associated with the macro- (e.g., protein, lipid, fiber) and micronutrient (e.g., iodine, iron, potassium, β-carotene, tocols
etc.) contents, flavor and texture enhancing properties (e.g., alginates, fucans, agar, carrageenans
etc.) of the various marine macroalgae and constituents, as well as potentially contributing to a reduction in diet-related chronic disease risk (
i.e., breast and colorectal cancers) in these populations [
1]. Marine macroalgae are also present, but much less ubiquitous, in the traditional diets of Icelandic, Welsh, Irish, North, Central and South American coastal peoples. Amongst these edible macroalgae are many species from the Rhodophyta such as “Nori” or “Kim/Gim” (
Pyropia tenera or
P. yezoensis) in Japan and Korea, “Hana Tsunomata™” (cultivated
Chondrus crispus) for the Japanese market, “Ceylon moss” (
Gracilaria edulis) in South-west and –east Asia, “Dulse” or “Dillisk/Dilleasc/Creathnach” (
Palmaria palmata) in Ireland, Scotland, Iceland, Norway, Atlantic Canada and U.S.A. While many of these red macroalgae are sun- or shade-dried, then toasted/roasted or rehydrated and consumed whole such as Nori, Dulse and Hana Tsunomata™; others are utilized as sources of hydrocolloid ingredients for the food industry, such as the carrageenophytes consisting of many members of the order Gigartinales, including “Irish moss” (
Chondrus crispus), “False Irish moss” (
Mastocarpus stellatus),
Eucheuma sp.,
Kappaphycus sp. and “Red String seaweed” (
Sarcodiotheca gaudichaudii); or even direct aqueous extraction of
C. crispus for use in regional cuisines such as the Seaweed Pie in Prince Edward Island, Canada or Irish Moss drinks in Central America and the Caribbean.
The first functional food or nutraceutical applications for edible macroalgae date back to approx. 1534 B.C. Egypt, where they were used for the treatment or prevention of breast cancer [
1], which has been more recently substantiated by a case-control study where intake of Gim was inversely related to breast cancer risk [
2]. Epidemiological data support the hypothesis of diet-related chronic disease risk reduction in populations known to consume macroalgae regularly in the diet: the age-standardized incidence of breast cancer in North America and Western Europe are approx. 76.7 and 89.9 per 100,000 compared to 25.3 and 31.0 in Eastern and Southeastern Asia [
3]. Similarly, the incidence of colorectal cancer in North America (35.3 and 25.7 per 100,000 for males and females) and Western Europe (41.2 and 26.3) are greater than in Eastern (21.5 and 14.8) and Southeastern Asia (15.2 and 12.9). Anticarcinogenic mechanisms contributing to the bioactivities of edible macroalgae include increased hepatic antioxidant enzyme activities [
4]; radio- and photo-protective effects of algal extracts against UVB (280–320 nm; [
5]), UVA (320–400 nm; [
6]) and ionizing sources of radiation [
4]; as well as induction of apoptosis and cell cycle arrest [
7]. Moreover, anti-oxidant and/or -mutagenic effects of edible macroalgae were reported from
in vitro and rodent models of colon and skin carcinogenesis, respectively, including tumour initiation suppression in the latter [
8,
9].
Porphyra umbilicalis extracts protected fibroblasts and keratinocytes against DNA damage and UVA irradiation as described by Schmid and coworkers [
10,
11]. Thus, there is growing interest in the nutraceutical, pharmacognosic and cosmeceutical applications of extracts from edible marine red macroalgae such as
C. crispus which is a formulation component contained in an international patent application for anti-aging skin cream uses [
12].
Many of the antioxidant and UV-protective bioactivities of edible red macroalgae and extracts above can be attributed to the efficacy of cellular constituents against oxidative stress resulting from exposure to temperature variances, tidal flows and UV-irradiation of these intertidal species [
13,
14,
15,
16]. For example, macroalgal tissue antioxidants (e.g., ascorbate and carotenoids) are typically reduced during Winter and Spring, and increase in Summer and Fall in concert with increased photosynthetically active radiation (PAR, 400–700 nm) and UVA and UVB-irradiation [
14,
17,
18]. Mycosporine-like amino acids (MAAs), comprised of an aminocyclo-hexenone or -hexenimine core conjugated with the nitrogen moiety of an amino-acid or –alcohol (
Figure 1), are most abundant in Rhodophyta (red) compared to Chlorophyta (green) and Pheophyta (brown) macroalgal species [
19,
20,
21,
22]. MAAs are noted to exhibit λ
max between 310–360 nm, thus, an UV-absorbing sunscreen protective role for these compounds in macroalgae, corals and marine animals can be deduced from the overlap with UVA and B wavelengths, as well as evidence that the MAA contents of
P. palmata and
Devaleraea ramentacea specimens wild-harvested prior to the break-up of Spring ice cover were decreased compared to counterparts harvested during the Summer [
17]. Previous work from this laboratory demonstrated that
P. palmata specimens wild-harvested from areas differing in topography exhibited varied MAA profiles: extracts from both low- and high-UV exposed
P. palmata contained palythine, shinorine, asterina-330, palythinol and porphyra-334, but the high-UV specimen alone, also contained usujirene [
13]. Interestingly, despite similar oxygen radical absorbance capacity (ORAC) antioxidant activities between the two
P. palmata extracts, that from the high-UV specimen was more inhibitory of B16-F1 murine skin melanoma cell proliferation. Previously, butanol extracts of wild-harvested high-UV exposed
P. palmata exhibited enhanced reducing activity and inhibition of HeLa cell proliferation
vs. a low-UV exposed counterpart [
14].
Clearly then, as photosynthetic, intertidal organisms, the composition of macroalgae within the same species may be highly variable when wild-harvested; thus, it is important that mariculture researchers and producers are cultivating macroalgae from uniform seed stocks in tanks with filtered seawater, fertilizer or other essential nutrients as well as controlled illumination [
12,
23,
24]. Management techniques such as these ensure not only a reliable, year-round supply of biomass with desired attributes such as the Hana Tsunomata™ produced by Acadian Seaplants, Nova Scotia, Canada, but also potentially give rise to genetic variants of interest from target species. A large body of knowledge exists of the MAA composition of wild-harvested red marine macroalgae from around the globe [
19,
21,
22,
25,
26,
27]; however, much less is known about Canadian and North American specimens [
13] and in particular, cultivated macroalgae [
23]. Thus, the objectives of the present study were to determine the MAA profiles and antioxidant activities of extracts from selected wild-harvested and cultivated Atlantic Canadian edible marine red macroalgae, and to determine the effects of these extracts on the proliferation of two human cancer cell lines, one adherent: cervical adenocarcinoma, HeLa cells, and the other suspended: histiocytic lymphoma, U-937 cells.
Figure 1.
The main mycosporine-like amino acids (MAAs) identified in Rhodophyta.
Figure 1.
The main mycosporine-like amino acids (MAAs) identified in Rhodophyta.
3. Discussion
This study is the first to report the MAA profiles of aqueous methanolic extracts from a cultivated
C. crispus specimen as well as wild-harvested
C. crispus specimens from low- and high-UV exposed locations, wild-harvested
M. stellatus which co-occurs with
C. crispus in the intertidal zone and
P. palmata, all edible red marine macroalgae from temperate zone locations in western Nova Scotia, and Wood and Grand Manan Islands, New Brunswick, Canada, respectively. We extend these findings by reporting the antioxidant capacity, comprising reducing activities and ORAC values of these extracts; as well as the HeLa (cervical) and U-937 (lymphocyte) human adenocarcinoma and histiocytic lymphoma cell antiproliferative activities of these red macroalgal extracts. Moreover, we describe the apoptotic effects of the cultivated
C. crispus and wild-harvested
P. palmata extacts on HeLa cells herein. We have previously reported that aqueous methanolic extracts of wild-harvested
P. palmata specimens from low-UV and high-UV exposed locations on Grand Manan Island (44°40.0’N, 66°45.0’W) exhibited different MAA profiles, with the low-UV sample containing five of the six MAAs herein, except for the low polarity usujirene, and the high-UV
P. palmata extract containing all six MAAs; however the ORAC values for the extracts were not different [
13]. It is noteworthy that the high-UV
P. palmata extract was more antiproliferative against B16-F1 murine skin melanoma cells over 24-48 h compared to the low-UV extract. Using HPLC-DAD chromatography, we determined that the wild-harvested low- and high-UV
P. palmata extracts both contained a majority of porphyra-334 and palythine, with smaller amounts of palythinol, shinorine and asterina-330. However, the amount of usujirene in the high-UV
P. palmata extract was likely underestimated due to the greater λ
max (356 nm) and molar extinction coefficient associated with the conjugated double-bond structure of this MAA (
Figure 1; [
13]). Thus, it is noteworthy that in the present study, LC/MS/MS multiple reaction monitoring indicated that usujirene was the predominant MAA in the wild-harvested
P. palmata specimen from the same high-UV location, with lower amounts of porphyra-334 and palythine, palythinol, asterina-330 and shinorine. These results with wild-harvested
P. palmata specimens from a temperature zone climate, are different from those of
P. palmata harvested from Arctic waters (Spitsbergen, Norway; 78°55.5’N, 11°56.0’E) containing a majority of porphyra-334, with smaller amounts of a compound with λ
max of 357 nm (possibly usujirene) and palythine, palythene (trans isomer of usujirene;
Figure 1), mycosporine-glycine and palythinol [
21]. Compared to other MAAs, usujirene is noted to absorb strongly in the UVA wavelength range which predominates at lower latitudes, in combination with stronger solar radiation, a shorter light path and thinner ozone layer [
13,
20]. Furthermore, it is noteworthy that porphyra-334 may undergo conversion to usujirene via the shikimic acid pathway [
29].
To the best of our knowledge, the present study is the first to elucidate the MAA profile of a cultivated specimen of
C. crispus. The carrageenophytes
C. crispus and
M. stellatus are not only similar in morphology, but also co-occur at the shoreline with
C. crispus inhabiting the low intertidal zone and
M. stellatus higher up on shore and in a mixed zone. Interestingly, these two species of red macroalgae (from two different families within the order Gigartinales) vary considerably in MAA synthesis and thereby, profiles:
C. crispus is noted for a high rate of synthesis of shinorine under UV-irradiation, and this MAA is amongst the first to be synthesized by this species [
20,
30]. Exposing
C. crispus to PAR + UV-irradiation results in the additional synthesis of palythine: when
C. crispus (containing traces of palythine; collected from the North Sea, 54°11’N, 7°53’E) was exposed to unfiltered light as well as light with UVA and UVB filters, shinorine was rapidly synthesized followed by a decline in this MAA, and induction of palythine and asterina-330 [
31]. When
C. crispus (containing palythine, asterina-330 and palythene) was exposed to UVB irradiation, shinorine was synthesized
de novo and levels of asterina-330 and palythene increased two-fold, while the amount of palythine remained unchanged [
30]. Thus, the predominance of palythine , followed by asterina-330 and lesser amounts of shinorine in the wild-harvested low- and high-UV
C. crispus extracts (from western Nova Scotia (43°82’N, 66°15’W) herein, are in agreement with an inverse relationship (
i.e., reciprocal changes reflecting a precursor-product relationship) between shinorine and palythine, as well as asterina-330 concentrations in this red macroalga [
20]. The greater MAA total peak area counts for the wild-harvested high-UV
C. crispus extract compared to the low-UV specimen are indicative of the increased oxidative stress, solar and UV-irradiation exposure associated with the shallow water habitat of the former, compared to the deeper water location of the latter. Moreover, the increased amounts of the most abundant MAAs palythine (λ
max 320 nm), asterina-330 (λ
max 330 nm) as well as minor amounts of porphyra-334 (λ
max 332 nm) and palythinol (λ
max 330 nm) but similar amounts of shinorine (λ
max 332) in the wild-harvested high-UV
C. crispus extract reflect the greater exposure to UVA-irradiation in the temperate zone shallow water depths of western Nova Scotia and thereby bioconversion of shinorine to form palythine and asterina-330 as above. It is noteworthy that the cultivated
C. crispus extract contained 2.44 × the MAA total peak area counts compared to the wild-harvested high-UV specimen, reflecting a greatly increased synthesis of shinorine and bioconversion of this MAA to yield palythine and asterina-330. The cultivated
C. crispus was very highly pigmented, appearing uniformly dark purple compared to the variegated red purple with some green, and pale green of the wild-harvested low-UV and high-UV
C. crispus, respectively. These pigmentation differences reflect the tissue levels of the key light-harvesting pigment in red macroalgae, (
R)-phycoerythrin, which is known to vary inversely with UV-irradiation exposure [
14]. Thus, the cultivation techniques used have uniquely maximized MAA synthesis of the selected
C. crispus seedstock in tanks with micro-filtered seawater and nutrients [
32]. Interestingly,
M. stellatus extracts are often comprised solely of shinorine [
21,
25,
30,
33]. For example,
M. stellatus (wild-harvested from the North Sea, as above) subjected to UVB-irradiation over 5 days, exhibited a 1.67 × increase in shinorine content without any trace of other MAAs [
30]. The extract from wild-harvested
M. stellatus from Wood Island, New Brunswick (44°37’N, 66°50’W) in the present study, contained not only a majority of shinorine, but also considerable amounts of palythine, asterina-330, usujirene and porphyra-334. Thus, it is likely that the lower latitude of growth of the
M. stellatus herein, compared to the North Sea, exposed the specimen to increased total solar and UVA-irradiation as discussed above, resulting in bioconversion of accumulated shinorine to form palythine and asterina-330, as well as conversion of porphyra-334 into usujirene since the latter absorbs more strongly in the UVA range than the former MAA [
20].
In the present study, the aqueous methanol extracts of the edible wild-harvested and cultivated red macroalgae exhibited variable antioxidant efficacies when expressed as single electron transfer reducing activity and hydrogen atom transfer ORAC values. Overall, the red macroalgal extracts exhibited weak reducing activities with 1 g of extract equivalent to milligrams of
l-ascorbic acid. This is not unexpected, as sun-drying and storage of macroalgae such as
P. palmata are noted to markedly reduce the antioxidant molecule content, including
l-ascorbate, α- and β-carotene [
14]. Alternatively, ORAC values represent the ability of chain-breaking antioxidants to quench free radicals through hydrogen atom transfer to the carbon or peroxyl radicals from AAPH decomposition and reaction with molecular oxygen. Differences in the antioxidant activities between the wild-harvested red macroalgal extracts are indicative of the endogenous cellular antioxidant capacity required to protect against environmental oxidative stresses associated with differences in intertidal zone habitat and UV-exposure. Previously, aqueous methanolic and 1-butanol soluble extracts from wild-harvested high- and low-UV
P. palmata specimens exhibited similar ORAC and AAPH-radical quenching activities, despite differing MAA profiles and greater reducing activity in the high-UV
P. palmata 1-butanol extract compared to a low-UV counterpart [
13,
14]. That the extract from the wild-harvested
P. palmata specimen (collected from the low-lying exposed eastern shore of Grand Manan Island with high UV-irradiation) exhibited the greatest reducing and second highest ORAC activities herein may be attributable to the total MAA content, but also to the predominance of usujirene in the MAA profile of this red macroalga. Nakayama and coworkers [
23] proposed that the strong antioxidant efficacy of usujirene may be attributable to hydrogen ion abstraction from the cycloheximine ring at C-4, C-6 or the methylene group at C-9 of the glycine residue with resonance stabilization from the conjugated double-bonds of the
cis-unsaturated chain at C-11 on the double-bonded nitrogen in series with the carbon ring double-bond structure. The wild-harvested
P. palmata extract herein was also characterized by the greatest amount of porphyra-334; this MAA demonstrated moderate inhibition of AAPH-induced lipid peroxidation when present in a 9.6:1 ratio mixture of porphyra-334:shinorine [
34].
The differences in intertidal zone habitats occupied by
M. stellatus compared to
C. crispus have been identified as conferring greater stress tolerance and a competitive advantage of the former over the latter, as evidenced by a greater total MAA content as well as endogenous antioxidant capacity comprising
l-ascorbate, α-tocopherol, β-carotene and antioxidant enzymes; [
16,
30]. Likewise, herein we report greater MAA total peak area counts for the wild-harvested
M. stellatus extract
vs. the high-UV and low-UV
C. crispus extracts, respectively, in addition to greater reducing and ORAC activities in the former compared to latter species. The stronger antioxidant efficacies of the wild-harvested
M. stellatus extract likely reflect the hydrogen ion abstraction mechanisms of the main constituent MAA, shinorine associated with the cycloheximine ring at C-4, C-6 or the methylene group at C-9 of the glycine residue with some resonance stabilization from the carbon ring double-bond structure, in addition to the efficacy of the usujirene content, as above [
23]. Moreover, Dunlap and coworkers [
35] reported the dose-dependent inhibition of AAPH-induced phosphatidylcholine peroxidation by shinorine. It is also plausible that palythine, which when present in a 27.9:9:4.3:1 ratio of mycosporine-glycine:palythine:palythinol:asterina-330, potentially contributed to the strong inhibition of AAPH-induced peroxidation of these MAAs [
34], played a role in the antioxidant effects of the wild-harvested
M. stellatus extract herein. On the other hand, the weaker antioxidant activities of both the wild-harvested high-UV and low-UV
C. crispus extracts are indicative of the efficacy of the main constituent MAA, palythine, as above, in addition to asterina-330 which exhibited little inhibition of AAPH-induced peroxidation when present in a 8:1 ratio of asterina-330:palythine [
34]. The antioxidant activities of the cultivated
C. crispus extract likely resulted from the efficacy of the predominant MAA, palythine as a hydrogen atom donor as well as that of shinorine, with resonance stabilization from the carbon ring double-bond structure in the latter MAA, as above. These lines of evidence from two different antioxidant mechanisms, suggest that the reducing and ORAC antioxidant activities of the wild-harvested
P. palmata,
M. stellatus, high- and low-UV
C. crispus and cultivated
C. crispus red macroalgal extracts can be attributed largely to the hydrogen atom transfer efficacies of usujirene, shinorine and palythine, with a minor contribution from asterina-330.
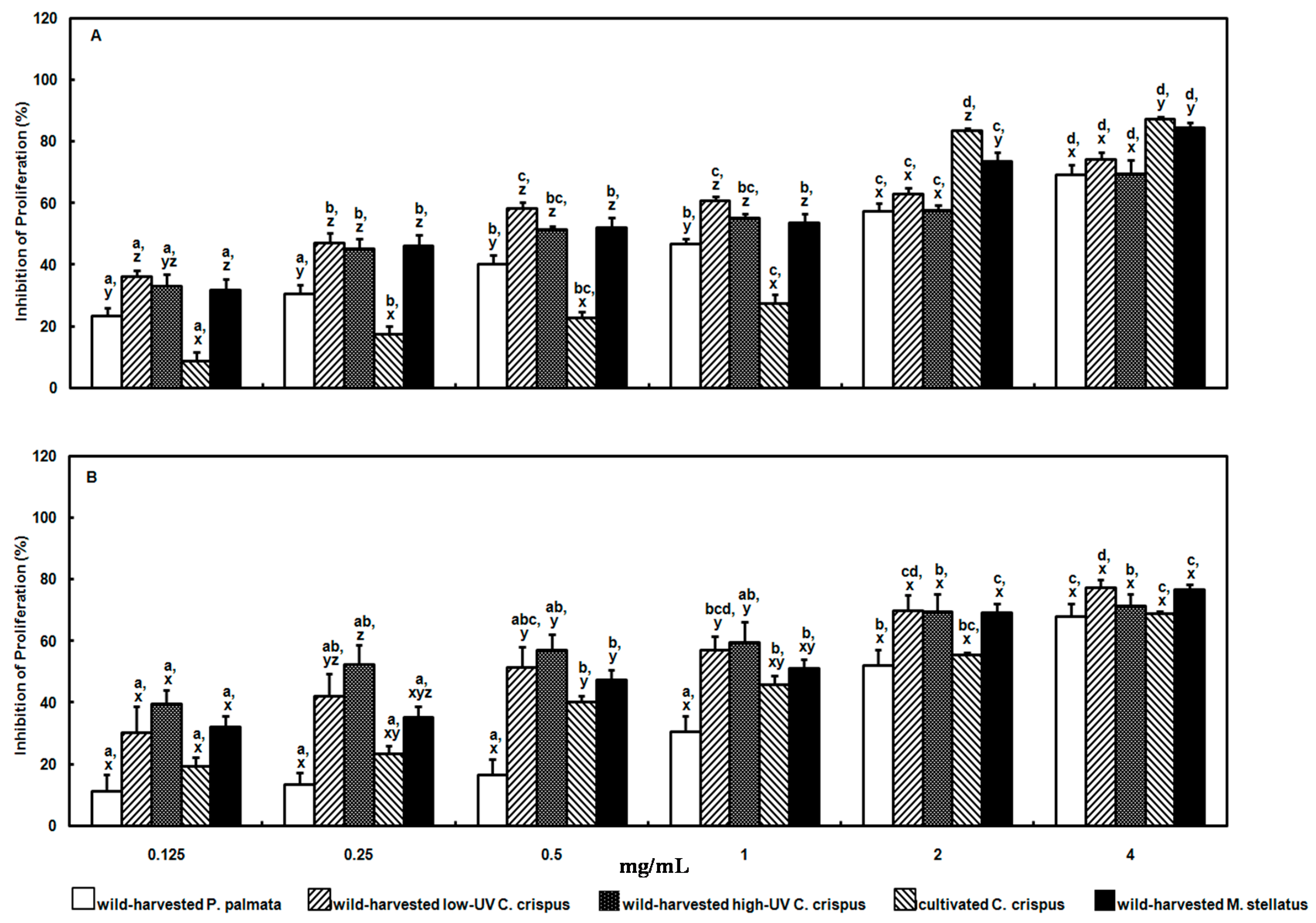
The aqueous methanolic extracts of the wild-harvested and cultivated red macroalgae herein exhibited dose- and species-dependent antiproliferative effects against human adenocarcinoma cervical (HeLa) and histiocytic lymphoma (U-937) cells
in vitro. The EC
50 values calculated for the wild-harvested
P. palmata, low-UV
C. crispus, high-UV
C. crispus, cultivated
C. crispus and wild-harvested
M. stellatus extract inhibition of HeLa cell proliferation after 24 h incubation were: 1.83, 0.457, 1.06, 1.73 and 0.747 mg/mL; whereas those for U-937 cells were: 2.51, 0.772, 0.344, 1.99 and 1.15 mg/mL. Previously, we reported that aqueous methanolic extracts from a high-UV exposed
P. palmata specimen exhibited greater antiproliferative effects against B16-F1 murine melanoma cells over 48 h than a low-UV exposed counterpart [
13]. It was hypothesized that these effects may be attributable to the passive uptake of the low-polarity, weakly acidic MAA, usujirene across cell membranes. Similarly, Moo-Puc and co-workers [
36] proposed that membrane permeation by low-polarity/lipophilic compounds was responsible for the cytotoxic and antiproliferative effects of Yucatán Rhodophyta extracts such as
Gracilaria cervicornis which exhibited an EC
50 value of 0.076 mg/mL against HeLa cells. Other workers have reported a dose-dependent and apparently saturable, active transport of the highly polar, strongly acidic MAA, shinorine across cell membranes to be antiproliferative against human skin carcinoma A431 cells [
33]. The above EC
50 values represent low antiproliferative efficacies in comparison to the U.S. National Cancer Institute standard of an EC
50 ≤ 30 μg/mL extract on cancer cells, which is not surprising, given the mixture of MAAs and varying concentrations present in our extracts. More recently, a case-control study revealed that the average intake and frequency of Gim consumption was inversely associated with breast cancer risk in pre- as well as post-menopausal women [
2]. It is noteworthy that several animal models of carcinogen-induced intestinal adenocarcinoma, as well as implanted sarcoma-180 ascites cell studies have attributed the anticarcinogenic efficacies of dietary red macroalgae in part to the sulfated polysaccharides, but also free radical scavenging and antioxidant small molecules [
2,
37,
38].
In the present study, the proliferation of both HeLa and U-937 cells were considerably inhibited by the wild-harvested M. stellatus extract containing the polar, strong acid shinorine, albeit the efficacy of this extract was not as strong in the latter cell line. Active transport of shinorine may also have been a factor in the slightly weaker antiproliferative effects of the cultivated C. crispus extract on HeLa and U-937 cells, given the reduced peak area counts for shinorine compared to that in the wild-harvested M. stellatus extract. Membrane permeation by the low-polarity, weakly acidic usujirene may also have played a role in the antiproliferative effects of the wild-harvested M. stellatus extract above. Similarly, usujirene may have been efficacious in the antiproliferative effects of the wild-harvested P. palmata extract against HeLa and U-037 cells. On the other hand, the antiproliferative efficacies of the wild-harvested high-UV and low-UV C. crispus extracts against HeLa and U-937 cells are unclear at present, but may potentially be associated with the trans-membrane movement of other polar, weakly acidic MAAs, namely palythine and asterina-330.
In other work, the proliferation of HT-29 colon cancer cells was strongly inhibited by 0.020 mg/mL of a methanolic extract from the Rhodophyta
Symphyocladia latiuscula, associated with an increased proportion of apoptotic cells [
7]. Induction of apoptosis (
i.e., programmed cell death) in the HT-29 cells was associated with activation of the caspase-3/7 cascade (aspartate-specific cysteine proteases) and poly (ADP-ribose) polymerase (PARP) activation. Apoptosis, also known as type I cell death in mammalian cells, is noted to be characterized by distinct alterations in the nucleus (comprising chromatin condensation and fragmentation), cell shrinkage and blebbing of the plasma membrane and ultimately, formation of apoptotic structures containing nuclear or cytosolic components [
39]. The mechanisms underlying apoptosis involve proteolytic activity of a family of caspases which participate in the initiation, execution and regulation of apoptosis, leading to breakdown of the cytoskeleton, disruption of cellular metabolism and fragmentation of nuclear material [
39]. Thus, the caspase family is noted to comprise upstream initiators (caspase-8, -9 and -10) which activate the downstream effectors of apoptosis (caspase-3, -6 and -7). Activation of caspases can occur by differing signaling routes: the extrinsic death receptor pathway which plays a role in tissue homeostasis and the immune system, or the intrinsic mitochondrial pathway which responds to a variety of extra- or intracellular insults such as DNA damage; it is this latter pathway which predominates in programmed cell death. Thus, it is noteworthy that both the wild-harvested
P. palmata and cultivated
C. crispus extracts increased caspase-3/7 activity in HeLa cells. Caspase-3/7 activation has been reported to be responsible for cleaving cell cycle regulators, including PARP, which induce cell cycle arrest, leading to induction of apoptosis as above [
7].
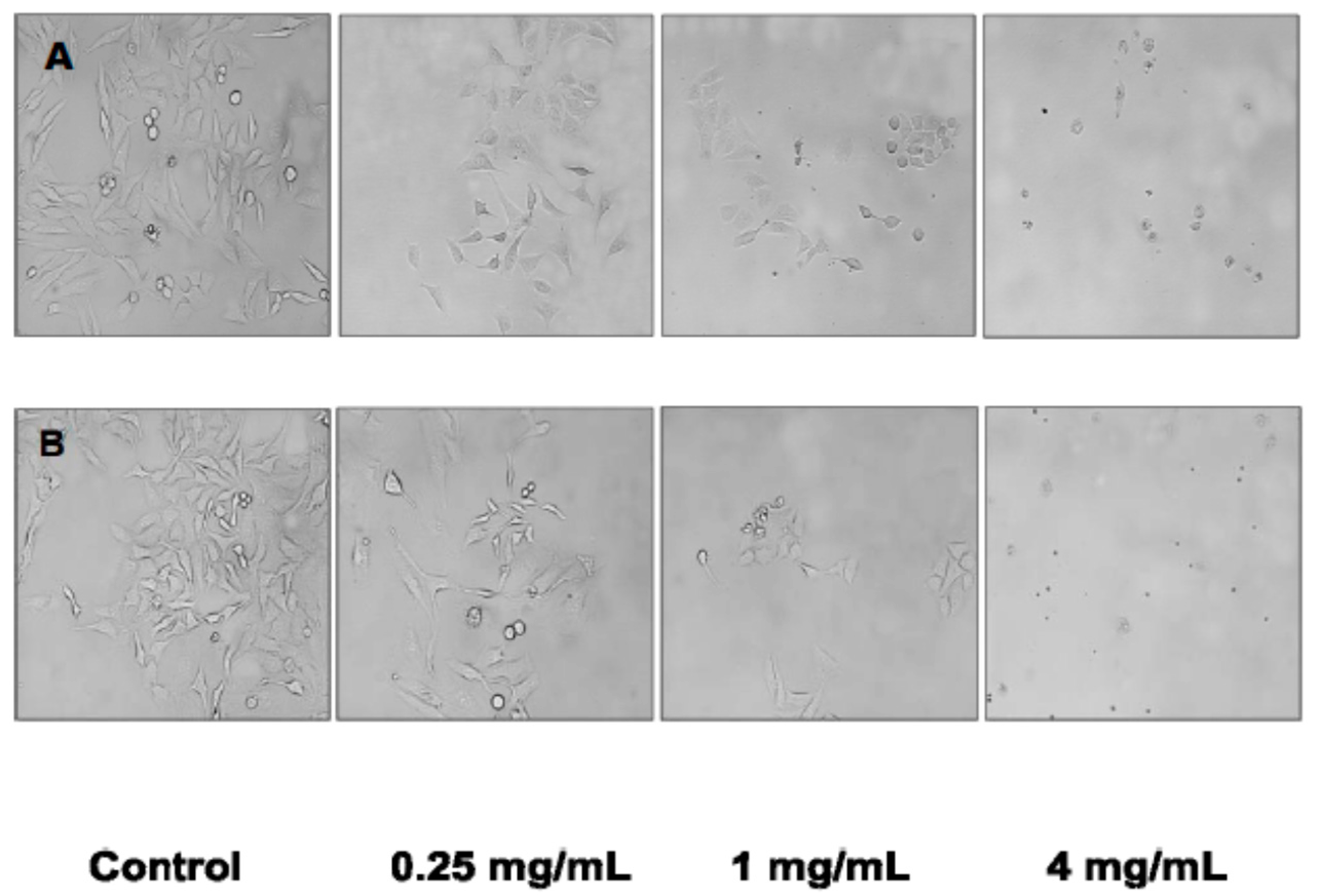
The induction of apoptosis in HeLa cells in the present study was confirmed by the dose-response changes in cell morphology as a result of incubation with the cultivated
C. crispus and wild-harvested
P. palmata extracts. Moreover, cell cycle analyses also confirmed that apoptosis played a role in the antiproliferative effects of the cultivated
C. crispus and wild-harvested
P. palmata extracts on HeLa cells with pronounced dose-dependent Sub G
1 phase (apoptotic) arrests after 24 h. Interestingly, while the induction of caspase-3/-7 activity in HeLa cells was stronger with the wild-harvested
P. palmata extract compared to that from cultivated
C. crispus and the untreated control cells, the accumulation of Sub G
1 apoptotic cells was greater in HeLa cells exposed to the latter, compared to the former extract. These differences may relate to the timing and duration of the respective phenomena, as apoptosis is noted to occur very rapidly, typically within hours [
39]; thus, caspase-3/-7 activation would be expected to precede mitochondrial and DNA fragmentation effects leading to cell cycle arrest. The lack of dose-dependent effects of the wild-harvested
M. stellatus, low-UV and high-UV
C. crispus extracts on HeLa cell caspase-3/-7 activity compared to those discussed above, does not eliminate the possibility that initiator caspase (caspase-8, -9 or -10) activities or other cell cycle regulators may have been affected. It is noteworthy that HeLa caspase-3/-7 activities were increased by the two red macroalgal extracts with the greatest MAA total peak area counts in the cultivated
C. crispus and the wild-harvested
P. palmata extracts.
In conclusion, the MAA profiles of aqueous methanol extracts from the edible wild-harvested and cultivated marine red macroalgae studied herein exhibited differences attributable to not only species-specific differences in MAA synthesis and bioconversion, but also UV-exposure in a temperate climate zone. Red macroalgal extract reducing and ORAC activities reflected the differing efficacies of component MAAs as electron and/or hydrogen atom donors and free radical quenchers; as well as the relative amounts of MAAs in individual extracts, with the greatest efficacies observed with the wild-harvested P. palmata, rich in usujirene, porphyra-334 and palythine, and wild-harvested M. stellatus extracts, rich in shinorine. The HeLa and U-937 cell antiproliferative effects of the wild-harvested P. palmata, M. stellatus and cultivated C. crispus extracts likely reflected passive uptake and active transport of usujirene and shinorine, respectively, across cell membranes as discussed above. The antiproliferative effects of the wild-harvested low-UV and high-UV C. crispus extracts remain unclear, but may have been due to transport of other polar MAAs across cell membranes. HeLa cell death via apoptosis was confirmed as the mechanism underlying the effects of the wild-harvested P. palmata and cultivated C. crispus extracts from not only increased effector caspase-3/-7 activities, but also cell-cycle arrest at Sub G1 in treated cells; cell morphologies also indicated characteristic apoptotic changes. Further work with purified MAAs will help to further elucidate the protective antioxidant and antiproliferative mechanisms of these unique UV-absorbing marine red macroalgal constituents. Moreover, animal model studies will determine the potential protective bioactivities of dietary wild-harvested and cultivated red macroalgae studied herein, and their constituents, against diet-related chronic diseases. This work will be instrumental in furthering the development of mariculture producers and processors of edible marine red macroalgae in the processed and functional food industries.