Discovery and Structure-Based Optimization of 6-Bromotryptamine Derivatives as Potential 5-HT2A Receptor Antagonists
Abstract
:1. Introduction
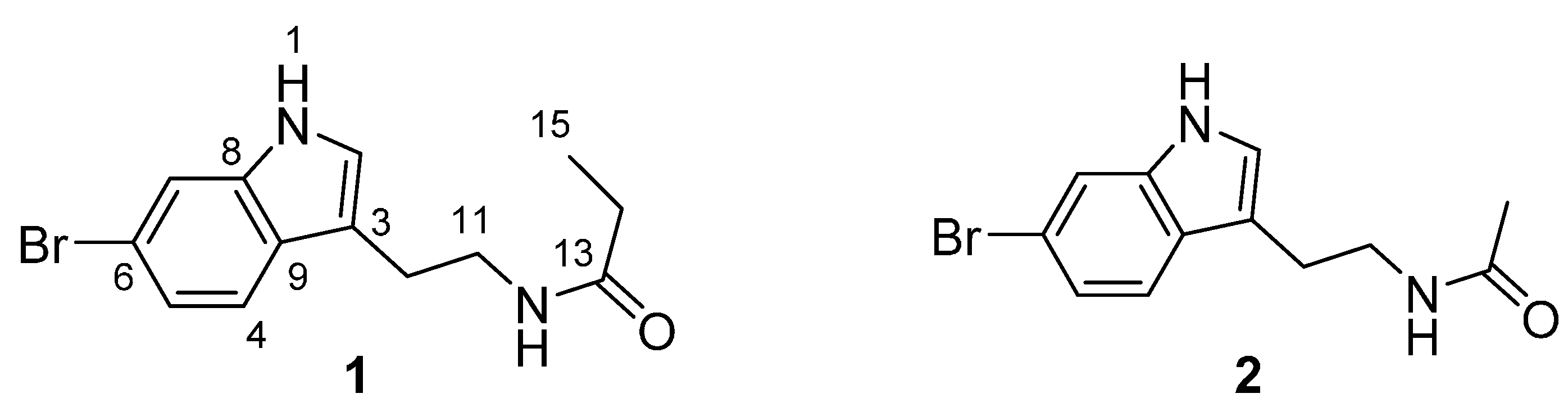
2. Results and Discussion
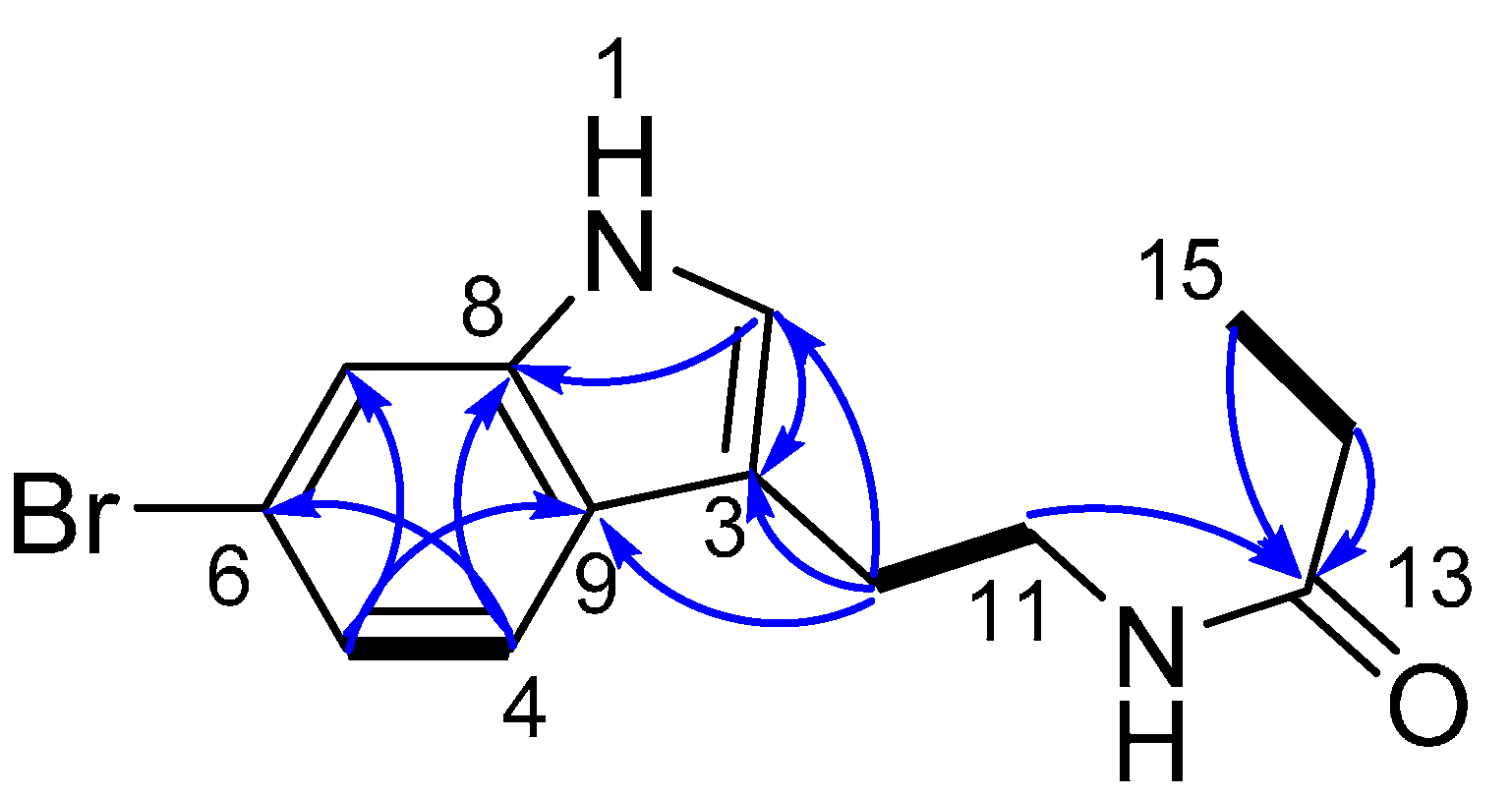
2.1. Structure Elucidation of 6-Bromotryptamine Derivatives 1–2
| Position | δC, Type | δH (J in Hz) | COSY | HMBC (H→C) |
|---|---|---|---|---|
| 2 | 124.3, CH | 7.07, s | 3, 8 | |
| 3 | 113.8, C | |||
| 4 | 120.8, CH | 7.47, d (8.4) | 5 | 6, 8 |
| 5 | 122.7, CH | 7.10, dd (8.5, 1.8) | 4 | 7, 9 |
| 6 | 115.7, C | |||
| 7 | 115.1, CH | 7.48, d (1.8) | 5, 9 | |
| 8 | 138.9, C | |||
| 9 | 127.3, C | |||
| 10 | 26.1, CH2 | 2.91, t (7.2) | 11 | 2, 3, 9, 11 |
| 11 | 41.3, CH2 | 3.44, t (7.3) | 10 | 3, 10,13 |
| 13 | 177.0, C | |||
| 14 | 30.3, CH2 | 2.16, q (7.6) | 15 | 13, 15 |
| 15 | 10.5, CH3 | 1.09, t (7.7) | 14 | 13, 14 |
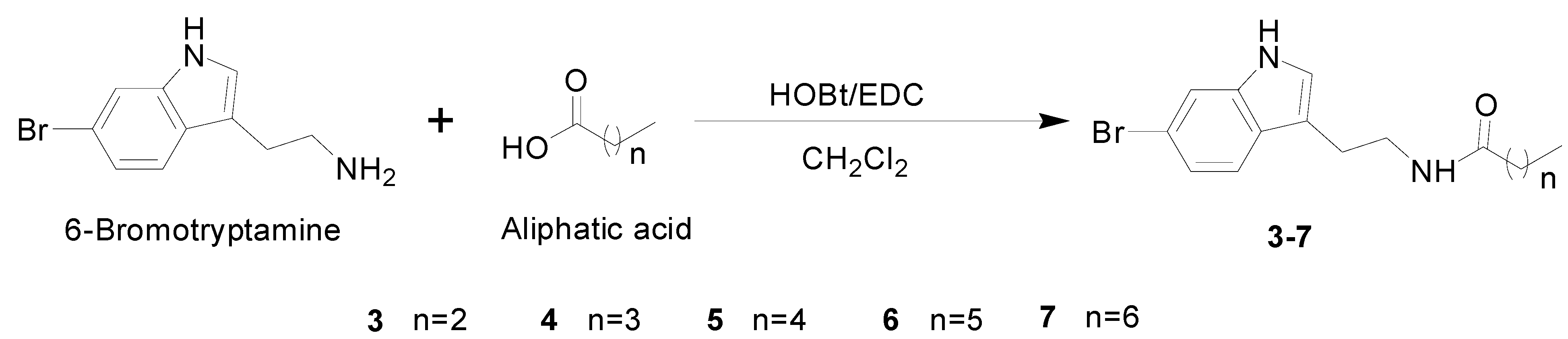
2.2. Syntheses of 6-Bromotryptamine Derivatives 3–7
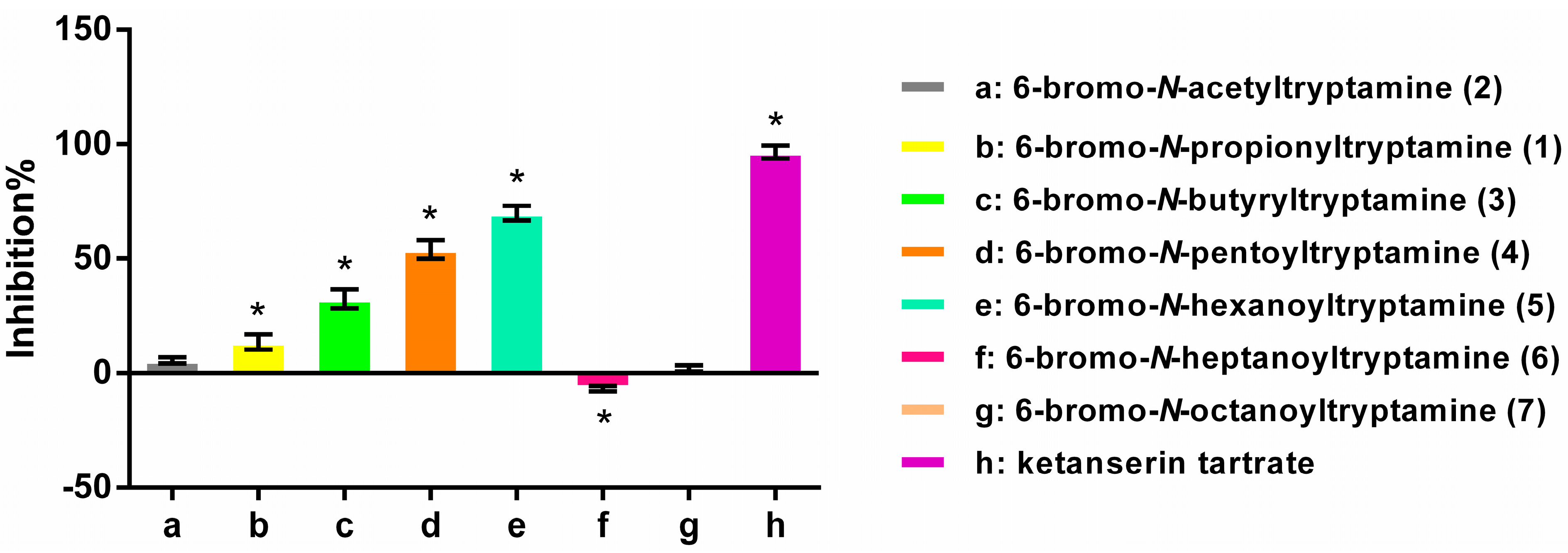
2.3. Structure-Activity Relationships
3. Experimental Section
3.1. General Experimental Procedures
3.2. Bacterial Material and Fermentation
3.3. Extraction and Isolation
3.4. General Procedure for the Synthesis and Isolation of Compounds 3–7
3.5. Antagonist Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Graeff, F.G.; Guimarães, F.S.; de Andrade, T.G.; Deakin, J.F. Role of 5-HT in stress, anxiety, and depression. Pharmacol. Biochem. Behav. 1996, 54, 129–141. [Google Scholar] [CrossRef]
- Oh, K.H.; Nam, Y.; Jeong, J.H.; Kim, I.K.; Sohn, U.D. The effect of DA-9701 on 5-hydroxytryptamine-induced contraction of feline esophageal smooth muscle cells. Molecules 2014, 19, 5135–5149. [Google Scholar] [CrossRef] [PubMed]
- Bombardi, C.; di Giovanni, G. Functional anatomy of 5-HT2A receptors in the amygdala and hippocampal complex: relevance to memory functions. Exp. Brain Res. 2013, 230, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.J.; Sorensen, S.M.; Kenne, J.H.; Carr, A.A.; Palfreyman, M.G. The role of 5-HT2A receptors in antipsychotic activity. Life Sci. 1995, 56, 2209–2222. [Google Scholar] [CrossRef]
- Bubar, M.J.; Cunningham, K.A. Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr. Top. Med. Chem. 2006, 6, 1971–1985. [Google Scholar] [CrossRef] [PubMed]
- Palangsuntikul, R.; Berner, H.; Berger, M.L.; Wolschann, P. Holographic quantitative structure-activity relationships of tryptamine derivatives at NMDA, 5HT1A and 5HT2A receptors. Molecules 2013, 18, 8799–8811. [Google Scholar] [CrossRef] [PubMed]
- Werneck, A.L.D.S.; Rosso, A.L.; Vincent, M.B. The use of an antagonist 5-HT2a/c for depression and motor function in Parkinson’disease. Arq. Neuropsiquiatr. 2009, 67, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Ebdrup, B.H.; Rasmussen, H.; Arnt, J.; Glenthøj, B. Serotonin 2A receptor antagonists for treatment of schizophrenia. Expert Opin. Investig. Drug. 2011, 20, 1211–1223. [Google Scholar] [CrossRef] [PubMed]
- Guella, G.; Mancini, I.; Duhet, D.; Richer de Forges, B.; Pietra, F. Ethyl 6 bromo-3-indolcarboxylate and 3-hydroxyacetal-6-bromoindole, novel bromoindoles from the sponge Pleroma menoui of the Coral Sea. Z. Naturforsch. 1989, 44c, 914–916. [Google Scholar]
- Fahy, E.; Potts, B.C.; Faulkner, D.J.; Smith, K. 6-Bromotryptamine derivatives from the Gulf of California tunicate Didemnum candidum. J. Nat. Prod. 1991, 54, 564–569. [Google Scholar] [CrossRef]
- Miyake, Y.; Kikugawa, Y. Synthesis of 6-methoxyindoles and indolines. Regioselective C-6 bromination of indolines and subsequent nucleophilic substitution with a methoxyl group. J. Heterocycl. Chem. 1983, 20, 349–352. [Google Scholar] [CrossRef]
- Yao, S.; Xie, H.; Zhang, L.; Meng, T.; Zhang, Y.; Wang, X.; Chen, L.; Pan, S.; Shen, J. Total synthesis and antidepressant activities of laetispicine and its derivatives. Molecules 2012, 17, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Mascuch, S.J.; Villa, F.A.; Byrum, T.; Teasdale, M.E.; Smith, J.E.; Preskitt, L.B.; Rowley, D.C.; Gerwick, L.; Gerwick, W.H. Honaucins A–C, potent inhibitors of inflammation and bacterial quorum sensing: Synthetic derivatives and structure-activity relationships. Chem. Biol. 2012, 19, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Efremov, I.V.; Vajdos, F.F.; Borzilleri, K.A.; Capetta, S.; Chen, H.; Dorff, P.H.; Dutra, J.K.; Goldstein, S.W.; Mansour, M.; McColl, A.; et al. Discovery and optimization of a novel spiropyrrolidine inhibitor of β-secretase (BACE1) through fragment-based drug design. J. Med. Chem. 2012, 55, 9069–9088. [Google Scholar] [CrossRef] [PubMed]
- Roelens, F.; Heldring, N.; Dhooge, W.; Bengtsson, M.; Comhaire, F.; Gustafsson, J.-Å.; Treuter, E.; de Keukeleire, D. Subtle side-chain modifications of the hop phytoestrogen 8-prenylnaringenin result in distinct agonist/antagonist activity profiles for estrogen receptors α and β. J. Med. Chem. 2006, 49, 7357–7365. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, K.J.; Broadhead, A.R.; Correa, L.D.; Scott, J.M.; Truong, Y.P.; Stearns, B.A.; Hutchinson, J.H.; Prasit, P.; Evans, J.F.; Lorrain, D.S. Therapeutic efficacy of AM156, a novel prostanoid DP2 receptor antagonist, in murine models of allergic rhinitis and house dust mite-induced pulmonary inflammation. Eur. J. Pharmacol. 2010, 638, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, J.M.; Christopoulos, A.; Ulven, T.; Royer, J.F.; Campillo, M.; Heinemann, A.; Pardo, L.; Kostenis, E. On the mechanism of interaction of potent surmountable and insurmountable antagonists with the prostaglandin D2 receptor CRTH2. Mol. Pharmacol. 2006, 69, 1441–1453. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; He, S.; Wu, W.; Jin, H.; Zhu, P.; Zhang, J.; Wang, T.; Yuan, Y.; Yan, X. Discovery and Structure-Based Optimization of 6-Bromotryptamine Derivatives as Potential 5-HT2A Receptor Antagonists. Molecules 2015, 20, 17675-17683. https://doi.org/10.3390/molecules200917675
Ding L, He S, Wu W, Jin H, Zhu P, Zhang J, Wang T, Yuan Y, Yan X. Discovery and Structure-Based Optimization of 6-Bromotryptamine Derivatives as Potential 5-HT2A Receptor Antagonists. Molecules. 2015; 20(9):17675-17683. https://doi.org/10.3390/molecules200917675
Chicago/Turabian StyleDing, Lijian, Shan He, Wei Wu, Haixiao Jin, Peng Zhu, Jinrong Zhang, Tingting Wang, Ye Yuan, and Xiaojun Yan. 2015. "Discovery and Structure-Based Optimization of 6-Bromotryptamine Derivatives as Potential 5-HT2A Receptor Antagonists" Molecules 20, no. 9: 17675-17683. https://doi.org/10.3390/molecules200917675






