pH-Dependence of the Aqueous Phase Room Temperature Brønsted Acid-Catalyzed Chemoselective Oxidation of Sulfides with H2O2
Abstract
:1. Introduction
2. Results and Discussion

| Entry | Catalyst Loading (%) | Conversion (%) b | Yield (%) b |
|---|---|---|---|
| 1 | 0.0 | 34.1 | 34.1 |
| 2 | 2.5 | 41.6 | 41.5 |
| 3 | 5.0 | 51.8 | 51.5 |
| 4 | 7.5 | 56.2 | 56.0 |
| 5 | 10.0 | 59.5 | 59.2 |
| 6 | 12.5 | 64.4 | 64.0 |
| 7 | 15.0 | 79.4 | 78.8 |
| 8 | 17.5 | 84.5 | 83.9 |
| 9 | 20.0 | 93.4 | 92.7 |
| 10 | 22.5 | 97.6 | 96.8 |
| 11 | 25.0 | 99.2 | 98.3 |

| Entry | Brønsted Acid | Amount (mmol) | pH Value b | Conversion (%) c | Yield (%) c |
|---|---|---|---|---|---|
| 1 |  | 0.25 | 1.28 | 99.6 | 99.3 |
| 2 | CH3SO3H | 0.25 | 1.25 | 99.7 | 98.8 |
| 3 | 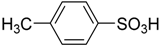 | 0.25 | 1.30 | 99.7 | 98.8 |
| 4 | HCOOH | 4.00 | 1.31 | 99.1 | 98.6 |
| 5 | CH3COOH | 5.50 | 1.35 | 99.1 | 98.9 |

| Entry | pH Value b | Conversion (%) c | Yield (%) c |
|---|---|---|---|
| 1 | 2.00 | 42.6 | 42.6 |
| 2 | 1.90 | 49.5 | 49.2 |
| 3 | 1.80 | 58.1 | 57.9 |
| 4 | 1.70 | 66.9 | 66.4 |
| 5 | 1.60 | 72.1 | 71.4 |
| 6 | 1.50 | 77.9 | 77.1 |
| 7 | 1.40 | 98.1 | 96.3 |
| 8 | 1.30 | 99.6 | 98.9 |
| 9 | 1.20 | 99.8 | 98.6 |
| 10 | 1.10 | 99.6 | 97.2 |
| 11 | 1.00 | 99.4 | 96.5 |
| Entry | Brønsted Acid | Conversion (%) c | Yield (%) c |
|---|---|---|---|
| 1 |  | 99.6 | 98.9 |
| 2 | CH3SO3H | 99.8 | 98.7 |
| 3 |  | 99.7 | 98.5 |
| 4 | 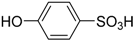 | 98.7 | 97.9 |
| 5 | HCOOH | 99.6 | 99.1 |
| 6 | CH3COOH | 99.2 | 98.6 |
| 7 | HOOCCOOH | 99.7 | 98.3 |
| 8 |  | 99.8 | 98.5 |
| 9 | CF3COOH | 98.8 | 97.7 |
| 10 | H2SO4 | 99.6 | 99.1 |
| 11 | HCL | 99.7 | 98.8 |
| 12 | H3PO4 | 99.5 | 98.9 |
| 13 | H3BO3 | 99.6 | 98.7 |
| 14 | 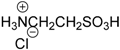 | 99.6 | 98.8 |
| 15 | 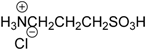 | 99.7 | 98.5 |
| Entry | Sulfide | Conversion (%) c | Yield (%) c |
|---|---|---|---|
| 1 | 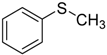 | 99.6 | 98.9 |
| 2 | 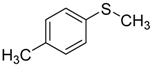 | 98.7 | 97.9 |
| 3 |  | 99.9 | 99.4 |
| 4 | 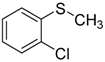 | 99.4 | 98.8 |
| 5 d | 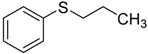 | 98.5 | 97.6 |
| 6 d |  | 98.1 | 97.4 |
| 7 d | 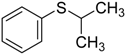 | 98.7 | 98.2 |
| 8 |  | 99.5 | 99.1 |
| 9 | 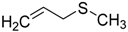 | 99.9 | 99.4 |
| 10 d | 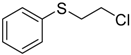 | 98.7 | 97.9 |
| 11 d |  | 96.8 | 95.6 |
| 12 |  | 99.2 | 98.6 |
3. Experimental Section
3.1. General Information
3.2. Materials
3.3. General Procedure for Oxidation of Sulfides Catalyzed by Brønsted Acid (1)
3.4. General Procedure for Oxidation of Sulfides Catalyzed by Brønsted Acid (2)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fernandez, I.; Khiar, N. Recent developments in the synthesis and utilization of chiral sulfoxides. Chem. Rev. 2003, 103, 3651–3705. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowska, K.; Kolarska, Z.; Mitka, K.; Kowalski, P. Oxidation of sulfides to sulfoxides. Part 2: Oxidation by hydrogen peroxide. Tetrahedron 2005, 61, 8315–8327. [Google Scholar] [CrossRef]
- Stingl, K.A.; Tsogoeva, S.B. Recent advances in sulfoxidation reactions: A metal-free approach. Tetrahedron: Asymmetry 2010, 21, 1055–1074. [Google Scholar] [CrossRef]
- Drabowicz, J.; Kielbasiński, P.; Zajac, A.; Wach-Panfilow, P. Synthesis of sulfides, sulfoxides and sulfones. In Comprehensive Organic Synthesis, 2nd ed.; Molander, G.A., Knochel, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 6, pp. 131–174. [Google Scholar]
- Drabowicz, J.; Kielbasinski, P.; Mikolajczyk, M. Synthesis of sulfoxides. In Syntheses of Sulphones, Sulphoxides and Cyclic Sulphides; Patai, S., Rappoport, Z., Eds.; Wiley: West Sussex, UK, 1994; Volume 3, pp. 109–254. [Google Scholar]
- Drabowicz, J.; Kielbasinski, P.; Mikolajczyk, M. Synthesis of sulfoxides. In Syntheses of Sulphones, Sulphoxides and Cyclic Sulphides; Patai, S., Rappoport, Z., Eds.; Wiley: West Sussex, UK, 1994; Volume 4, pp. 255–388. [Google Scholar]
- Drabowicz, J.; Mikolajczyk, M. Synthesis of sulfoxides. A review. Org. Prep. Proced. Int. 1982, 14, 45–89. [Google Scholar] [CrossRef]
- Le Maux, P.; Simonneaux, G. First enantioselective iron-porphyrin-catalyzed sulfide oxidation with aqueous hydrogen peroxide. Chem. Commun. 2011, 47, 6957–6959. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.M.; Roy, A.S.; Mondal, P.; Tuhina, K.; Mobarak, M.; Mondal, J. Selective oxidation of sulfides and oxidative bromination of organic substrates catalyzed by polymer anchored Cu(II) complex. Tetrahedron Lett. 2012, 53, 127–131. [Google Scholar] [CrossRef]
- Secci, F.; Frongia, A.; Piras, P.P. Ammonium salt catalyzed oxidation of organosulfides to organosulfoxydes. Tetrahedron Lett. 2014, 55, 603–605. [Google Scholar] [CrossRef]
- Gogoi, P.; Kalita, M.; Bhattacharjee, T.; Barman, P. Copper-Schiff base complex catalyzed oxidation of sulfides with hydrogen peroxide. Tetrahedron Lett. 2014, 55, 1028–1030. [Google Scholar] [CrossRef]
- Kon, Y.; Yokoi, T.; Yoshioka, M.; Tanaka, S.; Uesaka, Y.; Mochizuki, T.; Sato, K.; Tatsumi, T. Selective hydrogen peroxide oxidation of sulfides to sulfoxides or sulfones with MWW-type titanosilicate zeolite catalyst under organic solvent-free conditions. Tetrahedron 2014, 70, 7584–7592. [Google Scholar] [CrossRef]
- Huang, L.; Wang, S.S.; Zhao, J.W.; Cheng, L.; Yang, G.Y. Synergistic combination of multi-ZrIV cations and lacunary keggin germanotungstates leading to a gigantic Zr24-cluster-substituted polyoxometalate. J. Am. Chem. Soc. 2014, 136, 7637–7642. [Google Scholar] [CrossRef] [PubMed]
- Mirkhani, V.; Moghadam, M.; Tangestaninejad, S.; Mohammdpoor-Baltork, I.; Kargar, H.; Araghi, M. Highly efficient oxidation of sulfides with sodium periodate catalyzed by reusable silica supported Mn(Br8TPP)Cl and Mn(TPP)Cl catalysts under various reaction conditions. Appl. Catal. A Gen. 2009, 353, 61–67. [Google Scholar] [CrossRef]
- Rezaeifard, A.; Jafarpour, M. The catalytic efficiency of Fe-porphyrins supported on multi-walled carbon nanotubes in the heterogeneous oxidation of hydrocarbons and sulfides in water. Catal. Sci. Tech. 2014, 4, 1960–1969. [Google Scholar] [CrossRef]
- Hu, Y.L.; Fang, D.; Xing, R. Efficient and convenient oxidation of sulfides to sulfoxides with molecular oxygen catalyzed by Mn(OAc)2 in ionic liquid [C12mim][NO3]. RSC Adv. 2014, 4, 51140–51145. [Google Scholar] [CrossRef]
- Wang, Z.J.; Ghasimi, S.; Landfester, K.; Zhang, K.A.I. Highly porous conjugated polymers for selective oxidation of organic sulfides under visible light. Chem. Commun. 2014, 50, 8177–8180. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Zhang, X.; Reeson, T.C.; Chen, Y.S.; Zhang, J. Facile Control of the charge density and photocatalytic activity of an anionic indium porphyrin framework via in situ metalation. J. Am. Chem. Soc. 2014, 136, 15881–15884. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Li, F.M.; Zhao, W.L.; Tian, Y.H.; Chen, Y.; Cai, R.; Fu, W.F. Highly efficient and selective photocatalytic oxidation of sulfide by a chromophore-catalyst dyad of ruthenium-based complexes. Inorg. Chem. 2015, 54, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Chakraborty, D. Cu(II)-catalyzed oxidation of sulfides. Tetrahedron Lett. 2010, 51, 6255–6258. [Google Scholar] [CrossRef]
- Oble, J.; Riflade, B.; Noel, A.; Malacria, M.; Thorimbert, S.; Hasenknopf, B.; Lacote, E. Carbonyl-inserted organo-hybrids of a dawson-type phosphovanadotungstate: Scope and chemoselective oxidation catalysis. Org. Lett. 2011, 13, 5990–5993. [Google Scholar] [CrossRef] [PubMed]
- Rezaeifard, A.; Farshid, P.; Jafarpour, M.; Moghaddam, G.K. Silica-coated magnetite nanoparticles stabilized simple Mn-tetraphenylporphyrin for aqueous phase catalytic oxidations with tert-butyl hydroperoxide. RSC Adv. 2014, 4, 9189–9196. [Google Scholar] [CrossRef]
- Orive, J.; Larrea, E.S.; de Luis, R.F.; Iglesias, M.; Mesa, J.L.; Rojo, T.; Arriortua, M.I. Amine templated open-framework vanadium(III) phosphites with catalytic properties. Dalton Trans. 2013, 42, 4500–4512. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.H.; Guo, H.C.; Liu, S.Z.; Wang, M. Efficient soluble polymer-supported tartrate/Ti catalyst for asymmetric oxidation of prochiral sulfides. Tetrahedron Lett. 2007, 48, 8453–8455. [Google Scholar] [CrossRef]
- Pordea, A.; Creus, M.; Panek, J.; Duboc, C.; Mathis, D.; Novic, M.; Ward, T.R. Artificial metalloenzyme for enantioselective sulfoxidation based on vanadyl-loaded streptavidin. J. Am. Chem. Soc. 2008, 130, 8085–8088. [Google Scholar] [CrossRef] [PubMed]
- Mba, M.; Prins, L.J.; Zonta, C.; Cametti, M.; Valkonen, A.; Rissanen, K.; Licini, G. Ti(IV)-amino triphenolate complexes as effective catalysts for sulfoxidation. Dalton Trans. 2010, 39, 7384–7392. [Google Scholar] [CrossRef] [PubMed]
- Romanowski, G. Synthesis, characterization and catalytic activity in the oxidation of sulfides and styrene of vanadium(V) complexes with tridentate Schiff base ligands. J. Mol. Catal. A Chem. 2013, 368, 137–144. [Google Scholar] [CrossRef]
- Jaccob, M.; Comba, P.; Maurer, M.; Vadivelu, P.; Venuvanalingam, P. A combined experimental and computational study on the sulfoxidation by high-valent iron bispidine complexes. Dalton Trans. 2011, 40, 11276–11281. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Yuan, Z.B.; Carver, A.; Zhang, R. Visible light-promoted selective oxidation of sulfides to sulfoxides catalyzed by ruthenium porphyrins with iodobenzene diacetate. Appl. Catal. A Gen. 2014, 478, 275–282. [Google Scholar] [CrossRef]
- Yu, B.; Guo, C.X.; Zhong, C.L.; Diao, Z.F.; He, L.N. Metal-free chemoselective oxidation of sulfides by in situ generated Koser’s reagent in aqueous media. Tetrahedron Lett. 2014, 55, 1818–1821. [Google Scholar] [CrossRef]
- Khurana, J.M.; Nand, B. Aqueous sodium hypochlorite mediated chemoselective oxidation of chalcogenides to monoxides and dioxides by microwave exposure. Can. J. Chem. 2010, 88, 906–909. [Google Scholar] [CrossRef]
- Huang, J.Y.; Li, S.J.; Wang, Y.G. TEMPO-linked metalloporphyrins as efficient catalysts for selective oxidation of alcohols and sulfides. Tetrahedron Lett. 2006, 47, 5637–5640. [Google Scholar] [CrossRef]
- Alcon, M.J.; Corma, A.; Iglesias, M.; Sanchez, F. Homogeneous and encapsulated within the cavities of zeolite Y chiral manganese and copper complexes with C2-multidentate ligands as catalysts for the selective oxidation of sulphides to sulfoxides or sulfones. J. Mol. Catal. A Chem. 2002, 178, 253–266. [Google Scholar] [CrossRef]
- Yu, B.; Liu, A.H.; He, L.N.; Li, B.; Diao, Z.F.; Li, Y.N. Catalyst-free approach for solvent-dependent selective oxidation of organic sulfides with oxone. Green Chem. 2012, 14, 957–962. [Google Scholar] [CrossRef]
- Rostami, A.; Akradi, J. A highly efficient, green, rapid, and chemoselective oxidation of sulfides using hydrogen peroxide and boric acid as the catalyst under solvent-free conditions. Tetrahedron Lett. 2010, 51, 3501–3503. [Google Scholar] [CrossRef]
- Rostami, A.; Tahmasbi, B.; Abedi, F.; Shokri, Z. Magnetic nanoparticle immobilized N-propylsulfamic acid: The chemoselective, efficient, green and reusable nanocatalyst for oxidation of sulfides to sulfoxides using H2O2 under solvent-free conditions. J. Mol. Catal. A Chem. 2013, 378, 200–205. [Google Scholar] [CrossRef]
- Jeong, Y.C.; Huang, Y.D.; Choi, S.; Ahn, K.H. Synthesis of sterically controlled chiral beta-amino alcohols and their application to the catalytic asymmetric sulfoxidation of sulfides. Tetrahedron: Asymmetry 2005, 16, 3497–3501. [Google Scholar] [CrossRef]
- Aydin, A.E. Synthesis of novel beta-amino alcohols and their application in the catalytic asymmetric sulfoxidation of sulfides. Tetrahedron: Asymmetry 2013, 24, 444–448. [Google Scholar] [CrossRef]
- Romanowski, G.; Lis, T. Chiral oxidovanadium(V) complexes with tridentate Schiff bases derived from S-(+)-2-amino-1-propanol: Synthesis, structure, characterization and catalytic activity. Inorg. Chim. Acta 2013, 394, 627–634. [Google Scholar] [CrossRef]
- Adao, P.; Avecilla, F.; Bonchio, M.; Carraro, M.; Pessoa, J.C.; Correia, I. Titanium(IV)-salan catalysts for asymmetric sulfoxidation with hydrogen peroxide. Eur. J. Inorg. Chem. 2010, 2010, 5568–5578. [Google Scholar] [CrossRef]
- Nigel-Etinger, I.; Mahammed, A.; Gross, Z. Covalent versus non-covalent (biocatalytic) approaches for enantioselective sulfoxidation catalyzed by corrole metal complexes. Catal. Sci. Tech. 2011, 1, 578–581. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Iranpoor, N.; Jafari, A.A.; Riazymontazer, E. Metal-free chemoselective oxidation of sulfides to sulfoxides by hydrogen peroxide catalyzed by in situ generated dodecyl hydrogen sulfate in the absence of organic co-solvents. Adv. Synth. Catal. 2006, 348, 434–438. [Google Scholar] [CrossRef]
- Jafari, H.; Rostami, A.; Ahmad-Jangi, F.; Ghorbani-Choghamarani, A. Sulfamic acid-catalyzed oxidation of sulfides to sulfoxides and sulfones using H2O2: Green and chemoselective method. Synth. Commun. 2012, 42, 3150–3156. [Google Scholar] [CrossRef]
- Mokhtary, M.; Qandalee, M.; Niaki, M.R. Highly efficient selective oxygenation of sulfides to sulfoxides by oxalic acid dihydrate in the presence of H2O2. J. Chem. 2012, 9, 863–868. [Google Scholar] [CrossRef]
- Golchoubian, H.; Hosseinpoor, F. Effective oxidation of sulfides to sulfoxides with hydrogen peroxide under transition-metal-free conditions. Molecules 2007, 12, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Menova, P.; Kafka, F.; Dvorakova, H.; Gunnoo, S.; Sanda, M.; Cibulka, R. Pyrazinium salts as efficient organocatalysts of mild oxidations with hydrogen peroxide. Adv. Synth. Catal. 2011, 353, 865–870. [Google Scholar] [CrossRef]
- Zurek, J.; Cibulka, R.; Dvorakova, H.; Svoboda, J. N1,N10-Ethylene-bridged flavinium salts derived from l-valinol: Synthesis and catalytic activity in H2O2 oxidations. Tetrahedron Lett. 2010, 51, 1083–1086. [Google Scholar] [CrossRef]
- Marsh, B.J.; Carbery, D.R. Chemoselective sulfide oxidation mediated by bridged flavinium organocatalysts. Tetrahedron Lett. 2010, 51, 2362–2365. [Google Scholar] [CrossRef]
- Jurok, R.; Cibulka, R.; Dvorakova, H.; Hampl, F.; Hodacova, J. Planar chiral flavinium salts–Prospective catalysts for enantioselective sulfoxidation reactions. Eur. J. Org. Chem. 2010, 5217–5224. [Google Scholar] [CrossRef]
- Imada, Y.; Kitagawa, T.; Iwata, S.; Komiya, N.; Naota, T. Oxidation of sulfides with hydrogen peroxide catalyzed by synthetic flavin adducts with dendritic bis(acylamino)pyridines. Tetrahedron 2014, 70, 495–501. [Google Scholar] [CrossRef]
- Hartman, T.; Herzig, V.; Budesinsky, M.; Jindrich, J.; Cibulka, R.; Kraus, T. Flavin-cyclodextrin conjugates: Effect of the structure on the catalytic activity in enantioselective sulfoxidations. Tetrahedron Asymmetry 2012, 23, 1571–1583. [Google Scholar] [CrossRef]
- Zelenka, J.; Hartman, T.; Klimova, K.; Hampl, F.; Cibulka, R. Phase-transfer catalysis in oxidations based on the covalent bonding of hydrogen peroxide to amphiphilic flavinium salts. Chemcatchem 2014, 6, 2843–2846. [Google Scholar] [CrossRef]
- Menova, P.; Dvorakova, H.; Eigner, V.; Ludvik, J.; Cibulka, R. Electron-deficient alloxazinium salts: Efficient organocatalysts of mild and chemoselective sulfoxidations with hydrogen peroxide. Adv. Synth. Catal. 2013, 355, 3451–3462. [Google Scholar] [CrossRef]
- Mojr, V.; Herzig, V.; Budesinsky, M.; Cibulka, R.; Kraus, T. Flavin-cyclodextrin conjugates as catalysts of enantioselective sulfoxidations with hydrogen peroxide in aqueous media. Chem. Commun. 2010, 46, 7599–7601. [Google Scholar] [CrossRef] [PubMed]
- Sturala, J.; Bohacova, S.; Chudoba, J.; Metelkova, R.; Cibulka, R. Electron-deficient heteroarenium salts: An organocatalytic tool for activation of hydrogen peroxide in oxidations. J. Org. Chem. 2015, 80, 2676–2699. [Google Scholar] [CrossRef] [PubMed]
- Rahimizadeh, M.; Rajabzadeh, G.; Khatami, S.M.; Eshghi, H.; Shiri, A. TiO2 nanoparticles and Preyssler-type heteropoly acid modified nano-sized TiO2: A facile and efficient catalyst for the selective oxidation of sulfides to sulfones and sulfoxides. J. Mol. Catal. A Chem. 2010, 323, 59–64. [Google Scholar] [CrossRef]
- Veerakumar, P.; Lu, Z.Z.; Velayudham, M.; Lu, K.L.; Rajagopal, S. Alumina supported nanoruthenium as efficient heterogeneous catalyst for the selective H2O2 oxidation of aliphatic and aromatic sulfides to sulfoxides. J. Mol. Catal. A Chem. 2010, 332, 128–137. [Google Scholar] [CrossRef]
- Rajabi, F.; Naserian, S.; Primo, A.; Luque, R. Efficient and highly selective aqueous oxidation of sulfides to sulfoxides at room temperature catalysed by supported iron oxide nanoparticles on SBA-15. Adv. Synth. Catal. 2011, 353, 2060–2066. [Google Scholar] [CrossRef]
- Tundo, P.; Romanelli, G.P.; Vazquez, P.G.; Arico, F. Multiphase oxidation of alcohols and sulfides with hydrogen peroxide catalyzed by heteropolyacids. Catal. Commun. 2010, 11, 1181–1184. [Google Scholar] [CrossRef]
- Donoeva, B.G.; Trubitsina, T.A.; Maksimov, G.M.; Maksimovskaya, R.I.; Kholdeeva, O.A. Catalytic properties and stability of the heteropolytungstate P2W21O71(H2O)36− in H2O2-based oxidations. Eur. J. Inorg. Chem. 2009, 5142–5147. [Google Scholar] [CrossRef]
- Ezzat, R.; Narges, N. Keggin type heteropoly acid, encapsulated in metal-organic framework: A heterogeneous and recyclable nanocatalyst for selective oxidation of sulfides and deep desulfurization of model fuels. J. Mol. Catal. A Chem. 2015, 398, 17–25. [Google Scholar]
- Shaabani, A.; Rezayan, A.H. Silica sulfuric acid promoted selective oxidation of sulfides to sulfoxides or sulfones in the presence of aqueous H2O2. Catal. Commun. 2007, 8, 1112–1116. [Google Scholar] [CrossRef]
- Rostami, A.; Navasi, Y.; Moradi, D.; Ghorbani-Choghamarani, A. DABCO tribromide immobilized on magnetic nanoparticle as a recyclable catalyst for the chemoselective oxidation of sulfide using H2O2 under metal- and solvent-free conditions. Catal. Commun. 2014, 43, 16–20. [Google Scholar] [CrossRef]
- Islam, S.M.; Roy, A.S.; Mondal, P.; Salam, N.; Paul, S. Polymer-anchored Cu(II) complex as an efficient catalyst for selective and mild oxidation of sulfides and oxidative bromination reaction. Catal. Lett. 2013, 143, 225–233. [Google Scholar] [CrossRef]
- Sharma, R.K.; Pandey, A.; Gulati, S. Silica-supported molybdenum complex: A novel, selective and reusable organic-inorganic hybrid catalyst for eco-friendly oxidation of sulfides and olefins. Polyhedron 2012, 45, 86–93. [Google Scholar] [CrossRef]
- Tshentu, Z.R.; Togo, C.; Walmsley, R.S. Polymer-anchored oxovanadium(IV) complex for the oxidation of thioanisole, styrene and ethylbenzene. J. Mol. Catal. A Chem. 2010, 318, 30–35. [Google Scholar] [CrossRef]
- Khodaei, M.M.; Bahrami, K.; Arabi, M.S. Oxidation of sulfides to sulfoxides with H2O2/HNO3 reagent system. J. Sulfur Chem. 2010, 31, 83–88. [Google Scholar] [CrossRef]
- Liu, Z.M.; Zhao, H.; Li, M.Q.; Lan, Y.B.; Yao, Q.B.; Tao, J.C.; Wang, X.W. Chiral phosphoric acid-catalyzed asymmetric oxidation of aryl alkyl sulfides and aldehyde-derived 1,3-dithianes: using aqueous hydrogen peroxide as the terminal oxidant. Adv. Synth. Catal. 2012, 354, 1012–1022. [Google Scholar] [CrossRef]
- Rostami, A.; Hassanian, F.; Ghorbani-Choghamarani, A.; Saadati, S. Selective oxidation of sulfides to sulfoxides using H2O2 catalyzed by p-toluenesulfonic acid (p-TsOH) under solvent-free conditions. Phosphorus Sulfur 2013, 188, 833–838. [Google Scholar] [CrossRef]
- Lakouraj, M.M.; Hasantabar, V. Alumina-supported monoperphosphoric acid for selective oxidation of sulfides to sulfoxides. J. Sulfur Chem. 2011, 32, 93–98. [Google Scholar] [CrossRef]
- Zolfigol, M.A.; Khazaei, A.; Safaiee, M.; Mokhlesi, M.; Rostamian, R.; Bagheri, M.; Shiri, M.; Kruger, H.G. Application of silica vanadic acid as a heterogeneous, selective and highly reusable catalyst for oxidation of sulfides at room temperature. J. Mol. Catal. A Chem. 2013, 370, 80–86. [Google Scholar] [CrossRef]
- Shen, H.M.; Ji, H.B. Amino alcohol-modified beta-cyclodextrin inducing biomimetic asymmetric oxidation of thioanisole in water. Carbohydr. Res. 2012, 354, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.M.; Ji, H.B. Biomimetic asymmetric aldol reactions catalyzed by proline derivatives attached to beta-cyclodextrin in water. Tetrahedron Lett. 2012, 53, 3541–3545. [Google Scholar] [CrossRef]
- Shen, H.M.; Ji, H.B. Cyclodextrin-RuCl2(Arene)2 conjugates: Another way to enhance the enantioselectivity of aromatic ketones reduction by aromatic ligands’ volume. Tetrahedron 2013, 69, 8360–8367. [Google Scholar] [CrossRef]
- Karimi, B.; Khorasani, M. Selectivity adjustment of SBA-15 based tungstate catalyst in oxidation of sulfides by incorporating a hydrophobic organic group inside the mesochannels. ACS Catal. 2013, 3, 1657–1664. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, H.-M.; Zhou, W.-J.; Ma, X.; Wu, H.-K.; Yu, W.-B.; Ai, N.; Ji, H.-B.; Shi, H.-X.; She, Y.-B. pH-Dependence of the Aqueous Phase Room Temperature Brønsted Acid-Catalyzed Chemoselective Oxidation of Sulfides with H2O2. Molecules 2015, 20, 16709-16722. https://doi.org/10.3390/molecules200916709
Shen H-M, Zhou W-J, Ma X, Wu H-K, Yu W-B, Ai N, Ji H-B, Shi H-X, She Y-B. pH-Dependence of the Aqueous Phase Room Temperature Brønsted Acid-Catalyzed Chemoselective Oxidation of Sulfides with H2O2. Molecules. 2015; 20(9):16709-16722. https://doi.org/10.3390/molecules200916709
Chicago/Turabian StyleShen, Hai-Min, Wen-Jie Zhou, Xin Ma, Hong-Ke Wu, Wu-Bin Yu, Ning Ai, Hong-Bing Ji, Hong-Xin Shi, and Yuan-Bin She. 2015. "pH-Dependence of the Aqueous Phase Room Temperature Brønsted Acid-Catalyzed Chemoselective Oxidation of Sulfides with H2O2" Molecules 20, no. 9: 16709-16722. https://doi.org/10.3390/molecules200916709






