Blumea balsamifera Oil for the Acceleration of Healing of Burn Injuries
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Different Treated Groups of BBO on General Observation in Rats
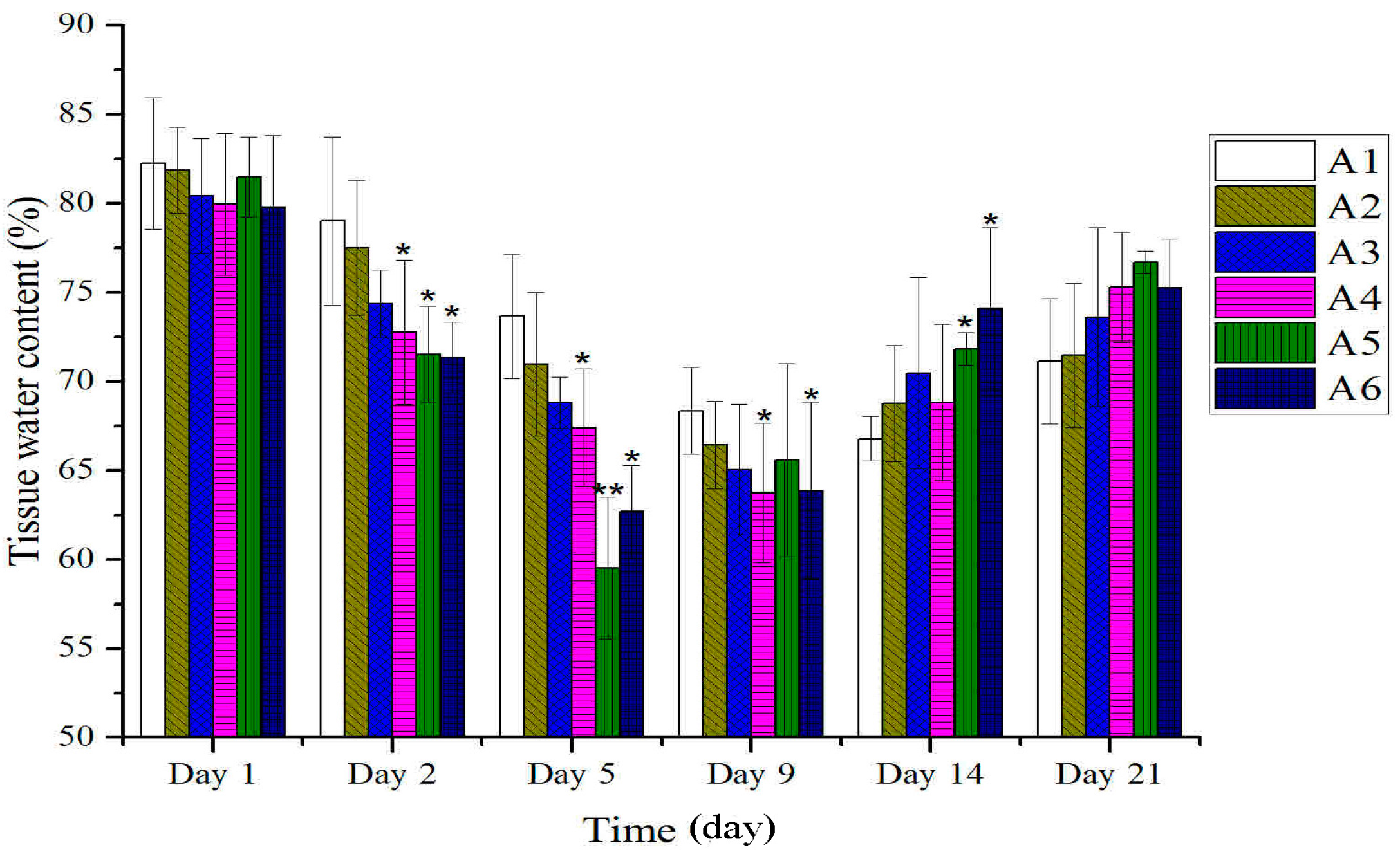
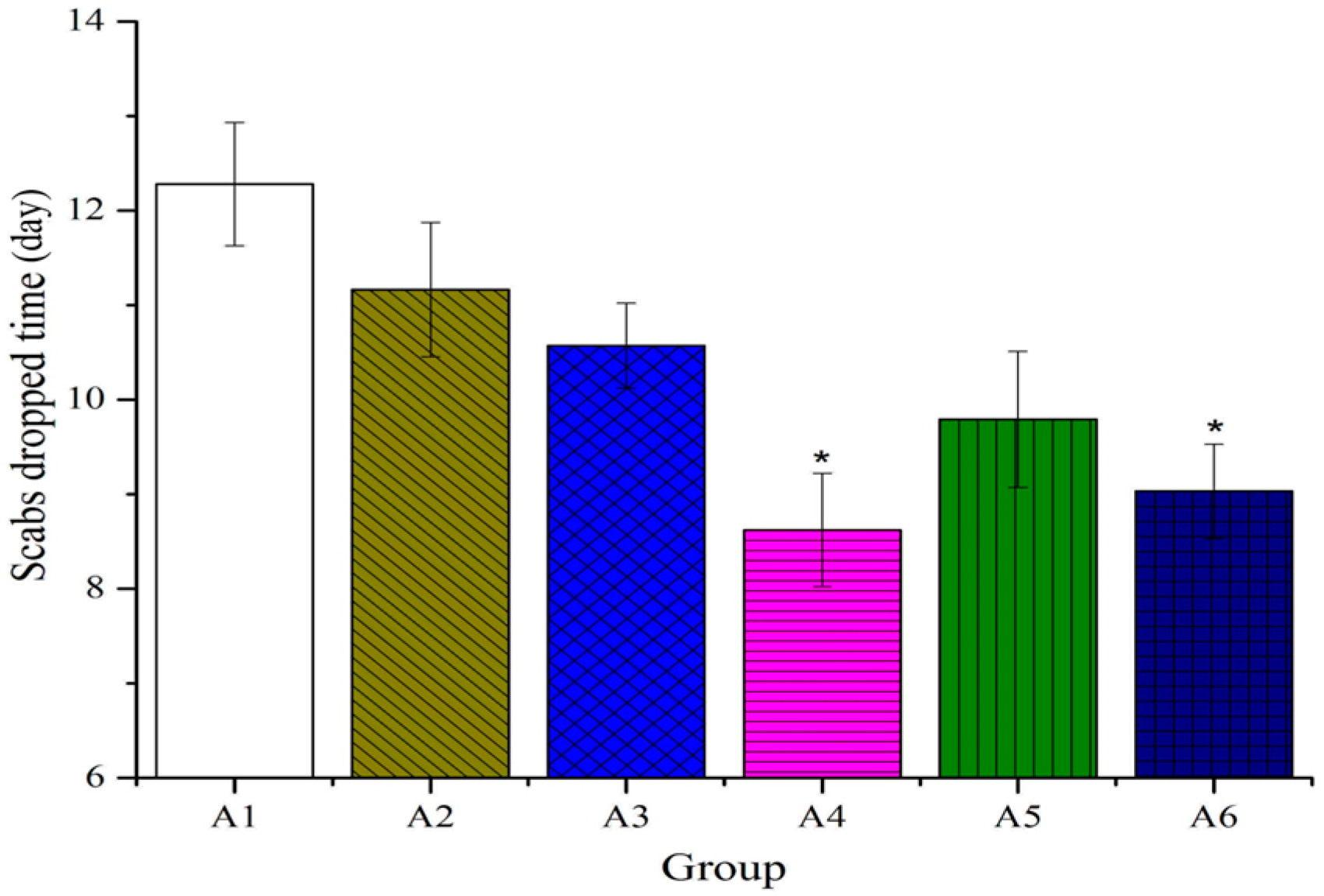
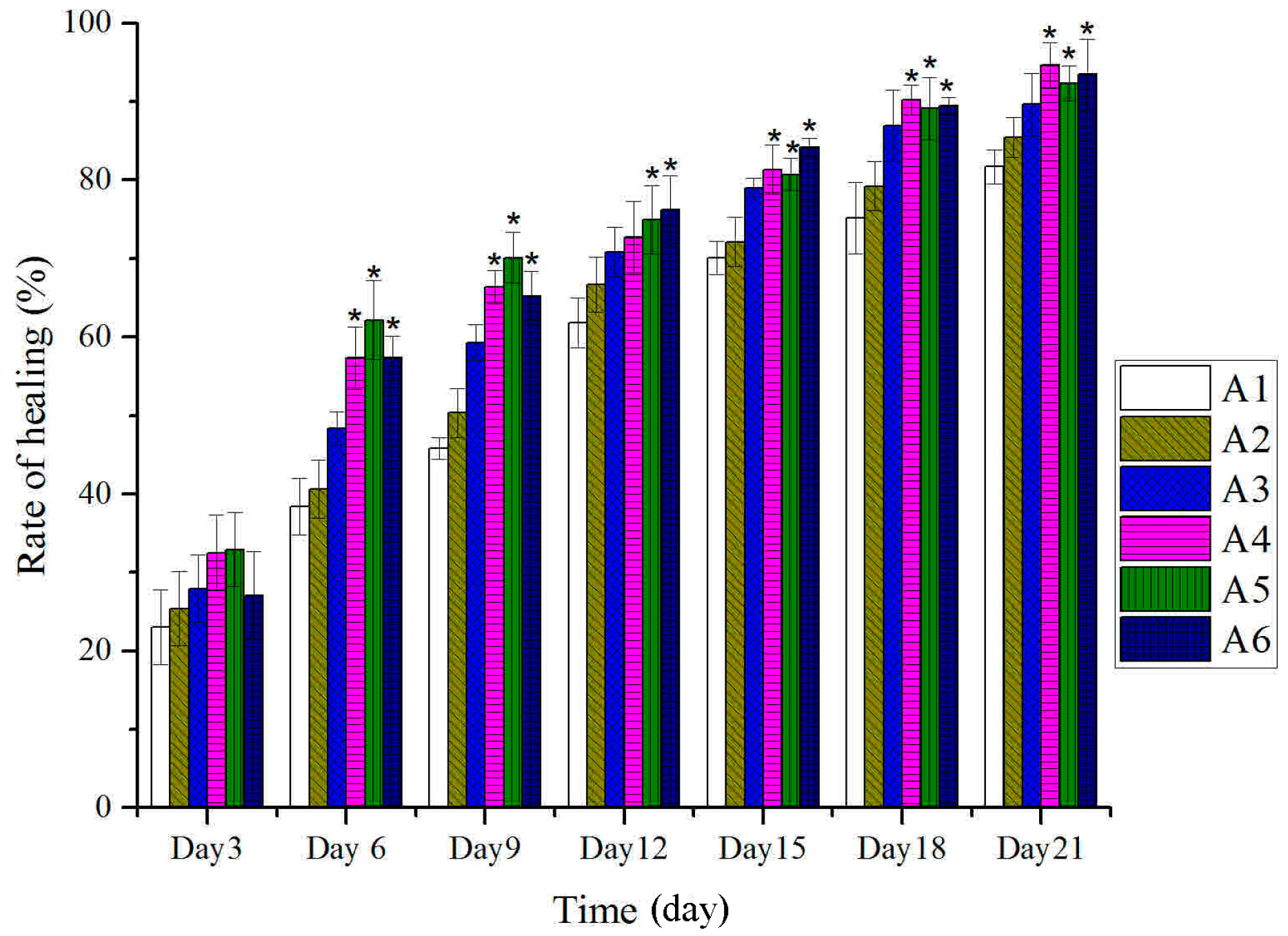
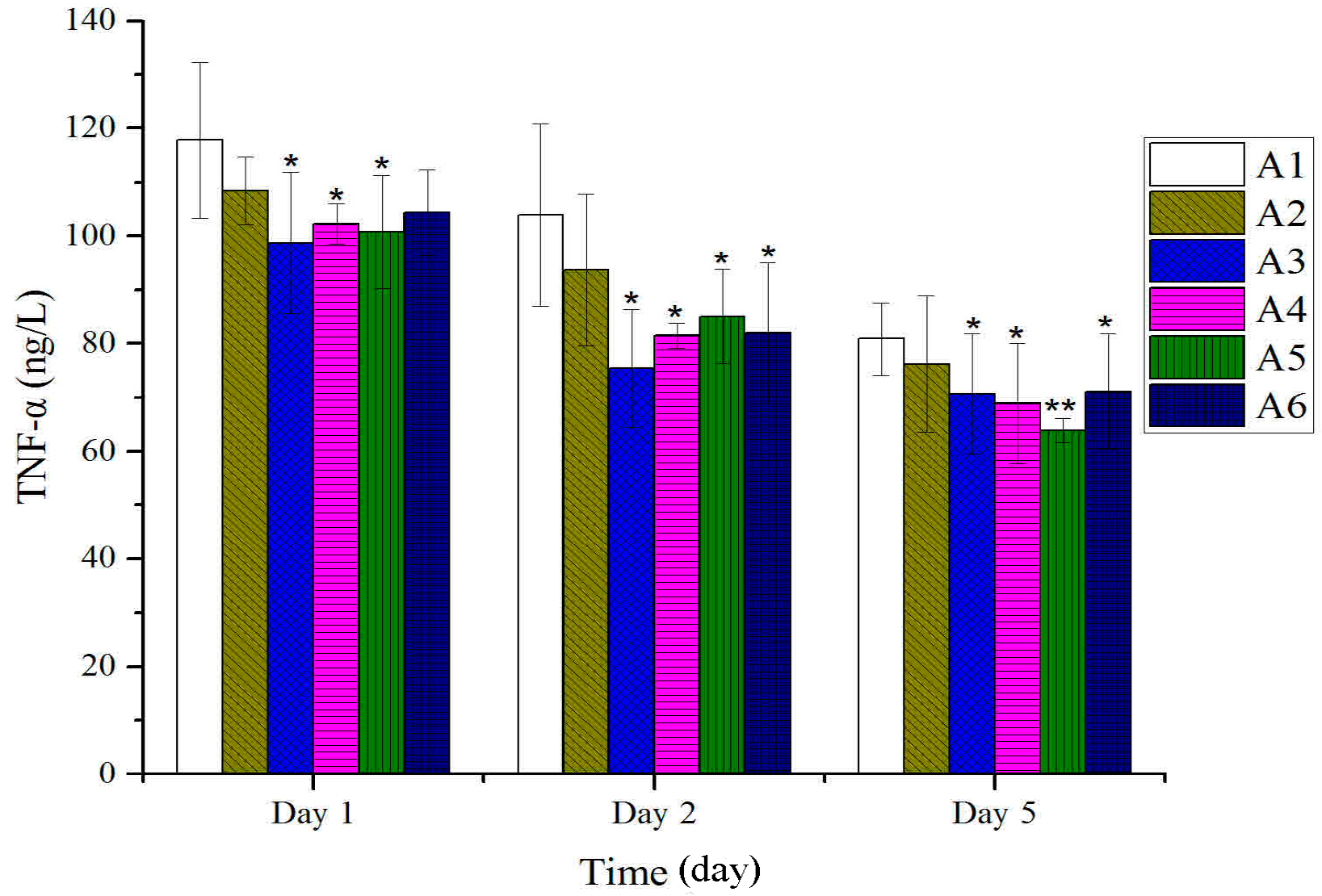
2.2. Effect of Different Treated Groups of BBO on Plasma IL-1 and TNF-α Level in Rats

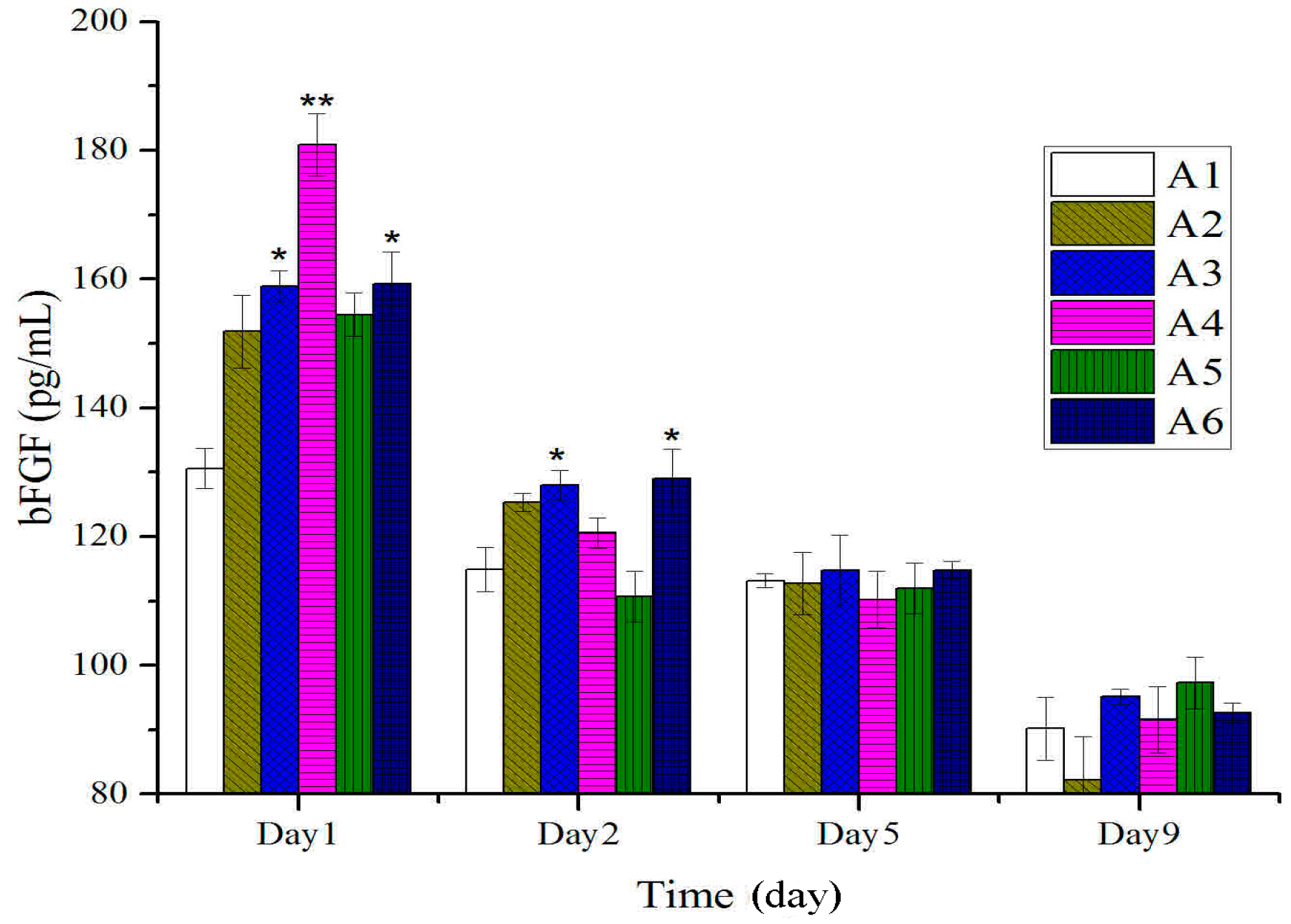
2.3. Expressions of bFGF in Tissues after Treatment with BBO in Rats
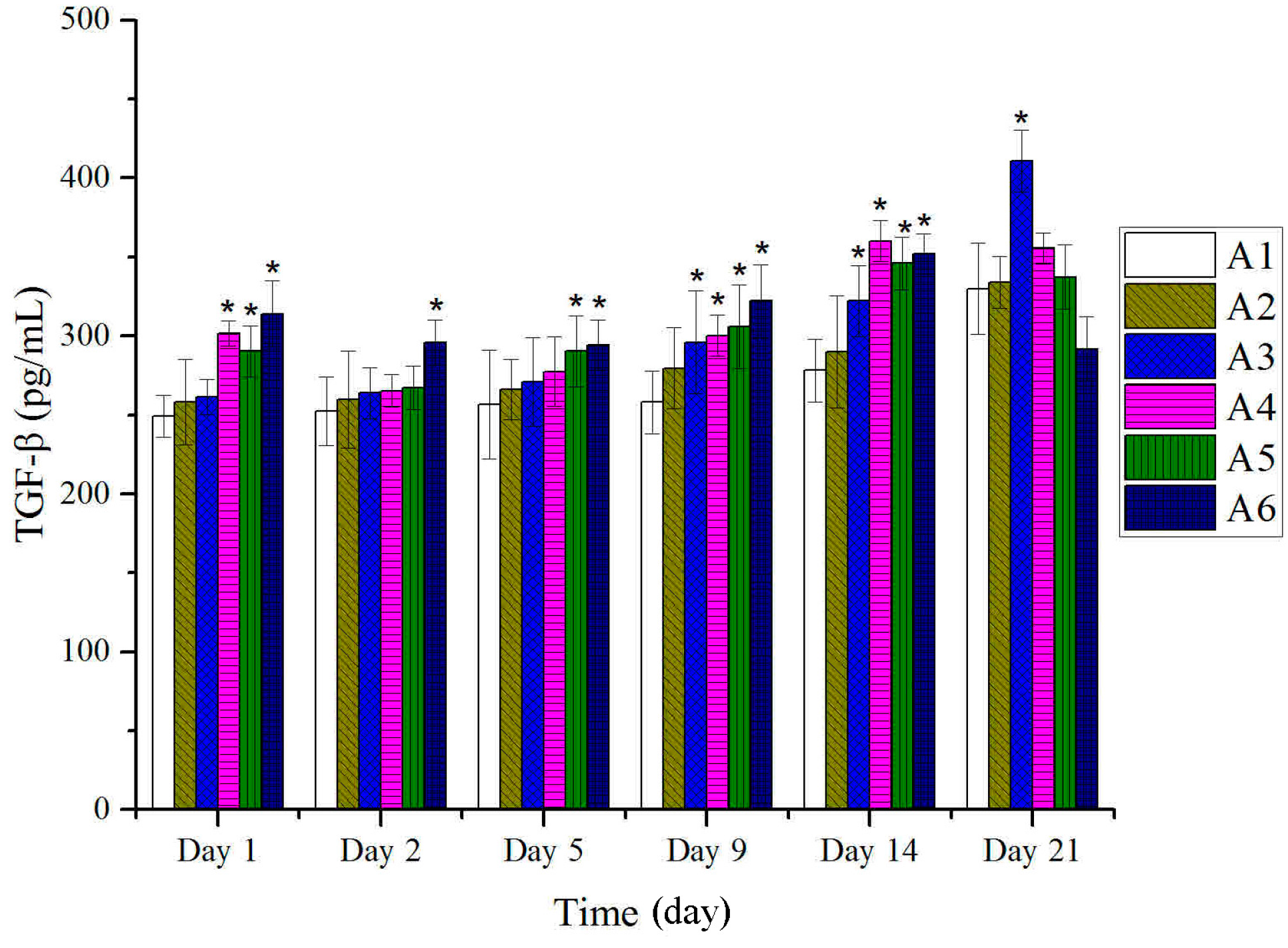
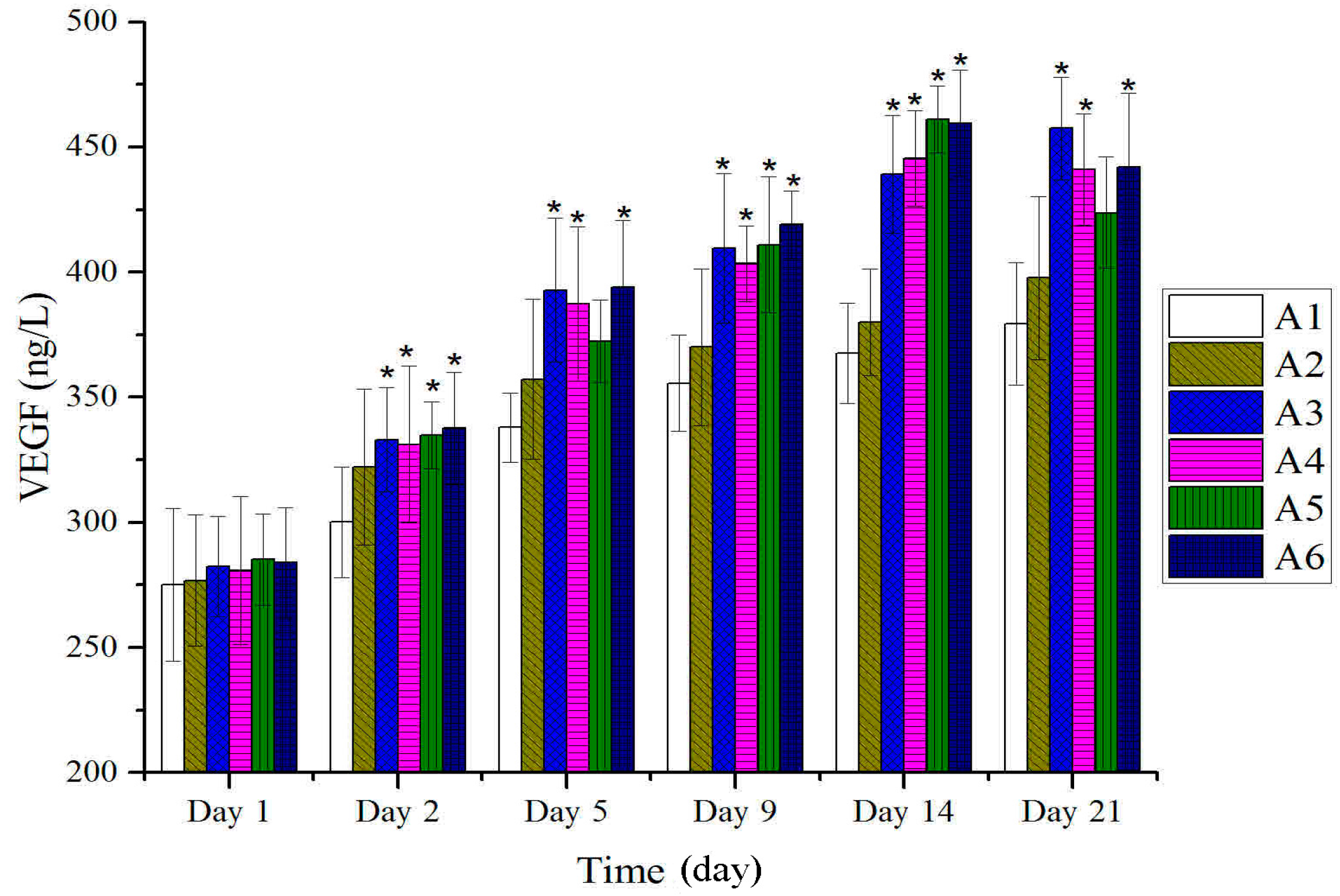
2.4. Expressions of TGF-β and VEGF in Tissues after Treatment with BBO in Rats
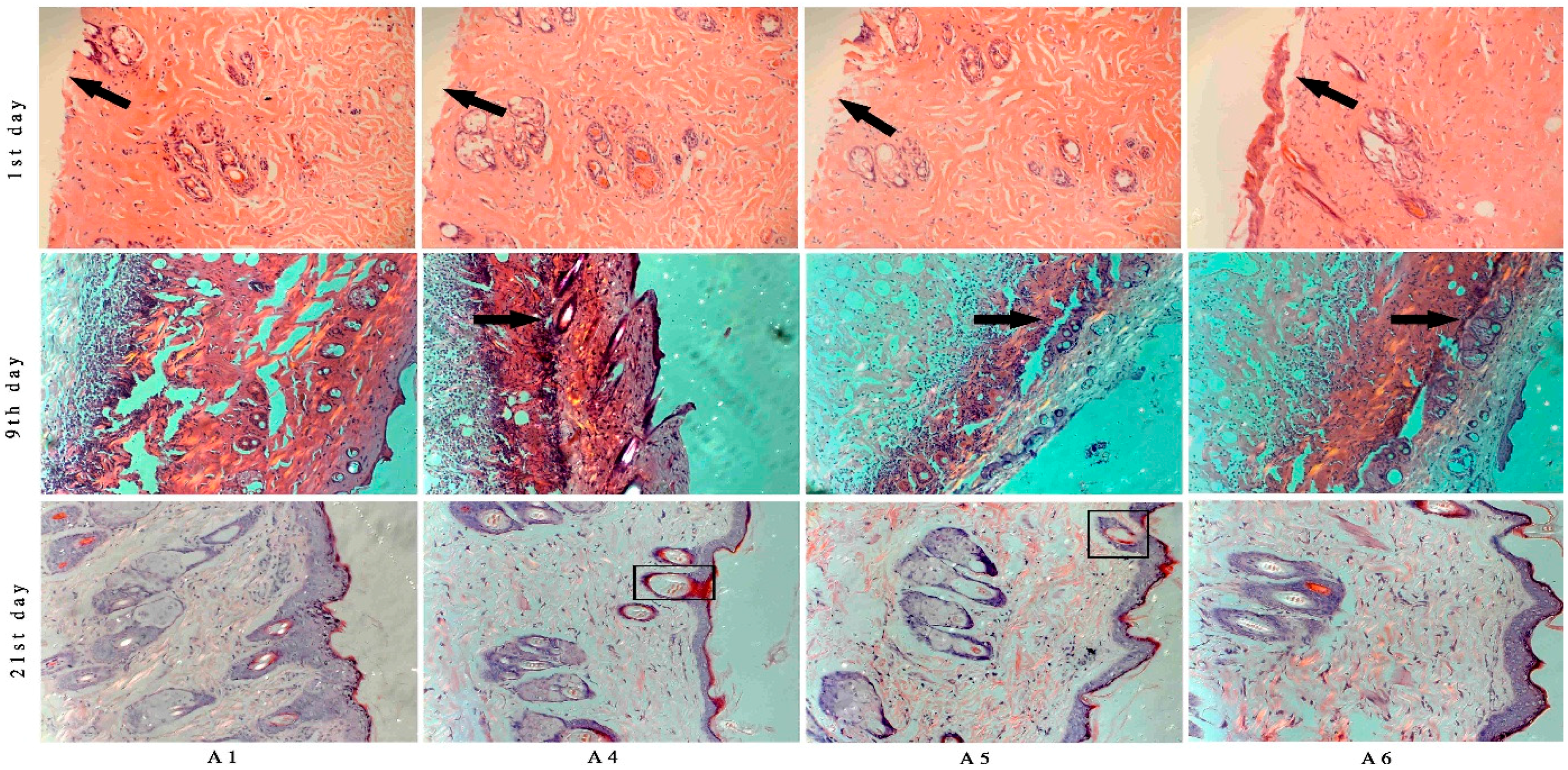
2.5. Histological Analysis
2.6. Discussion
3. Experimental Section
3.1. Materials
3.2. Experimental Animals
3.3. Animal Model
| Group No. | Treatments | Doses (mg/kg) |
|---|---|---|
| A1 | Control | 0 |
| A2 | 80% ethanol (vehicle) | 1500 |
| A3 | Moist exposed burn ointment (standard) | 1500 |
| A4 | BBO (100%) | 1500 |
| A5 | BBO (50%) | 1500 |
| A6 | BBO (10%) | 1500 |
3.4. General Observation
3.5. Sample Collection
3.6. Clinical Chemistry
3.7. Histopathologic Evaluation
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wasserman, D. Criteria for burn severity: Epidemiology, prevention, organization of management. Pathol. Biol. 2002, 50, 65–73. [Google Scholar]
- Ma, X.L. The experience of nursing the severe burn patients with sepsis. Chin. J. Med. Guide 2012, 3, 481. (In Chinese) [Google Scholar] [CrossRef]
- Li, A. The Treatment of Burns, 2nd ed.; People’s Medical Publishing House: Beijing, China, 1995. [Google Scholar]
- Tyack, Z.F.; Ziviani, J. What influences the functional outcome of children at 6 months post-burn? Burns 2003, 29, 433–444. [Google Scholar] [CrossRef]
- Griffiths, H.R.; Thornton, K.L.; Clements, C.M.; Burge, T.S.; Kay, A.R.; Young, A.E. The cost of a hot drink scald. Burns 2006, 32, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N. Effect of silver on burn wound infection control and healing: Review of the literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Z.; Abu, S.F.; Chong, N.J. A systematic review of silver-containing dressings and topical silver agents (used with dressings) for burn wonds. Burns 2012, 38, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.F.; Cuttle, L.; Kempf, M. Cytotoxicity of topical anti-microbial agents used in burn wounds in Australasia. ANZ J. Surg. 2004, 74, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Kearns, R.D.; Cairns, C.B.; Holmes, J.H.; Rich, P.B.; Cairns, B.A. Chemical burn care: A review of best practices. EMS World 2014, 43, 40–45. [Google Scholar] [PubMed]
- Rowan, M.P.; Cancio, L.C.; Burmeister, D.M.; Rose, L.F.; Natesan, S.; Chan, R.K.; Christy, R.J.; Chung, K.K. Burn wound healing and treatment: Review and advancements. Crit. Care 2015, 19, 243. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Upadhyay, N.K.; Sawhney, R.C.; Kumar, R. A poly herbal formulation accelerates normal and impaired diabetic wound healing. Wound Repair Regen. 2008, 16, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Udupa, S.; Udupa, L. Evaluation of antioxidant and wound healing effects of alcoholic and aqueous extract of Ocimum sanctum Linn in rats. Evid. Based Complement. Altern. Med. 2008, 5, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.S.; Sandiford, S.; Maxwell, A. Evaluation of the wound- healing activity of ethanolic extract of Morinda citrifolia L. leaf. Evid. Based Complement. Altern. Med. 2009, 6, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.T.; Hughes, M.A.; Cherry, G.W. Enhanced proliferation of fibroblasts and endothelial cells treated with an extract of the leaves of Chromolaena odorata (Eupolin), an herbal remedy for treating wounds. Plast. Reconstr. Surg. 1998, 101, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.X.; Wang, W.Q.; Mo, T.H.; Yuan, Y. Clonal diversity and structure in natrual populations of Blumea balsamifera. Guihaia 2010, 2, 13–15. (In Chinese) [Google Scholar]
- Yuan, Y.; Pang, Y.X.; Wang, W.Q.; Yu, J.B. Investigation on the plants resources of Blumea balsamifera (L.) DC. in China. J. Trop. Org. 2011, 2, 78–82. (In Chinese) [Google Scholar]
- Guan, L.L.; Pang, Y.X.; Wang, D.; Wu, K.Y. Research progress on Chinese minority medicine of Blumea balsamifera L. DC. J. Plant Genet. Res. 2012, 4, 695–698. [Google Scholar]
- Jiangsu New Medical College. Dictionary of Chinese Materia Medica; Shanghai Science and Technology Press: Shanghai, China, 1977. [Google Scholar]
- Guizhou Institute of Traditional Chinese Medicine. Guizhou Chinese Medicine Resources; Chinese Medical Science Press: Beijing, China, 1992; p. 262. [Google Scholar]
- Li, J.; Zhao, G.Z.; Chen, H.H.; Wang, H.B.; Qin, S.; Zhu, W.Y.; Xu, L.H.; Jiang, C.L.; Li, W.J. Antitumour and antimicrobial activities of endophytic streptomycetes from pharmaceutical plants in rainforest. Lett. Appl. Microbiol. 2008, 47, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Osaki, N.; Koyano, T.; Kowithayakorn, T.; Hayashi, M.; Komiyama, K.; Ishibashi, M. Sesquiterpenoids and plasmin-inhibitory flavonoids from Blumea balsamifera. J. Nat. Prod. 2005, 68, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Fazilatun, N.; Nornisah, M.; Zhari, I. Superoxide radical scavenging properties of extracts and flavonoids isolated from the leaves of Blumea balsamifera. Pharm. Biol. 2005, 43, 15–20. [Google Scholar] [CrossRef]
- Chen, M.; Qin, J.J.; Fu, J.J.; Hu, X.J.; Liu, X.H.; Zhang, W.D.; Jin, H.Z. Blumeaenes A-J, sesquiterpenoid esters from Blumea balsamifera with NO inhibitory activity. Planta Med. 2010, 76, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Yamada, Y.; Komiyama, K.; Hayashi, M.; Ishibashi, M.; Yoshida, T.; Koyano, T.; Kam, T.S.; Murata, K.; Sugahara, K.; et al. Dihydroflavonol BB-1, an extract of natural plant Blumea balsamifera, abrogates TRAIL resistance in leukemia cells. Blood 2006, 107, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Norikura, T.; Kojima-Yuasa, A.; Shimizu, M.; Huang, X.D.; Xu, S.H.; Kametani, S.; Rho, S.N.; Kennedy, D.O.; Matsui-Yuasa, I. Anticancer activities and mechanisms of Blumea balsamifera extract in hepatocellular carcinoma Cells. Am. J. Chin. Med. 2008, 36, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, X.S.; Zhao, C. Chemical components of volatile oil from foliumet caumen Blumea balsamifera originated from Guizhou. J. Instrum. Anal. 2001, 20, 76–78. (In Chinese) [Google Scholar]
- Md Nazrul, I.B.; Jasim, U.C.; Jaripa, B. Chemical components in volatile oil from Blumea balsamifera (L.) DC. Bangladesh J. Bot. 2009, 38, 107–109. [Google Scholar]
- Pang, Y.X.; Fan, Z.W.; Wang, D.; Yang, Q.; Wang, K.; Chen, X.L.; Hu, X.; Yu, F.L.; Chen, Z.X. External application of the volatile oil from Blumea balsamifera may be safe for liver—A study on its chemical composition and hepatotoxicity. Molecules 2014, 19, 18479–18492. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2010; p. 82. [Google Scholar]
- Pang, Y.X.; Wang, D.; Fan, Z.W.; Chen, X.L.; Yu, F.L.; Hu, X.; Wang, K.; Yuan, L. Blumea balsamifera—A phytochemical and pharmacological review. Molecules 2014, 19, 9453–9477. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.X.; Wang, D.; Hu, X.; Wang, H.; Fu, W.; Fan, Z.W.; Chen, X.L.; Yu, F.L. Effect of volatile oil from Blumea balsamifera (L.) DC. leaves on wound healing in mice. J. Tradit. Chin. Med. 2014, 34, 716–724. [Google Scholar] [CrossRef]
- Dorsett-Martin, W.A. Rat models of skin wound healing: A review. Wound Repair Regen. 2004, 12, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Jandera, V.; Hudson, D.A.; de Wet, P.M.; Innes, P.M.; Rode, H. Cooling the burn wound: Evaluation of different modalities. Bums 2000, 26, 265–270. [Google Scholar] [CrossRef]
- Thomas, G.W.; Rael, L.T.; Bar-Or, R.; Shimonkevitz, R.; Mains, C.W.; Slone, D.S.; Craun, M.L.; Bar-Or, D. Mechanisms of delayed wound healing by commonly used antiseptics. J. Trauma 2009, 66, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Cho Lee, A.R.; Leem, H.; Lee, J.; Park, K.C. Reversal of silver sulfadiazine-impaired wound healing by epidermal growth factor. Biomaterials 2005, 26, 4670–4676. [Google Scholar] [CrossRef] [PubMed]
- Arturson, G. Pathophysiology of the burn wound and pharmacological treatment. The Rudi Hermans lecture, 1995. Burns 1996, 22, 255–274. [Google Scholar] [CrossRef]
- Lootens, L.; Brusselaers, N.; Beele, H.; Monstrey, S. Keratinocytes in the treatment of severe burn injury: An update. Int. Wound J. 2012, 10, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Eigenbrod, T.; Nunez, G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J. Immuol. 2009, 183, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.; Cahill, C.M. TNF-alpha and neuropathic pain—A review. J. Neuroinflamm. 2010, 27, 2–11. [Google Scholar]
- Ashcroft, G.S.; Jeong, M.J.; Ashworth, J.J.; Hardman, M.; Jin, W.; Moutsopoulos, N.; Wild, T.; McCartney-Francis, N.; Sim, D.; McGrady, G.; et al. Tumor necrosis factor-alpha (TNF-α) is a therapeutic target for impaired cutaneous wound healing. Wound Repair Regen. 2012, 20, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Yoo, G. Efects of adipose-derived stromal cells and of their extract on wound healing in a mouse model. J. Korean Med. Sci. 2010, 25, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Akita, S.; Akino, K.; Imaizumi, T.; Hirana, A. A basic fibroblast growth factor improved the quality of skin grafting in burn patient. Burns 2005, 31, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-fibroblast interactions in wound healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Desmouliere, A.; Geinoz, A.; Gabbiani, F.; Gabbiani, G. Transforming growth factor-beta 1 induces alpha-smooth muscle act in expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 1993, 122, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.B.; Huh, C.G.; Becker, D.; Geiser, A.; Lyght, M.; Flanders, K.C.; Roberts, A.B.; Sporn, M.B.; Ward, J.M.; Karlsson, S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl. Acad. Sci. USA 1993, 90, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.W.; Cachianes, G.; Kuang, W.J.; Goeddel, D.V.; Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989, 246, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
- Nissen, N.N.; Polverini, P.J.; Koch, A.E.; Volin, M.V.; Gamelli, R.L.; DiPietro, L.A. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am. J. Pathol. 1998, 152, 1445–1452. [Google Scholar] [PubMed]
- Infanger, M.; Schmidt, O.; Kossmehl, P.; Grad, S.; Ertel, W.; Grimm, D. Vascular endothelial growth factor serum level is strongly enhanced after burn injury and correlated with local and general tissue edema. Burns 2004, 30, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Corral, C.J.; Siddiqui, A.; Wu, L.; Farrell, C.L.; Lyons, D.; Mustoe, T.A. Vascular endothelial growth factor is more important than basic fibroblastic growth factor during is chemic wound healing. Arch. Surg. 1999, 134, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.C.; Miguel, C.; Domingues, I.; Calado, A.; Zhu, Z.; Wu, Y.; Dias, S. VEGF and VEGFR-2 (KDR) internalization is required for endothelial recovery during wound healing. Exp. Cell Res. 2007, 313, 1561–1574. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.H.; Lin, S.; Xiao, D.; Zheng, X.; Gu, Y.; Guo, S. Evaluation of the wound healing potential of resina Draconis (Dracaena cochinchinensis) in animal models. Evid. Based Complement. Altern. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Wilgus, T.A. Immune cells in the healing skin wound: Influential players at each stage of repair. Pharmacol. Res. 2008, 58, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.L.; Zhou, Y.; Ge, W.C.; Yuan, K. Phytochemical compositions of volatile oil form Blumea balsamifera and their biological activities. Pharmacogn. Mag. 2014, 10, 346–352. [Google Scholar] [PubMed]
- Fu, W.J.; Wang, D.; Pang, Y.X.; Wang, D.; Huang, Z.; Nie, H.; Yu, F.L.; Zhang, Y.B. Effect of Blumea balsamifera oil on percutaneous absorption of salbutamol sulfate. Chin. J. Exp. Tradit. Med. Formula 2013, 19, 174–177. (In Chinese) [Google Scholar]
- Almeida, J.R.; Souza, G.R.; Silva, J.C.; Saraiva, S.R.; Junior, R.G.; Quintans, J.S.; Barrrto, R.S.; Bonjardim, L.R.; Cavalcanti, S.C.; Quintans, L.J., Jr. Borneol, a bicyclic monoterpene alcohol, reduces nociceptive behavior and inflammatory response in mice. Sci. World J. 2013. [Google Scholar] [CrossRef] [PubMed]
- Chien, T.C.; Lo, S.F.; Ho, C.L. Chemical composition and anti-inflammatory activity of Chamaecyparis obtuse f.formasana wood essential oil from Taiwan. Nat. Prod. Commun. 2014, 9, 723–729. [Google Scholar] [PubMed]
- Wu, H.Y.; Tang, Y.; Gao, L.Y.; Sun, W.X.; Yang, S.B.; Zhang, Z.P.; Liao, Q.G.; Luo, C.X.; Zhu, D.Y. The synergetic effect of edaravone and borneol in the rat model of ischemic stroke. Eur. J. Pharmacol. 2014, 740, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zuo, J.; Li, X. Chen, J.W. Anti-arthritic and anti-oxidative effect of ethyl acetate fraction of Blumea balsamlfera residues in rat adjuvant-induced arthritis. Zhongguo Zhong Yao Za Zhi 2014, 39, 3819–3823. [Google Scholar] [PubMed]
- Su, Y.; Zhu, W.J.; Fang, M.; Zhou, L.L. Study on protective effect and mechanism of Fufang Tangshang Gao on deep grade II skin burn in rats. Zhongguo Zhong Yao Za Zhi 2012, 37, 2143–2146. (In Chinese) [Google Scholar] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Z.-W.; Pang, Y.-X.; Wang, K.; Yu, F.-L.; Wang, D.; Yang, Q.; Ma, Q.-S.; Li, X.-T.; Zou, J.; Zhang, W.-Q.; et al. Blumea balsamifera Oil for the Acceleration of Healing of Burn Injuries. Molecules 2015, 20, 17166-17179. https://doi.org/10.3390/molecules200917166
Fan Z-W, Pang Y-X, Wang K, Yu F-L, Wang D, Yang Q, Ma Q-S, Li X-T, Zou J, Zhang W-Q, et al. Blumea balsamifera Oil for the Acceleration of Healing of Burn Injuries. Molecules. 2015; 20(9):17166-17179. https://doi.org/10.3390/molecules200917166
Chicago/Turabian StyleFan, Zuo-Wang, Yu-Xin Pang, Kai Wang, Fu-Lai Yu, Dan Wang, Quan Yang, Qing-Song Ma, Xiao-Ting Li, Jin Zou, Wen-Qing Zhang, and et al. 2015. "Blumea balsamifera Oil for the Acceleration of Healing of Burn Injuries" Molecules 20, no. 9: 17166-17179. https://doi.org/10.3390/molecules200917166





