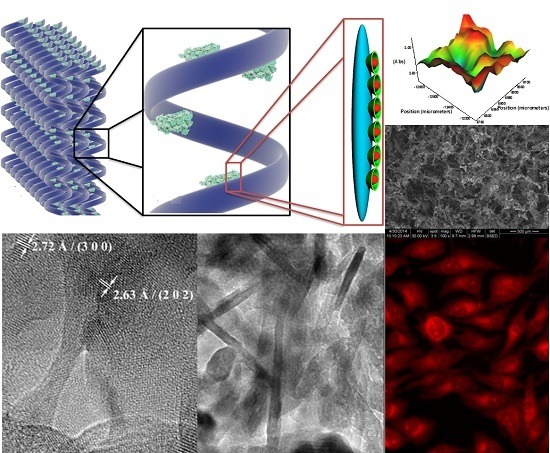
Biocompatible 3D Matrix with Antimicrobial Properties
Abstract
:1. Introduction
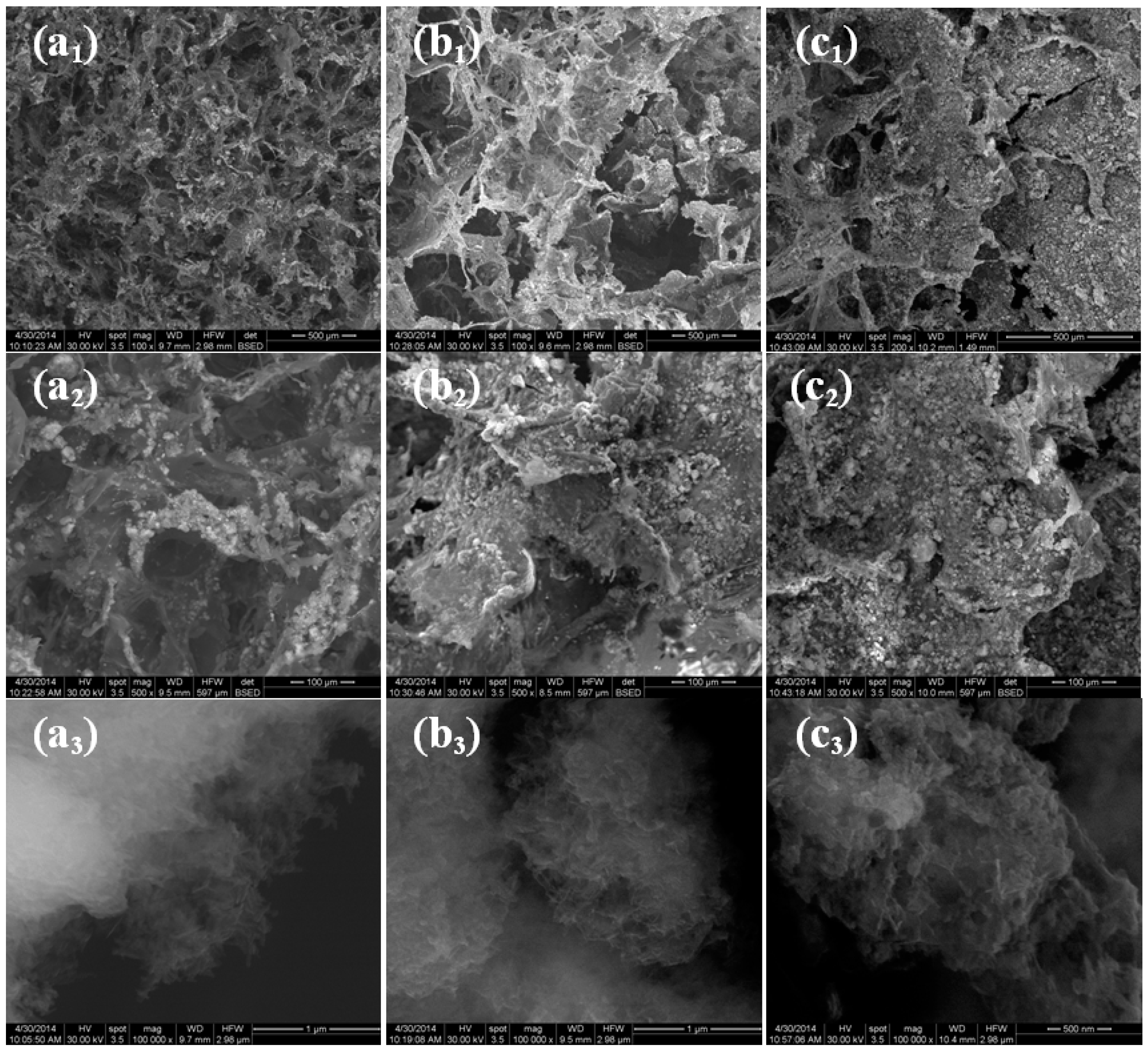
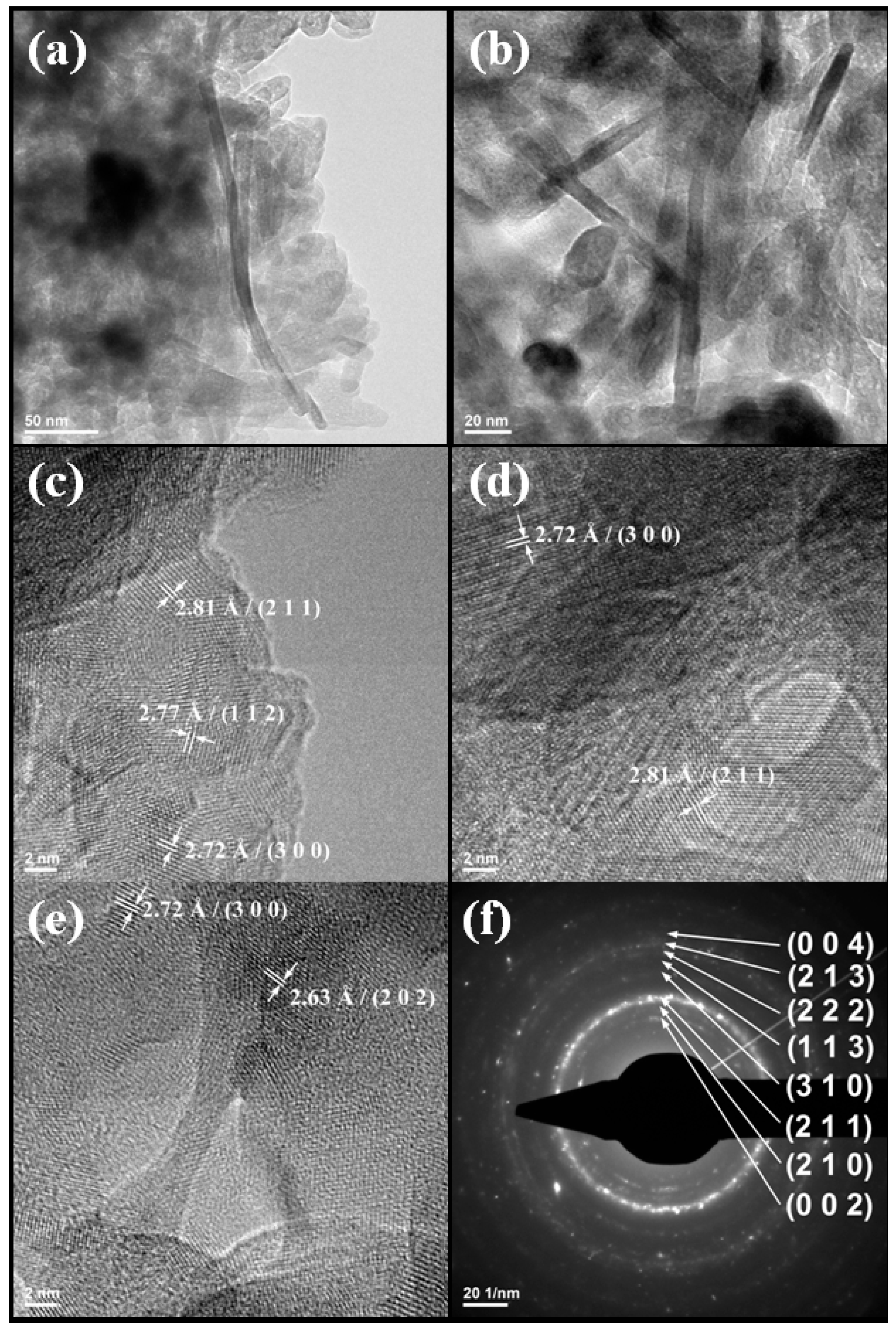
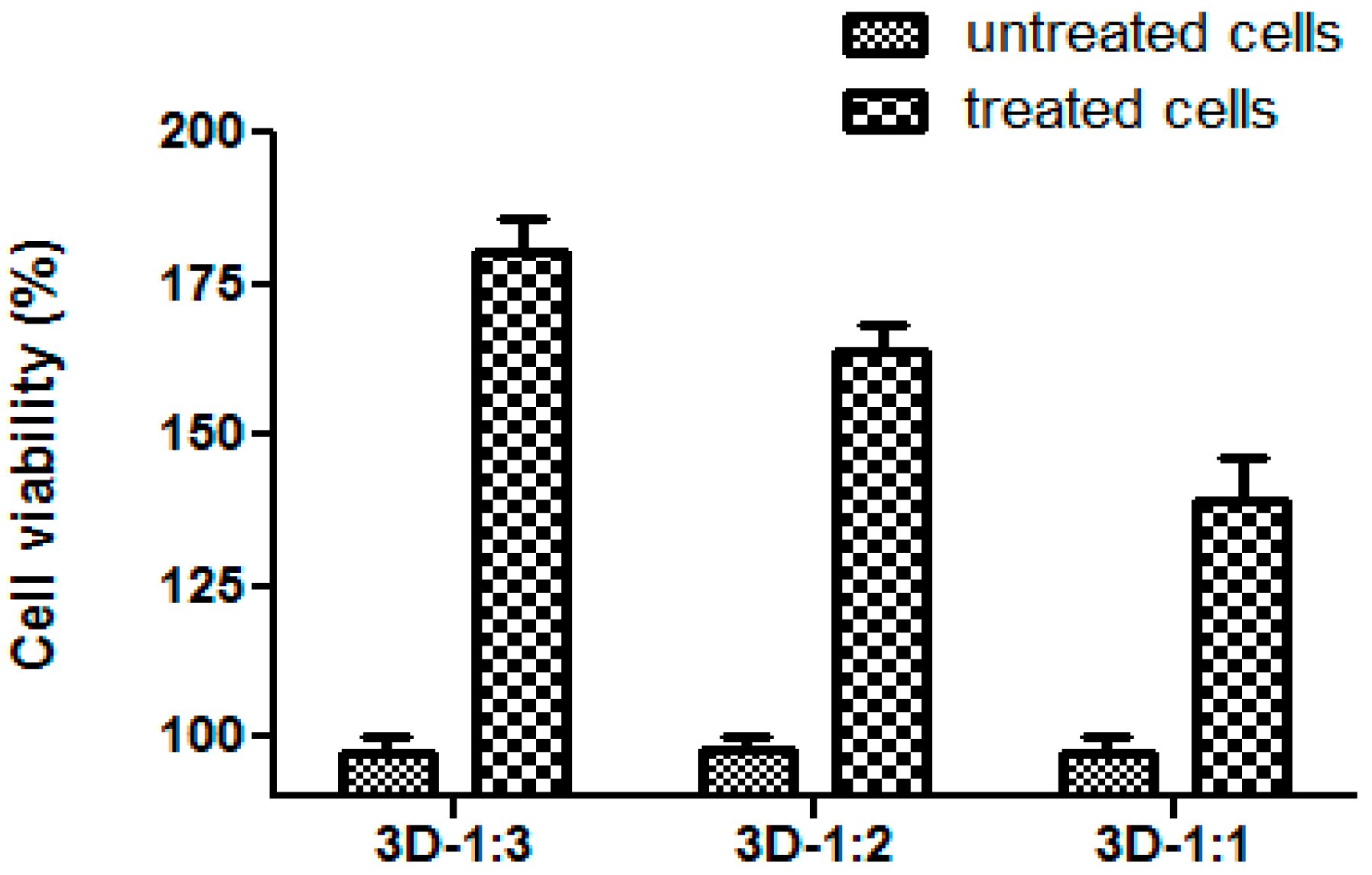
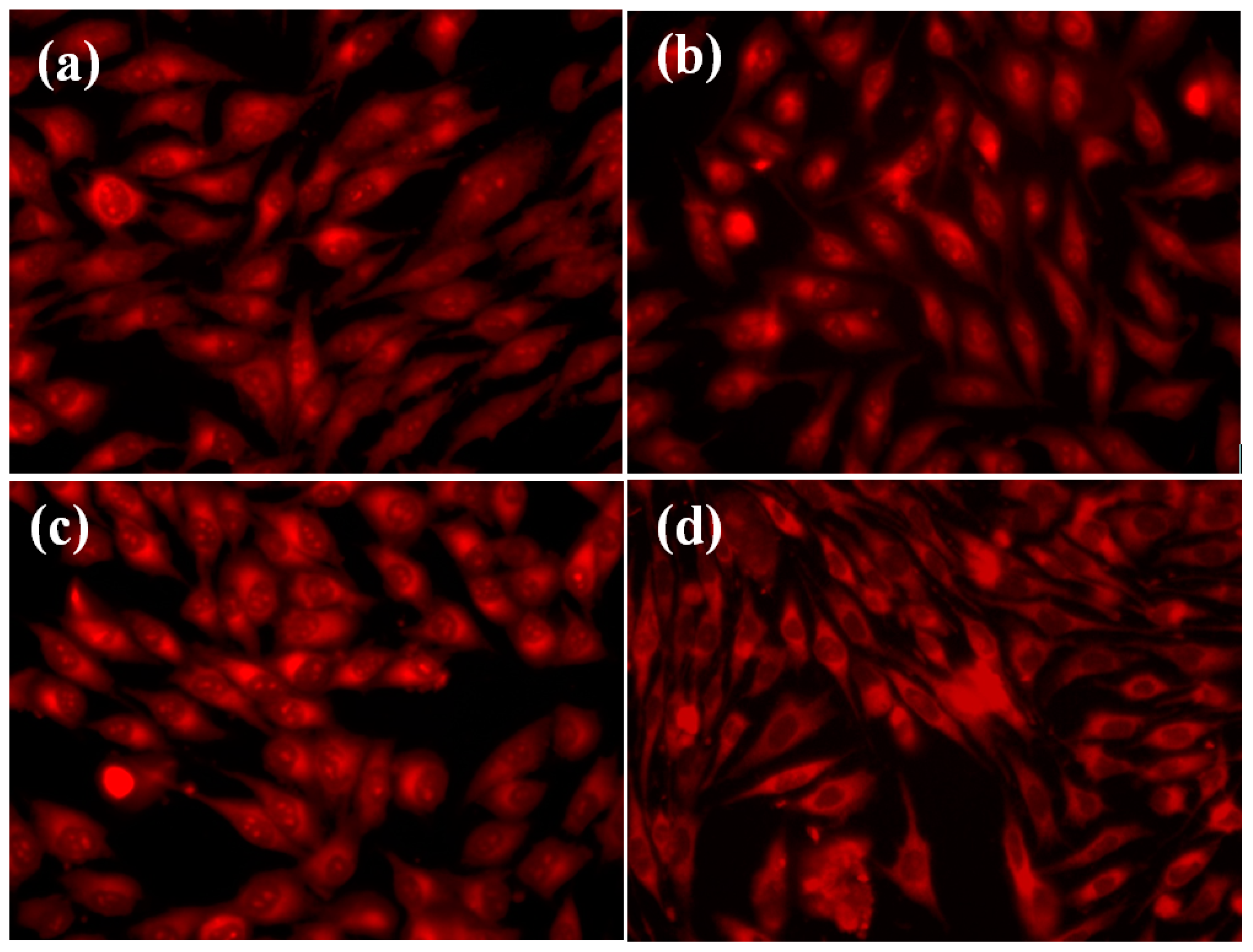
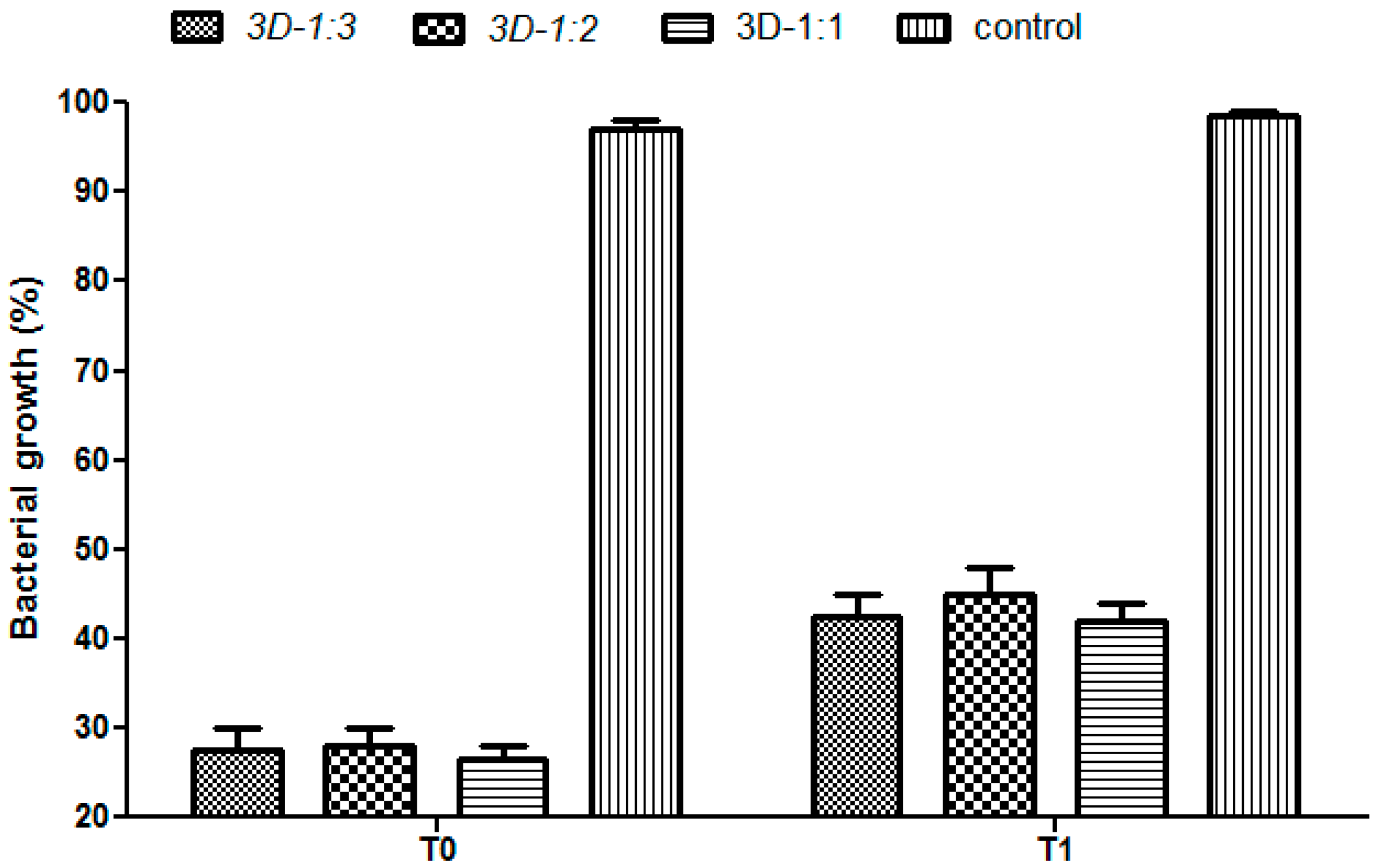
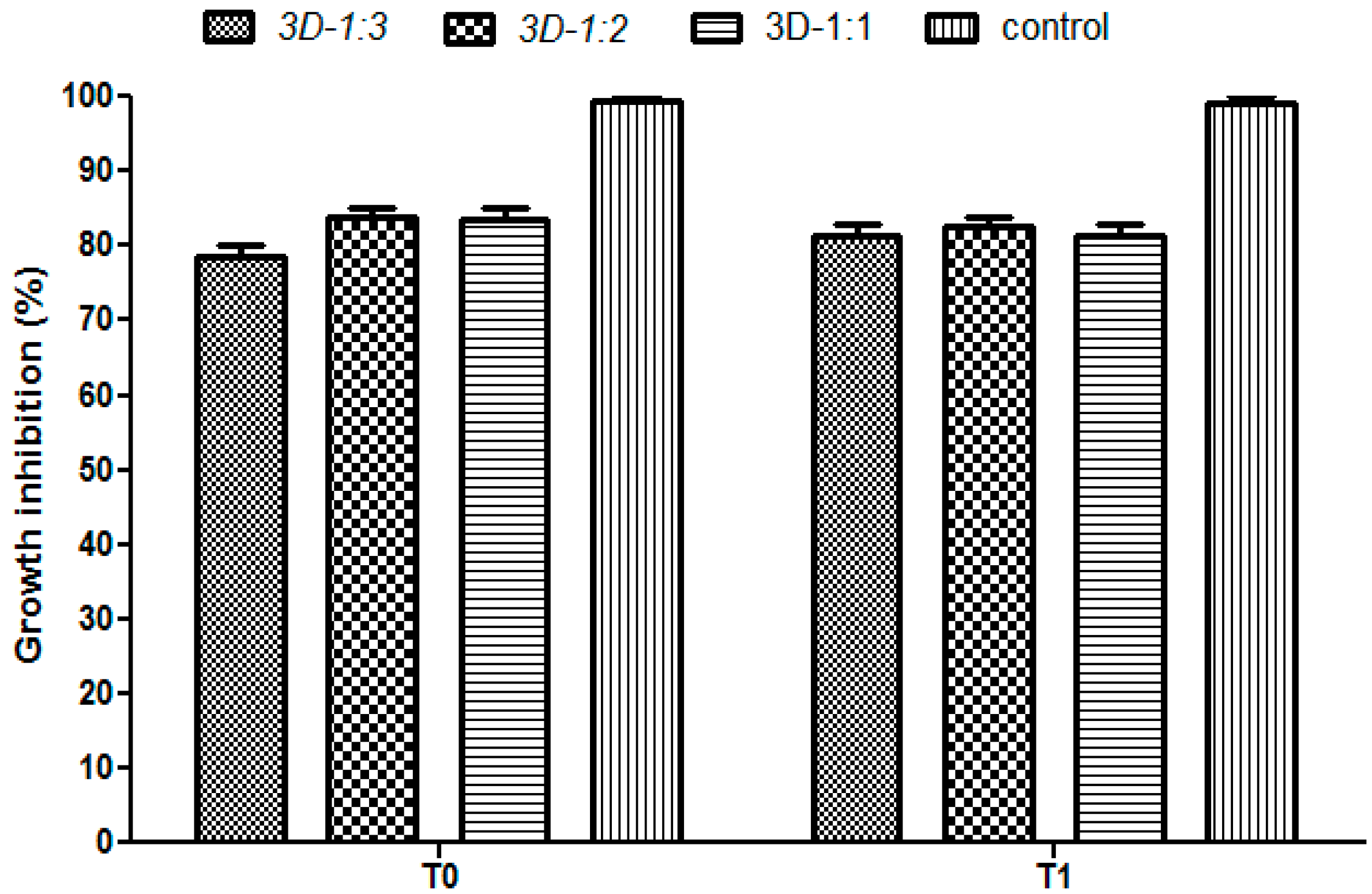
2. Results and Discussion

3. Materials and Methods
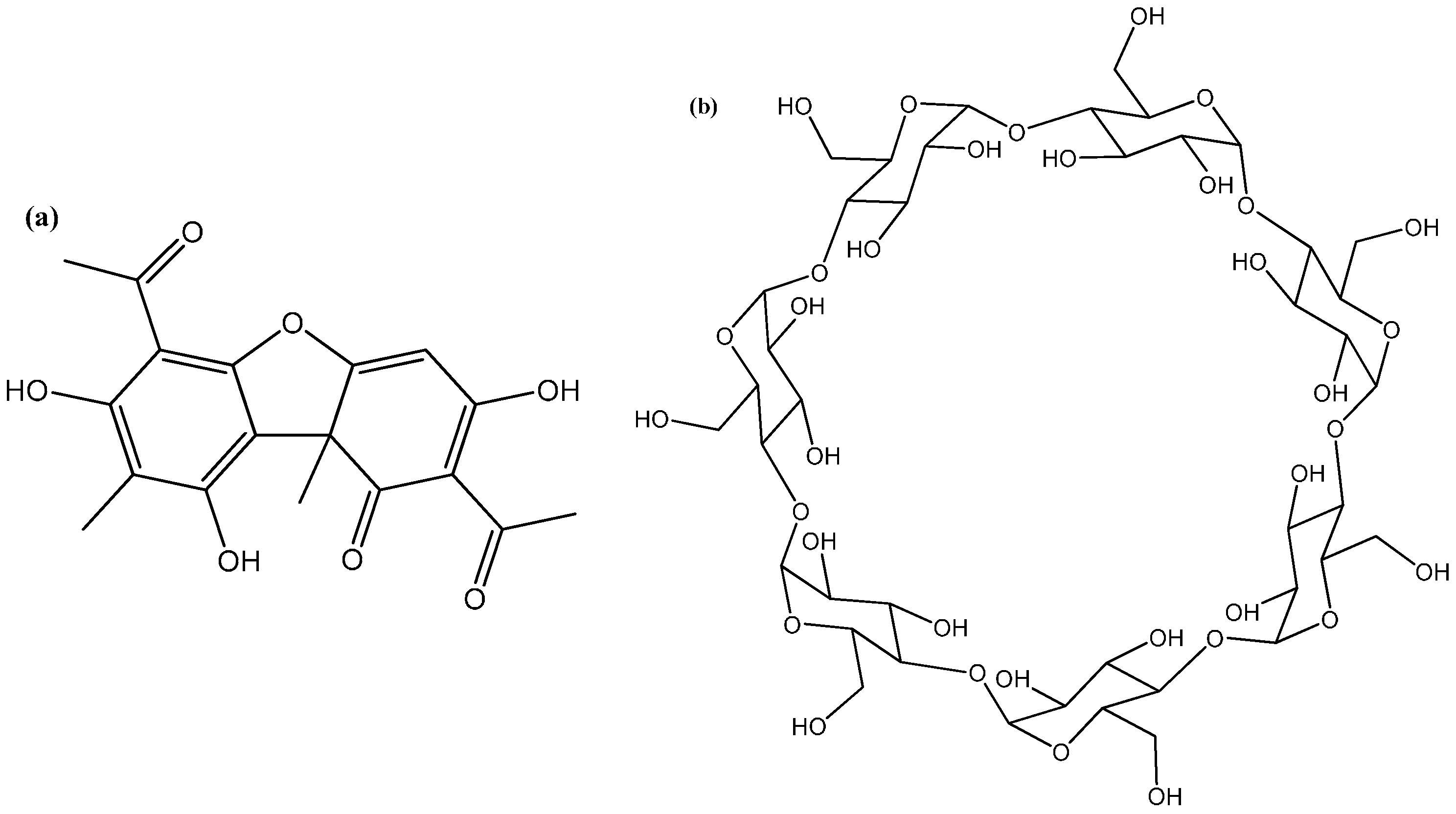
3.1. Materials
3.2. Fabrication of 3D Anti-Infective Regeneration Matrix
3.3. Characterization
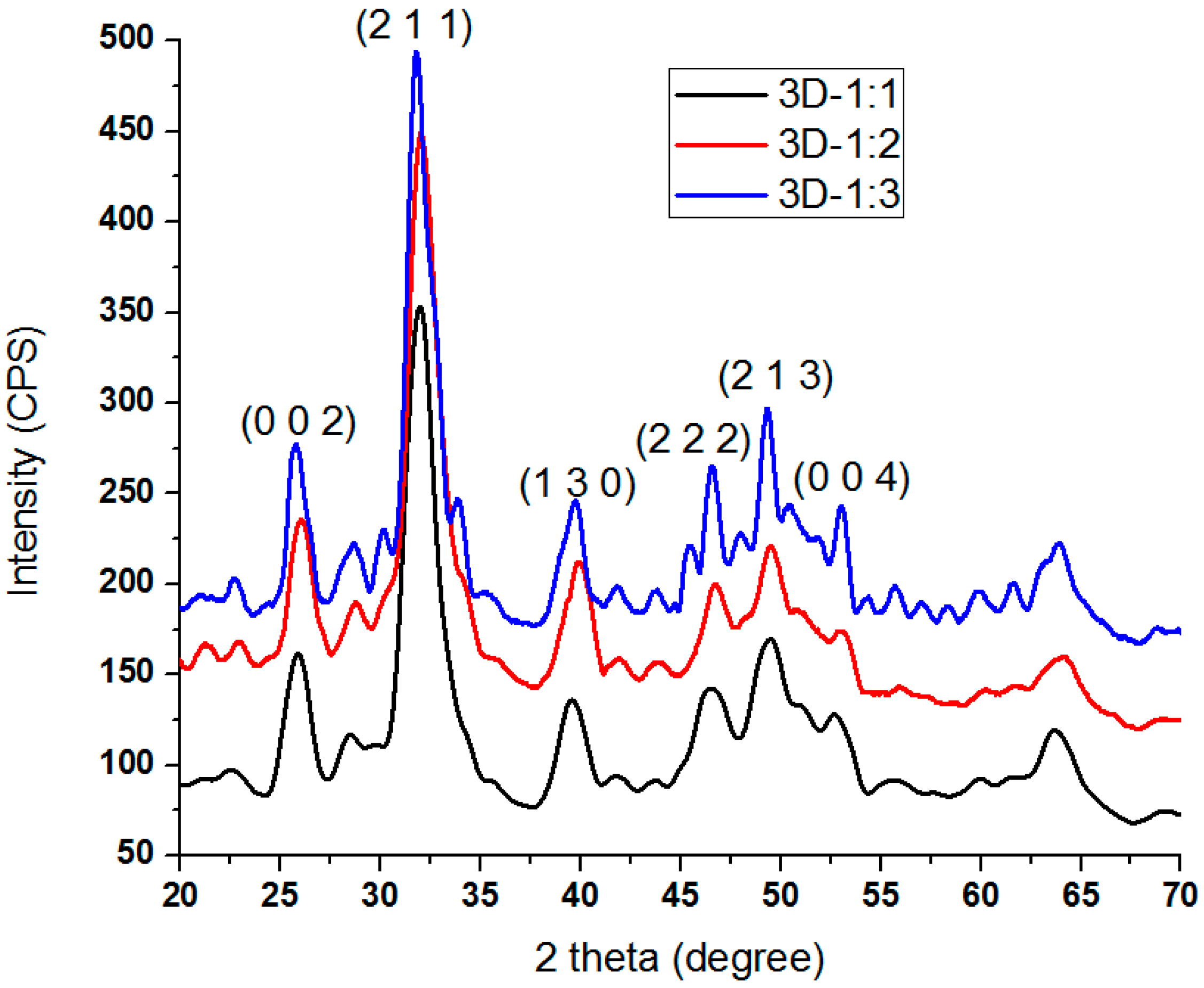
3.3.1. XRD
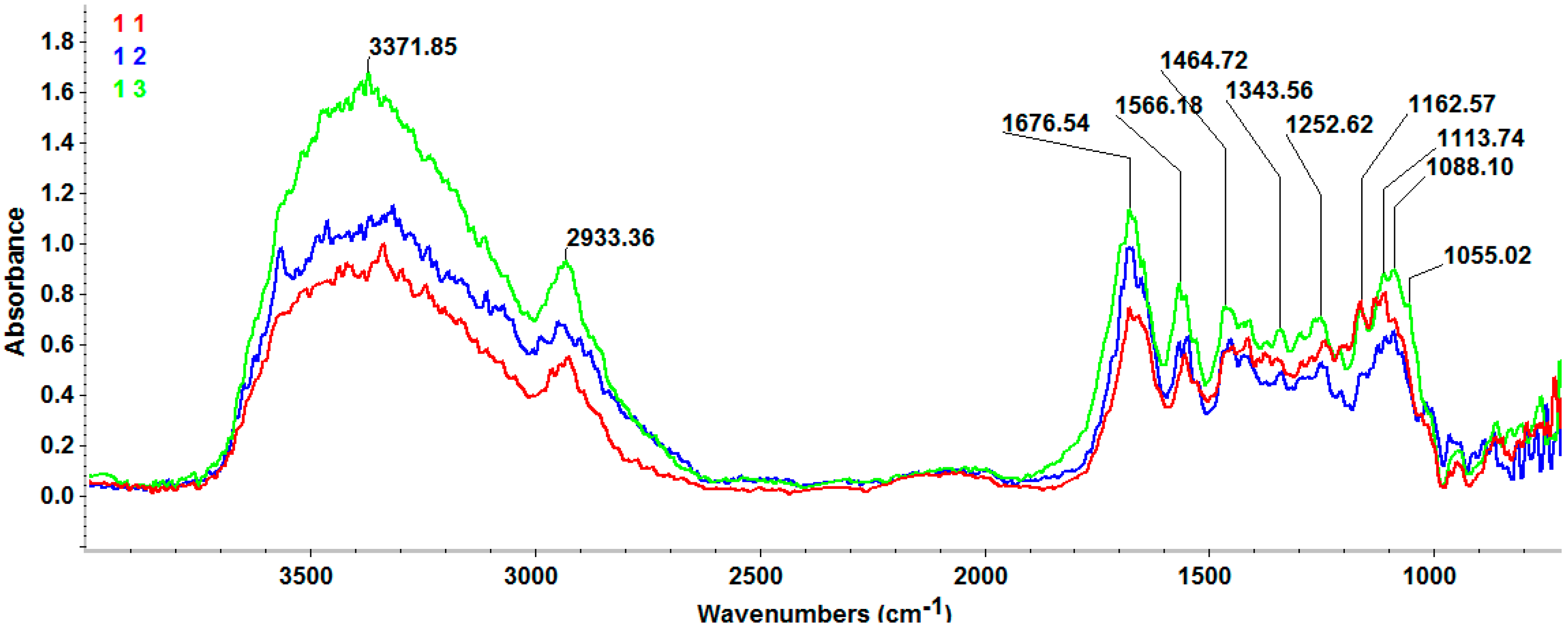
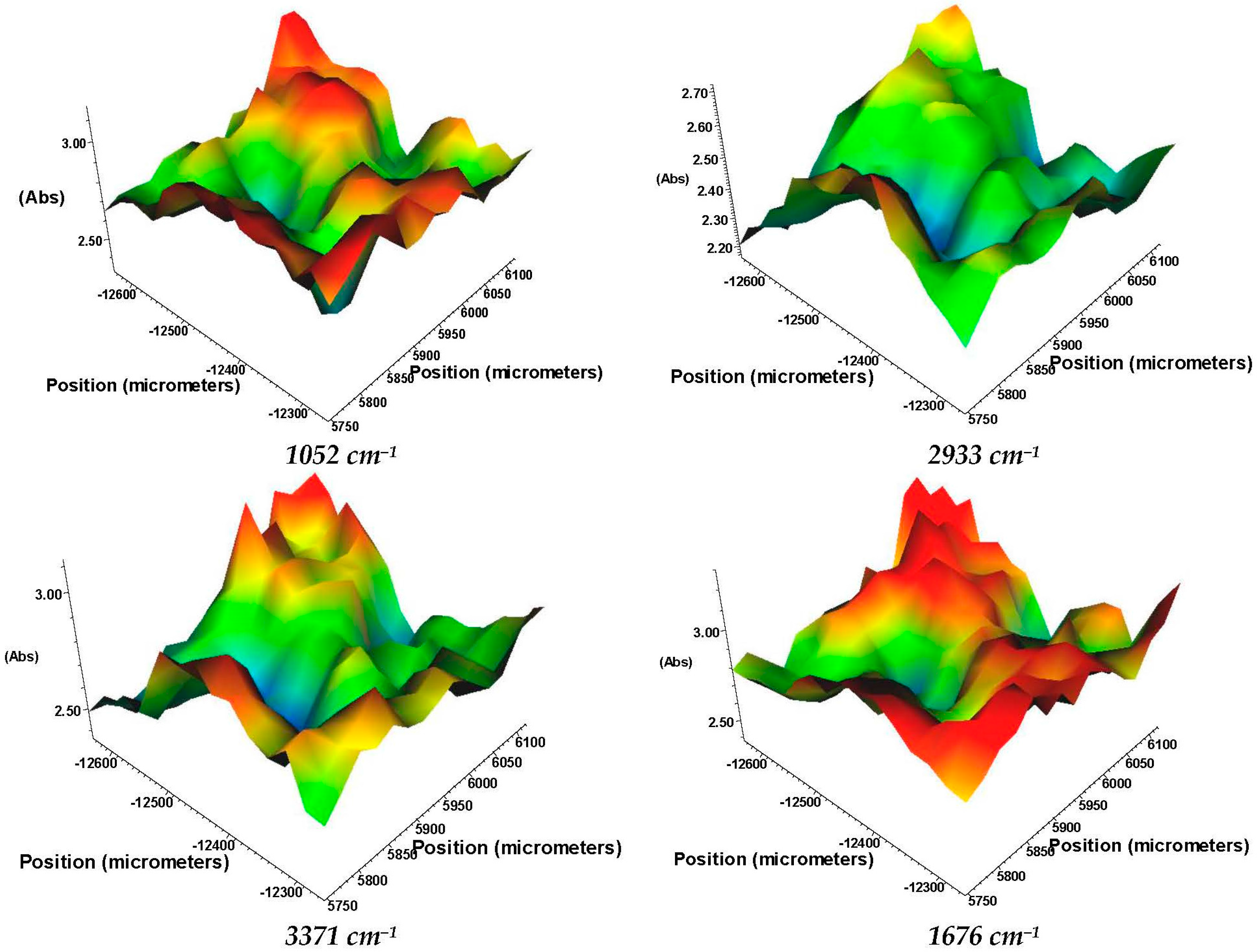
3.3.2. FT-IR
3.3.3. SEM
3.3.4. TEM
3.4. In Vitro Biocompatibility Test
3.5. Antimicrobial Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ottaviani, G.; Jaffe, N. The epidemiology of osteosarcoma. In Pediatric and Adolescent Osteosarcoma; Jaffe, N., Bruland, O.S., Bielack, S., Eds.; Springer: Philadelphia, PA, USA, 2010; Volume 152, pp. 3–13. [Google Scholar]
- SarcomaHelp.org. Available online: http://sarcomahelp.Org/research/osteosarcoma-cip4.Html (accessed on 10 March 2015).
- Gill, J.; Ahluwalia, M.K.; Geller, D.; Gorlick, R. New targets and approaches in osteosarcoma. Pharmacol. Ther. 2013, 137, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Voicu, G.; Cricǎ, L.E.; Fufǎ, O.; Moraru, L.I.; Popescu, R.C.; Purcel, G.; Stoilescu, M.C.; Grumezescu, A.M.; Bleotu, C.; Holban, A.M.; et al. Magnetite nanostructures functionalized with cytostatic drugs exhibit great anti-tumoral properties without application of high amplitude alternating magnetic fields. Romanian J. Morphol. Embryol. 2014, 55, 357–362. [Google Scholar]
- Popescu, R.C.; Andronescu, E.; Grumezescu, A.M. In vivo evaluation of Fe3O4 nanoparticles. Romanian J. Morphol. Embryol. 2014, 55, 1013–1018. [Google Scholar]
- Abdal-hay, A.; Salam Hamdy, A.; Morsi, Y.; Abdelrazek Khalil, K.; Hyun Lim, J. Novel bone regeneration matrix for next-generation biomaterial using a vertical array of carbonated hydroxyapatite nanoplates coated onto electrospun nylon 6 nanofibers. Mater. Lett. 2014, 137, 378–381. [Google Scholar] [CrossRef]
- Briceno, S.; Silva, P.; Bramer-Escamilla, W.; Zabala, J.; Alcala, O.; Guari, Y.; Larionova, J.; Long, J. Magnetic water-soluble rhamnose- coated Mn1−xCoxFe2O4 nanoparticles as potential heating agents for hyperthermia. Biointerface Res. Appl. Chem. 2015, 5, 910–915. [Google Scholar]
- Fufă, O.; Andronescu, E.; Grumezescu, V.; Holban, A.; Mogoantă, L.; Mogoșanu, G.; Socol, G.; Iordache, F.; Chifiriuc, M.C.; Grumezescu, A.M. Silver nanostructurated surfaces prepared by maple for biofilm prevention. Biointerface Res. Appl. Chem. 2015, 5, 1011–1017. [Google Scholar]
- Cristea, A.D.; Popa, M.; Chirifiuc, M.C.; Marutescu, L.; Lazar, V.; Suciu, I.; Iliescu, A.; Dimitriu, B.; Perlea, P. The antimicrobial efficiency of endodontic irrigation solutions on bacterial biofilm. A literature review. Biointerface Res. Appl. Chem. 2015, 5, 963–969. [Google Scholar]
- Lazar, V. Quorum sensing in biofilms—How to destroy the bacterial citadels or their cohesion/power? Anaerobe 2011, 17, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Pammi, M.; Liang, R.; Hicks, J.; Mistretta, T.A.; Versalovic, J. Biofilm extracellular DNA enhances mixed species biofilms of Staphylococcus epidermidis and Candida albicans. BMC Microbiol. 2013, 13, 257. [Google Scholar] [CrossRef] [PubMed]
- Von Eiff, C.; Jansen, B.; Kohnen, W.; Becker, K. Infections associated with medical devices: Pathogenesis, management and prophylaxis. Drugs 2005, 65, 179–214. [Google Scholar] [CrossRef] [PubMed]
- Istrate, C.M.; Holban, A.M.; Grumezescu, A.M.; Mogoantă, L.; Mogoşanu, G.D.; Savopol, T.; Moisescu, M.; Iordache, M.; Vasile, B.S.; Kovacs, E. Iron oxide nanoparticles modulate the interaction of different antibiotics with cellular membranes. Romanian J. Morphol. Embryol. 2014, 55, 849–856. [Google Scholar]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Holban, A.M.; Grumezescu, A.M.; Andronescu, E.; Grumezescu, V.; Chifiriuc, C.M.; Rǎdulescu, R. Magnetite–Usnicacid nanostructured bioactive material with antimicrobial activity. Rev. Romana Mater. Romanian J. Mater. 2013, 43, 402–407. [Google Scholar]
- Holban, A.M.; Gestal, M.C.; Grumezescu, A.M. New molecular strategies for reducing implantable medical devices associated infections. Curr. Med. Chem. 2014, 21, 3375–3382. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, B.; Ionescu, D.; Gheorghe, I.; Mihaescu, G.; Bleotu, C.; Sakizlian, M.; Banu, O. Staphylococcus aureus virulence phenotypes among romanian population. Biointerface Res. Appl. Chem. 2015, 5, 945–948. [Google Scholar]
- Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface R. Soc. 2006, 3, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Abdal-hay, A.; Pant, H.R.; Lim, J.K. Super-hydrophilic electrospun nylon-6/hydroxyapatite membrane for bone tissue engineering. Eur. Polym. J. 2013, 49, 1314–1321. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Kruger, T.E.; Miller, A.H.; Wang, J. Collagen scaffolds in bone sialoprotein-mediated bone regeneration. Sci. World J. 2013, 2013, Article ID 812718. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, T.; Nakamura, T.; Kuremoto, K.; Notazawa, S.; Nakahara, T.; Hashimoto, Y.; Satoh, T.; Shimizu, Y. Development of β-tricalcium phosphate/collagen sponge composite for bone regeneration. Dent. Mater. J. 2006, 25, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.I.; Graves, S.E.; Wong, A.T.C.; Triffitt, J.T.; Francis, M.J.O.; Czernuszka, J.T. Investigation into the formation and mechanical properties of a bioactive material based on collagen and calcium phosphate. J. Mater. Sci. Mater. Med. 1993, 4, 107–110. [Google Scholar] [CrossRef]
- Moldovan, L.; Craciunescu, O.; Oprita, E.I.; Balan, M.; Zarnescu, O. Collagen-chondroitin sulfate-hydroxyapatite porous composites: Preparation, characterization and in vitro biocompatibility testing. Romanian Biotechnol. Lett. 2009, 14, 4459–4466. [Google Scholar]
- Chen, G.; Lv, Y.; Dong, C.; Yang, L. Effect of internal structure of collagen/hydroxyapatite scaffold on the osteogenic differentiation of mesenchymal stem cells. Curr. Stem Cell Res. Ther. 2015, 10, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Lee, H.T.; Reinhardt, R.A.; Marky, L.A.; Wang, D. Novel biomineral-binding cyclodextrins for controlled drug delivery in the oral cavity. J. Control. Release 2007, 122, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-M.; Wiswall, A.T.; Rutledge, J.E.; Akhter, M.P.; Cullen, D.M.; Reinhardt, R.A.; Wang, D. Osteotropic β-cyclodextrin for local bone regeneration. Biomaterials 2008, 29, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Ruszczak, Z.; Friess, W. Collagen as a carrier for on-site delivery of antibacterial drugs. Adv. Drug Deliv. Rev. 2003, 55, 1679–1698. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, M.; Patel, S.; MacFarlane, R.J.; Haddad, F.S. Biodegradable antibiotic delivery systems. J. Bone Joint Surg. Br. Vol. 2011, 93, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Nie, X.; Zou, M.; Shi, Y.; Cheng, G. Recent advances in materials for extended-release antibiotic delivery system. J. Antibiot. 2011, 64, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Iconaru, S.L.; Pasuk, I.; Vasile, B.S.; Lupu, A.R.; Hermenean, A.; Dinischiotu, A.; Predoi, D. Structural properties of silver doped hydroxyapatite and their biocompatibility. Mater. Sci. Eng. C 2013, 33, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Lala, S.; Brahmachari, S.; Das, P.K.; Das, D.; Kar, T.; Pradhan, S.K. Biocompatible nanocrystalline natural bonelike carbonated hydroxyapatite synthesized by mechanical alloying in a record minimum time. Mater. Sci. Eng. C 2014, 42, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dong, X.; Bian, M.; Zhao, J.; Zhang, Y.; Sun, Y.; Chen, J.; Wang, X. Solution combustion method for synthesis of nanostructured hydroxyapatite, fluorapatite and chlorapatite. Appl. Surf. Sci. 2014, 314, 1026–1033. [Google Scholar] [CrossRef]
- Wenpo, F.; Gaofeng, L.; Shuying, F.; Yuanming, Q.; Keyong, T. Preparation and characterization of collagen–hydroxyapatite/pectin composite. Int. J. Biol. Macromol. 2015, 74, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gasga, J.; Martínez-Piñeiro, E.L.; Rodríguez-Álvarez, G.; Tiznado-Orozco, G.E.; García-García, R.; Brès, E.F. XRD and FTIR crystallinity indices in sound human tooth enamel and synthetic hydroxyapatite. Mater. Sci. Eng. C 2013, 33, 4568–4574. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, A.M.; Andronescu, E.; Holban, A.M.; Ficai, A.; Ficai, D.; Voicu, G.; Grumezescu, V.; Balaure, P.C.; Chifiriuc, C.M. Water dispersible cross-linked magnetic chitosan beads for increasing the antimicrobial efficiency of aminoglycoside antibiotics. Int. J. Pharm. 2013, 454, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, V.; Socol, G.; Grumezescu, A.M.; Holban, A.M.; Ficai, A.; Truşcǎ, R.; Bleotu, C.; Balaure, P.C.; Cristescu, R.; Chifiriuc, M.C. Functionalized antibiofilm thin coatings based on PLA–PVA microspheres loaded with usnic acid natural compounds fabricated by maple. Appl. Surf. Sci. 2014, 302, 262–267. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Z.; Li, X.; Li, S.; Zhou, Z.; Zhang, Y.; Feng, Q.L.; Yu, B. In vitro biocompatibility and osteoblast differentiation of an injectable chitosan/nano-hydroxyapatite/collagen scaffold. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Tazi, N.; Zhang, Z.; Messaddeq, Y.; Almeida-Lopes, L.; Zanardi, L.; Levinson, D.; Rouabhia, M. Hydroxyapatite bioactivated bacterial cellulose promotes osteoblast growth and the formation of bone nodules. AMB Express 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.D.; Issa, J.P.M.; Oliveira, R.R.D.; Iyomasa, M.M.; Siéssere, S.; Regalo, S.C.H. Biomaterials applied to the bone healing process. Int. J. Morphol. 2007, 25, 839–846. [Google Scholar] [CrossRef]
- Wahl, D.A.; Czernuszka, J.T. Collagen-hydroxyapatite composites for hard tissue repair. Eur. Cells Mater. 2006, 11, 43–56. [Google Scholar]
- Francolini, I.; Norris, P.; Piozzi, A.; Donelli, G.; Stoodley, P. Usnic acid, a natural antimicrobial agent able to inhibit bacterial biofilm formation on polymer surfaces. Antimicrob. Agents Chemother. 2004, 48, 4360–4365. [Google Scholar] [CrossRef] [PubMed]
- Milo, R.; Jorgensen, P.; Moran, U.; Weber, G.; Springer, M. BioNumbers—The database of key numbers in molecular and cell biology. Nucl. Acids Res. 2010, 38 (Suppl 1), D750–D753. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, V.; Holban, A.M.; Grumezescu, A.M.; Socol, G.; Ficai, A.; Vasile, B.S.; Truscǎ, R.; Bleotu, C.; Lazar, V.; Chifiriuc, C.M.; et al. Usnic acid-loaded biocompatible magnetic PLGA-PVA microsphere thin films fabricated by maple with increased resistance to staphylococcal colonization. Biofabrication 2014, 6, 035002. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, A.M.; Saviuc, C.; Chifiriuc, M.C.; Hristu, R.; Mihaiescu, D.E.; Balaure, P.; Stanciu, G.; Lazar, V. Inhibitory activity of Fe3O4/oleic acid/usnic acid-core/shell/extra-shell nanofluid on S. aureus biofilm development. IEEE Trans. Nanobiosci. 2011, 10, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, A.; Cotar, A.; Andronescu, E.; Ficai, A.; Ghitulica, C.; Grumezescu, V.; Vasile, B.; Chifiriuc, M. In vitro activity of the new water-dispersible Fe3O4@usnic acid nanostructure against planktonic and sessile bacterial cells. J. Nanopart. Res. 2013, 15, 1–10. [Google Scholar] [CrossRef]
- Francolini, I.; Taresco, V.; Crisante, F.; Martinelli, A.; D’Ilario, L.; Piozzi, A. Water soluble usnic acid-polyacrylamide complexes with enhanced antimicrobial activity against Staphylococcus epidermidis. Int. J. Mol. Sci. 2013, 14, 7356–7369. [Google Scholar] [CrossRef] [PubMed]
- Ficai, M.; Andronescu, E.; Ficai, D.; Voicu, G.; Ficai, A. Synthesis and characterization of COLL–PVA/HA hybrid materials with stratified morphology. Colloids Surf. B Biointerfaces 2010, 81, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.G.; Oliveira, M.A.; Alves, R.d.S.; Menezes, P.d.P.; Serafini, M.R.; de Souza Araújo, A.A.; Bezerra, D.P.; Quintans Júnior, L.J. Encapsulation of carvacrol, a monoterpene present in the essential oil of oregano, with β-cyclodextrin, improves the pharmacological response on cancer pain experimental protocols. Chem. Biol. Interact. 2015, 227, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Mihai Grumezescu, A.; Andronescu, E.; Georgiana Albu, M.; Ficai, A.; Bleotu, C.; Dragu, D.; Lazar, V. Wound dressing based collagen biomaterials containing usnic acid as quorum sensing inhibitor agent: Synthesis, characterization and bioevaluation. Curr. Org. Chem. 2013, 17, 125–131. [Google Scholar] [CrossRef]
- Chang, M.C.; Tanaka, J. FT-IR study for hydroxyapatite/collagen nanocomposite cross-linked by glutaraldehyde. Biomaterials 2002, 23, 4811–4818. [Google Scholar] [CrossRef]
- Sample Availability: Samples are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ion, A.; Andronescu, E.; Rădulescu, D.; Rădulescu, M.; Iordache, F.; Vasile, B.Ș.; Surdu, A.V.; Albu, M.G.; Maniu, H.; Chifiriuc, M.C.; et al. Biocompatible 3D Matrix with Antimicrobial Properties. Molecules 2016, 21, 115. https://doi.org/10.3390/molecules21010115
Ion A, Andronescu E, Rădulescu D, Rădulescu M, Iordache F, Vasile BȘ, Surdu AV, Albu MG, Maniu H, Chifiriuc MC, et al. Biocompatible 3D Matrix with Antimicrobial Properties. Molecules. 2016; 21(1):115. https://doi.org/10.3390/molecules21010115
Chicago/Turabian StyleIon, Alberto, Ecaterina Andronescu, Dragoș Rădulescu, Marius Rădulescu, Florin Iordache, Bogdan Ștefan Vasile, Adrian Vasile Surdu, Madalina Georgiana Albu, Horia Maniu, Mariana Carmen Chifiriuc, and et al. 2016. "Biocompatible 3D Matrix with Antimicrobial Properties" Molecules 21, no. 1: 115. https://doi.org/10.3390/molecules21010115