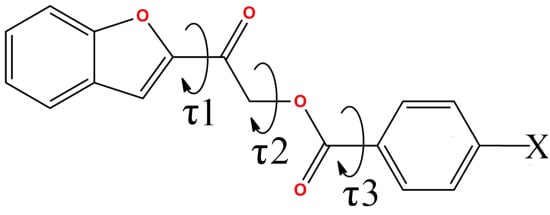
Benzofuranyl Esters: Synthesis, Crystal Structure Determination, Antimicrobial and Antioxidant Activities
Abstract
:1. Introduction
2. Results and Discussion
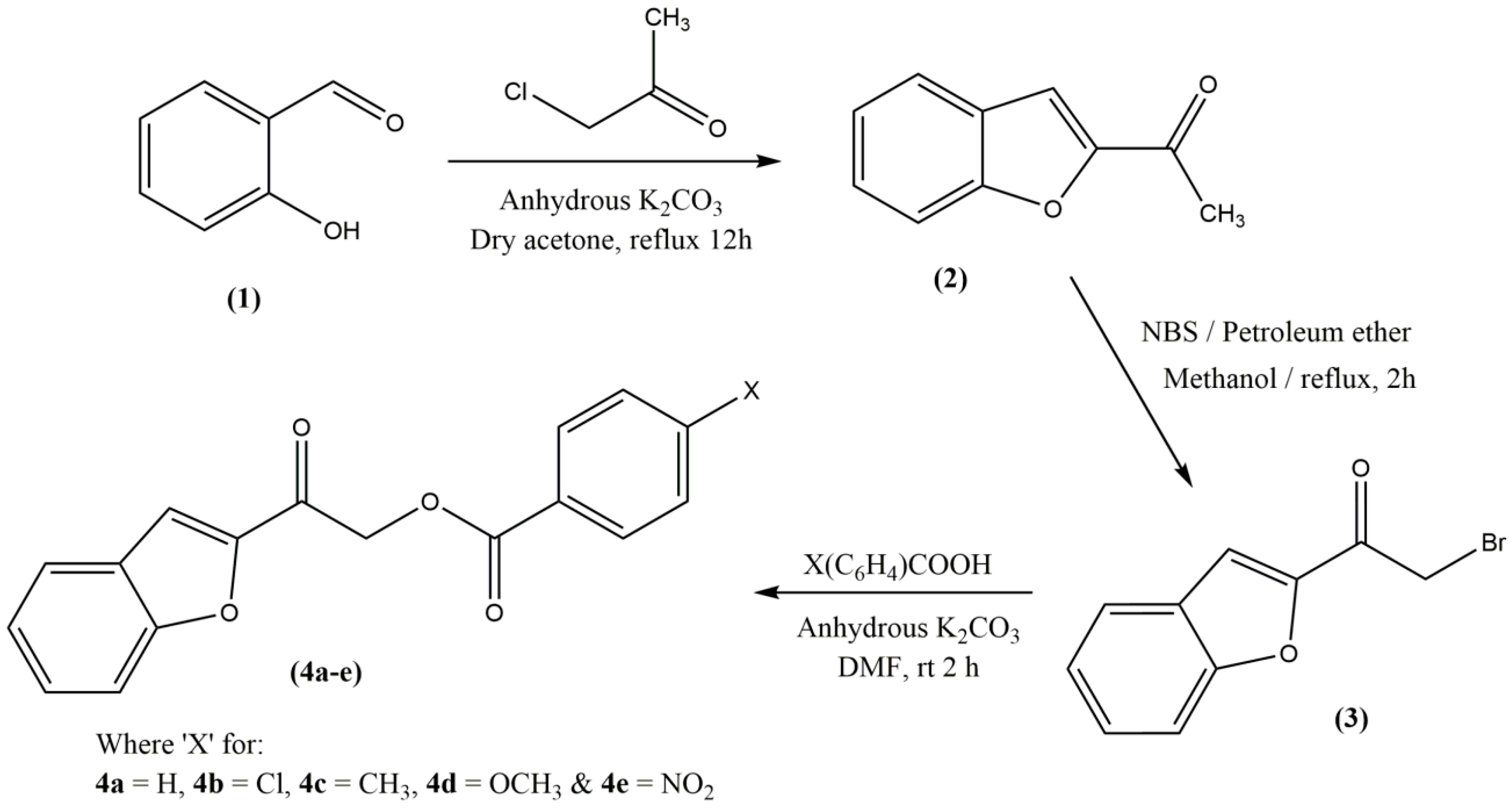
2.1. Chemistry
2.2. X-ray Crystal Structure Description
| Compound | 4a | 4b | 4c | 4d | 4e |
|---|---|---|---|---|---|
| CCDC Deposition Number | 1,037,756 | 1,037,759 | 1,037,762 | 1,037,763 | 1,037,764 |
| Molecular Formula | C17H12O4 | C17H11ClO4 | C18H14O4 | C18H14O5 | C17H11NO6 |
| Molecular Weight | 280.27 | 314.71 | 294.29 | 310.29 | 325.27 |
| Crystal System | Monoclinic | Monoclinic | Monoclinic | Triclinic | Triclinic |
| Space Group | P21/n | C2/c | P21/n | ||
| a (Å) | 10.2230(9) | 33.483(5) | 12.5266(13) | 6.9335(6) | 6.5424(10) |
| b (Å) | 8.4353(7) | 5.3687(8) | 6.7689(7) | 8.4724(7) | 13.197(2) |
| c (Å) | 16.1205(15) | 26.404(4) | 18.0335(19) | 12.8702(11) | 16.973(3) |
| α (°) | 90 | 90 | 90 | 103.539(2) | 79.694(3) |
| β (°) | 98.300(2) | 114.833(3) | 108.165(2) | 95.789(2) | 87.211(3) |
| γ (°) | 90 | 90 | 90 | 92.664(2) | 85.715(3) |
| V (Å3) | 1375.6(2) | 4307.6(10) | 1452.9(3) | 729.36(11) | 1436.9(4) |
| Z | 4 | 12 | 4 | 2 | 4 |
| Dcalc (g·cm−3) | 1.353 | 1.456 | 1.345 | 1.413 | 1.504 |
| Crystal Dimension (mm) | 0.25 × 0.36 × 0.48 | 0.08 × 0.17 × 0.46 | 0.14 × 0.24 × 0.46 | 0.20 × 0.27 × 0.41 | 0.12 × 0.20 × 0.41 |
| µ (mm−1) | 0.10 | 0.28 | 0.10 | 0.10 | 0.12 |
| Tmin/Tmax | 0.901/0.976 | 0.857/0.977 | 0.903/0.987 | 0.899/0.980 | 0.903/0.986 |
| Reflections Measured | 15,170 | 17,994 | 16,236 | 14,866 | 37,178 |
| Indices Range (h, k, l) | −14, 9; | −40, 40; | −17, 16; | −9, 9; | −7, 7; |
| −9, 11; | −6, 6; | −9, 9; | −11, 11; | −15, 15; | |
| −22, 22 | −32, 32 | −25, 24 | −18, 18 | −20, 20 | |
| θ Limit (°) | 2.2–30.2 | 1.7–26.0 | 1.8–30.0 | 1.6–30.2 | 1.6–25.0 |
| Unique Reflections | 4037 | 4235 | 4234 | 4283 | 4946 |
| Observed Reflections (I > 2σ(I)) | 2630 | 2044 | 2835 | 3078 | 3889 |
| Parameters | 190 | 397 | 200 | 209 | 433 |
| Goodness of Fit on F2 | 1.04 | 0.98 | 1.03 | 1.07 | 1.11 |
| R1,wR2 [I > 2σ(I)] | 0.047, 0.162 | 0.044, 0.143 | 0.045, 0.148 | 0.045, 0.149 | 0.065, 0.216 |
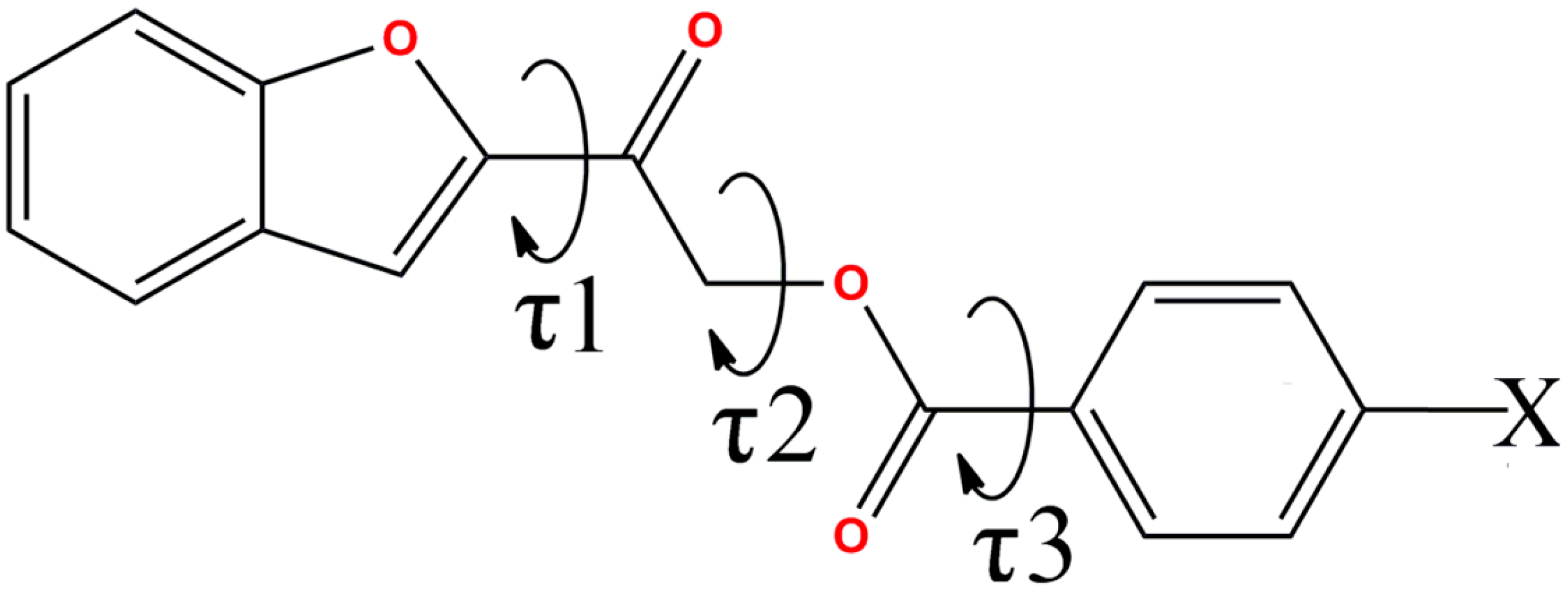
| Compound | O1–C8–C9–O3 (τ1,°) | C9–C10–O2–C11 (τ2,°) | O2–C11–C12–C13 (τ3,°) |
|---|---|---|---|
| 4a | −176.92(14) | 75.19(17) | 171.53(13) |
| 4b | 5.3(4), −175(2) | 162.5(2), −70.2(13) | −176.7(2), −172(2) |
| 4c | −3.5(2) | 78.36(16) | −171.25(12) |
| 4d | −2.62(19) | 79.49(15) | −169.34(11) |
| 4e | 7.2(4), 2.5(4) | 177.4(2), 179.6(3) | −175.4(3), −175.6(3) |

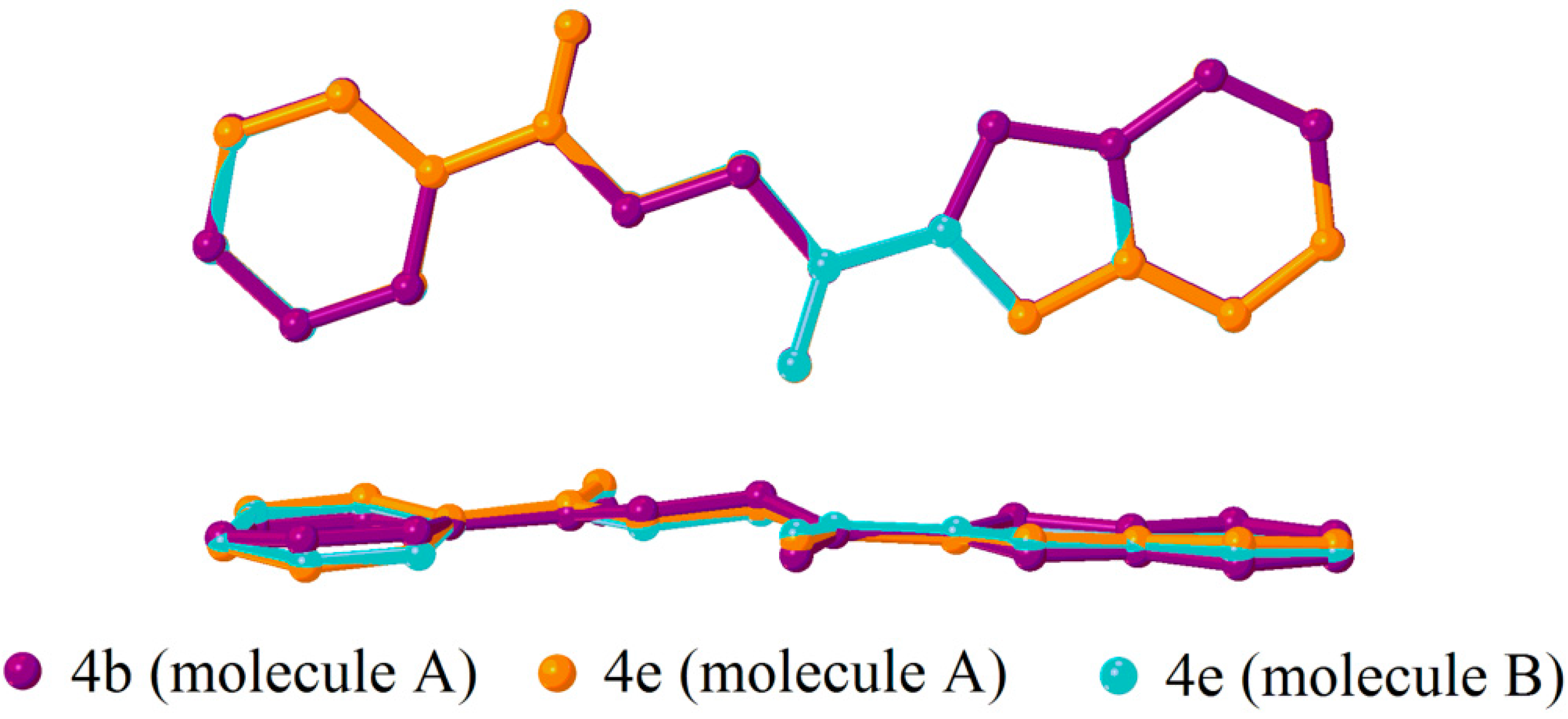
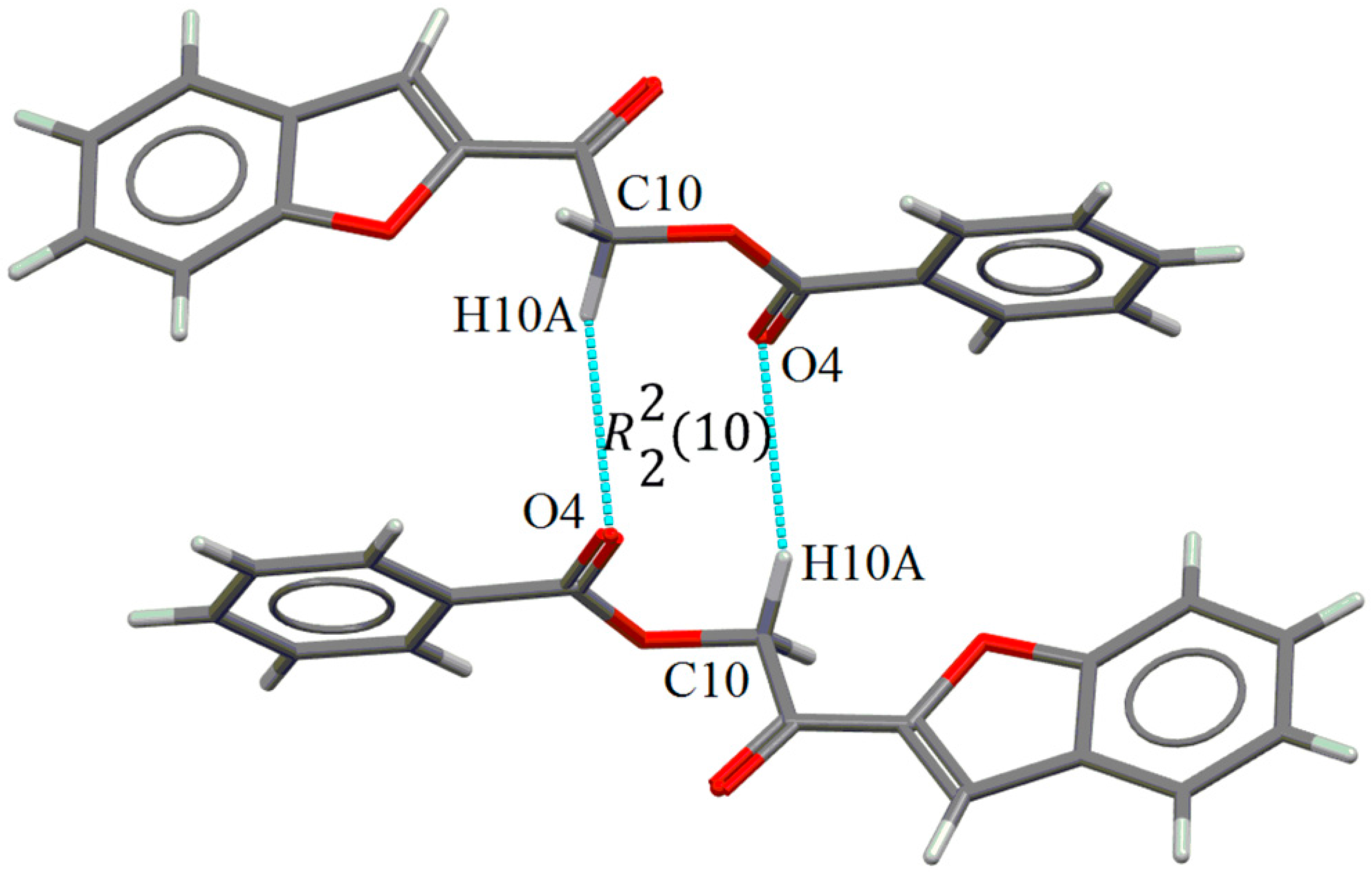
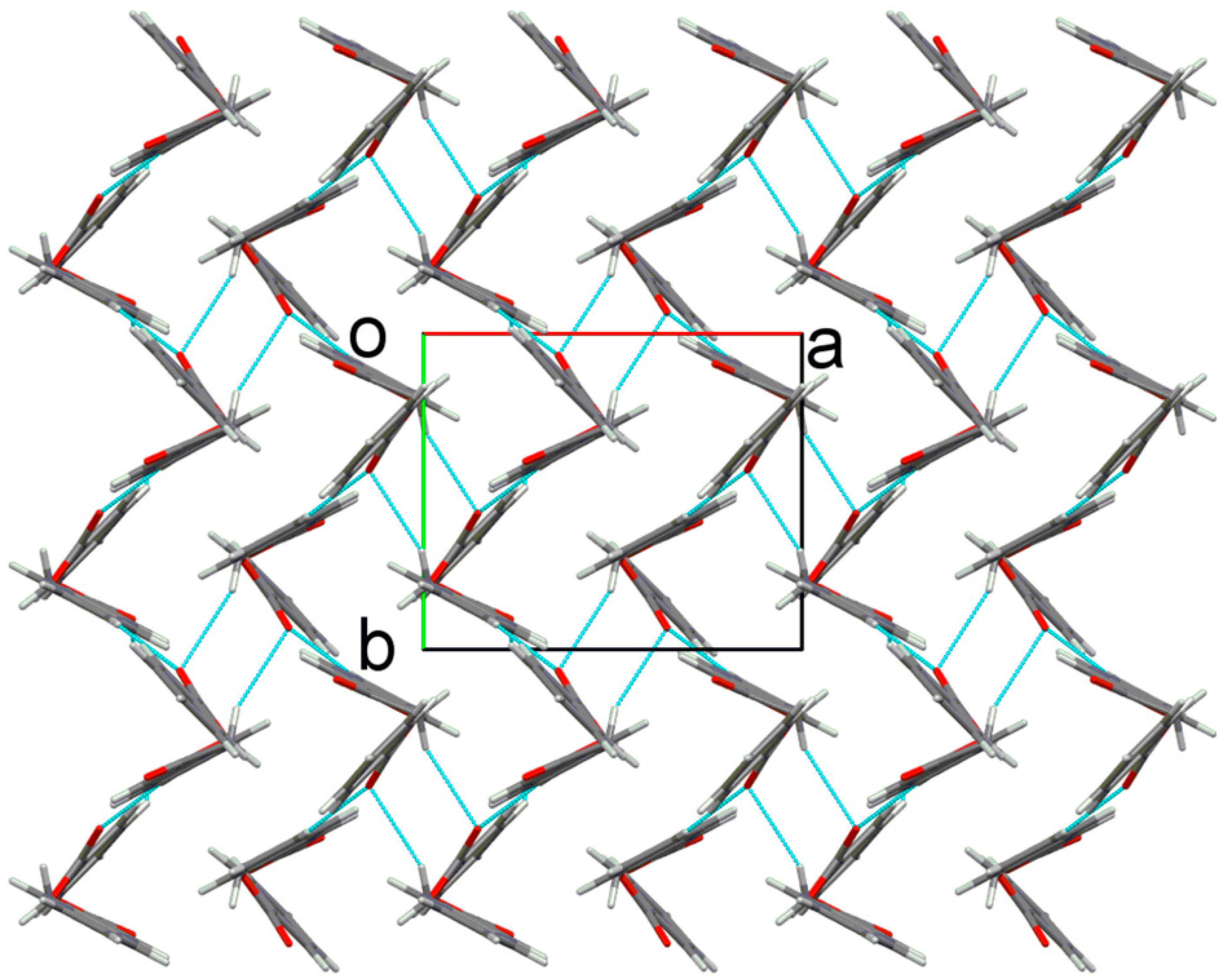

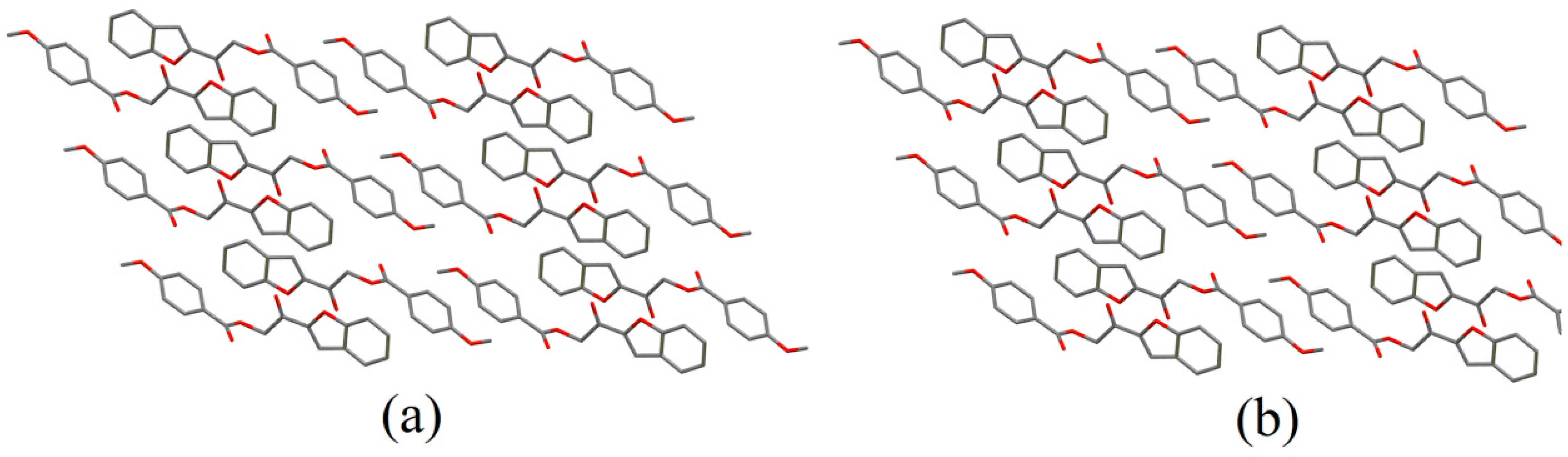
2.3. Antimicrobial Activities of the Synthesized Compound
| Compound | Gram Negative | Gram Positive | Yeast | |||||
|---|---|---|---|---|---|---|---|---|
| Escherichia coli ATCC 25922 | Klebsiella pneumoniae ATCC 13883 | Pseudomonas aeruginosa ATCC 27853 | Salmonella typhimurium ATCC 14028 | Bacillus licheniformis ATCC 12759 | Streptococcus mutans ATCC 25175 | Staphylococcus aureus ATCC 700699 | Candida albicans ATCC 10231 | |
| 4a | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
| 4b | - | - | - | - | - | - | - | - |
| 4c | 500 | 500 | 500 | 500 | - | 500 | 500 | 500 |
| 4d | 500 | 500 | 500 | 250 | 250 | 250 | 250 | 250 |
| 4e | 250 | 250 | 250 | 125 | 250 | 125 | 250 | 125 |
| Tetracycline | 0.977 | 1.953 | 7.813 | 0.977 | 1.953 | 0.244 | 31.25 | 0.122 |
2.4. Antioxidant Activities of the Synthesized Compound
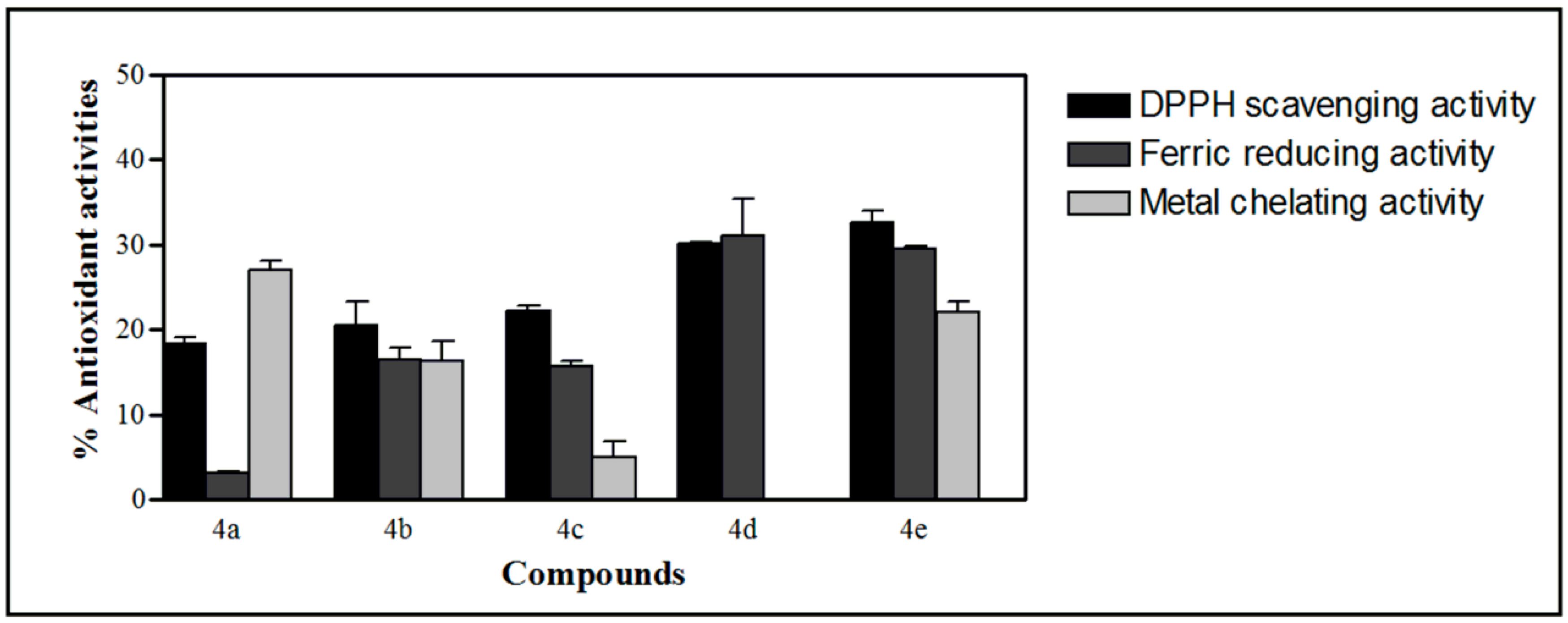
3. Experimental Section
3.1. Instrumentation
3.2. X-ray Diffraction Analysis
3.3. Synthesis
3.3.1. Synthesis of 1-(Benzofuran-2-yl)ethanone (2) and -(Benzofuran-2-yl)-2-bromoethan-1-one (3)
3.3.2. General Procedure for the Synthesis of 2‐(1‐Benzofuran‐2‐yl)‐2‐oxoethyl 4 (Unsubstituted/substituted)benzoates 4(a–e)
3.4. Antimicrobial Activities
3.5. Antioxidant Activities
3.5.1. Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Assay
3.5.2. Ferric Reducing Antioxidant Power (FRAP) Assay
3.5.3. Metal Chelating (MC) Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nagahara, T.; Yokoyama, Y.; Inamura, K.; Katakura, S.; Komoriya, S.; Yamaguchi, H.; Hara, T.; Iwamoto, M. Dibasic (amidinoaryl)propanoic acid derivatives as novel blood coagulation factor Xa inhibitors. J. Med. Chem. 1994, 37, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Rees, C.W.; Scriven, E.F.V. Comprehensive Heterocyclic Chemistry II; Pergamon Press: Oxford, UK, 1996; Volume 2, pp. 259–321. [Google Scholar]
- Furan. Encyclopædia Britannica. Available online: http://global.britannica.com/EBchecked/topic/222526/furan (accessed on 25 January 2015).
- Fuganti, C.; Serra, S. A new approach to 2-aryl-7-alkoxy-benzofurans: Synthesis of ailanthoidol, a natural neolignan. Tetrahedron Lett. 1998, 39, 5609–5610. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Abdel-Aziz, H.A.; Ahmed, E.M. Synthesis and antimicrobial evaluation of 1-(benzofuran-2-yl)-4-nitro-3-arylbutan-1-ones and 3-(benzofuran-2-yl)-4,5-dihydro-5-aryl-1-[4-(aryl)-1,3-thiazol-2-yl]-1H-pyrazoles. Eur. J. Med. Chem. 2009, 44, 2632–2635. [Google Scholar] [CrossRef] [PubMed]
- Gubin, J.; de Vogelaer, H.; Inion, H.; Houben, C.; Lucchetti, J.; Mahaux, J.; Rosseels, G.; Peiren, M.; Clinet, M.; Polster, P. Novel heterocyclic analogues of the new potent class of calcium entry blockers: 1-[[4-(Aminoalkoxy)phenyl]sulfonyl]indolizines. J. Med. Chem. 1993, 36, 1425–1433. [Google Scholar]
- Pietinen, P.; Stumpf, K.; Mannisto, S.; Kataja, V.; Uusitupa, M.; Adlercreutz, H. Serum enterolactone and risk of breast cancer: A case-control study in eastern Finland. Cancer Epidemiol. Biomark Prev. 2001, 10, 339–344. [Google Scholar]
- Silva, D.H.S.; Pereira, F.C.; Zanoni, M.V.B.; Yoshida, M. Lipophyllic antioxidants from Iryanthera juruensis fruits. Phytochemistry 2001, 57, 437–442. [Google Scholar] [CrossRef]
- Lambert, J.D.; Meyers, R.O.; Timmermann, B.N.; Dorr, R.T. Tetra-O-methylnordihydroguaiaretic acid inhibits melanoma in vivo. Cancer Lett. 2001, 171, 47–56. [Google Scholar] [CrossRef]
- Brader, P.G.; Greger, H.; Bacher, M.; Kalchhauser, H.; Hofer, O.; Vajrodaya, S.J. Bisamides, lignans, triterpenes, and insecticidal Cyclopenta[b]benzofurans from Aglaia species. Nat. Prod. 1998, 61, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, R.; Nagao, T.; Okabe, H.; Nakano, Y.; Matsunaga, H.; Katano, M.; Mori, M. Antiproliferative constituents in umbelliferae plants. III. Constituents in the root and the ground part of Anthriscus sylvestris Hoffm. Chem. Pharm. Bull. 1998, 46, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.; Charlton, J.L.; Cow, C. Antiviral activity of arylnaphthalene and aryldihydronaphthalene lignans. Can. J. Chem. 2000, 78, 553–561. [Google Scholar]
- Zacchino, S.; Rodriguez, G.; Pezzenati, G.; Orellana, G.; Enriz, R.; Gonzalez, S.M. In vitro evaluation of antifungal properties of 8.O.4′-neolignans. Nat. Prod. 1997, 60, 659–662. [Google Scholar]
- Chen, C.C.; Hsin, W.C.; Ko, F.N.; Huang, Y.L.; Ou, J.C.; Teng, C.M. Antiplatelet arylnaphthalide lignans from Justicia procumbens. J. Nat. Prod. 1996, 59, 1149–1150. [Google Scholar]
- Day, S.H.; Chiu, N.Y.; Tsao, L.T.; Wang, J.P.; Lin, C.N. New lignan glycosides with potent antiinflammatory effect, isolated from Justicia ciliata. J. Nat. Prod. 2000, 63, 1560–1562. [Google Scholar]
- Cho, J.Y.; Kim, A.R.; Yoo, E.S.; Baik, K.U.; Park, M.H. Immunomodulatory effect of arctigenin, a lignan compound, on tumour necrosis factor-alpha and nitric oxide production, and lymphocyte proliferation. J. Pharm. Pharmacol. 1999, 51, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.S. Lignans, neolignans, and related compounds. Nat. Prod. Rep. 1995, 12, 183–205. [Google Scholar] [CrossRef] [PubMed]
- Den Tonkelaar, I.; Keinan-Boker, L.; Van’t Veer, P.; Arts, C.J.M.; Adlercreutz, H.; Thijssen, J.H.H.; Peeters, P.H.M. Urinary phytoestrogens and postmenopausal breast cancer risk. Cancer Epidemiol. Biomark Prev. 2001, 10, 223–228. [Google Scholar]
- Oter, O.; Ertekin, K.; Kirilmis, C.; Koca, M.; Ahmedzade, M. Characterization of a newly synthesized fluorescent benzofuran derivative and usage as a selective fiber optic sensor for Fe (III). Sens. Act. B Chem. 2007, 122, 450–456. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Introduction to Spectroscopy, 4th ed.; Brook/Cole Cengage Learning: Belmont, CA, USA, 2001. [Google Scholar]
- Aslam, S.N.; Stevenzon, P.C.; Phythian, S.J.; Veith, N.C.; Hall, D.R. Synthesis of cicerfuran, an antifungal benzofuran, and some related analogues. Tetrahedron 2006, 62, 4214–4226. [Google Scholar] [CrossRef]
- Kirilmis, C.; Ahmedzade, M.; Servi, S.; Koca, M.; Kizirgil, A.; Kazaz, C. Synthesis and antimicrobial activity of some novel derivatives of benzofuran: Part 2. The synthesis and antimicrobial activity of some novel 1-(1-benzofuran-2-yl)-2-mesitylethanone derivatives. Eur. J. Med. Chem. 2008, 43, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Galal, S.A.; El-All, A.S.A.; Hegab, K.H.; Magd-El-Din, A.A.; Youssef, N.; El-Diwani, H.I. Novel antiviral benzofuran-transition metal complexes. Eur. J. Med. Chem. 2010, 45, 3035–3046. [Google Scholar] [CrossRef] [PubMed]
- Salomé, C.; Narbonne, V.; Ribeiro, N.; Thuaud, F.; Serova, M.; de Gramont, A.; Faivre, S.; Raymond, E.; Désaubry, L. Benzofuran derivatives as a novel class of inhibitors of mTOR signaling. Eur. J. Med. Chem. 2014, 74, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.S.C.; Chia, T.S.; Chandraju, S.; Ooi, C.W.; Quah, C.K.; Fun, H.K. Conformational studies of 2-(4-bromophenyl)-2-oxoethylbenzoates. Z. Kristallogr. 2014, 229, 328–342. [Google Scholar]
- Fun, H.K.; Arshad, S.; Garudachari, B.; Isloor, A.M.; Satyanarayan, M.N. 2-Oxo-2-phenyl-ethyl benzoate. Acta Cryst. 2011, E67, o1528. [Google Scholar] [CrossRef]
- Fun, H.K.; Loh, W.S.; Garudachari, B.; Isloor, A.M.; Satyanarayan, M.N. 2-(4-Bromo-phenyl)-2-oxoethyl 4-meth-oxy-benzoate. Acta Cryst. 2011, E67, o1529. [Google Scholar] [CrossRef]
- Bruker. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Harish Kumar, D.R.; Karvekar, M.D. Synthesis of Benzofuran Derivatives and their Evaluation of Antimicrobial Activity. E-J.Chem. 2010, 7, 636–640. [Google Scholar] [CrossRef]
- Kumar, C.S.C.; Fun, H.K.; Tursun, M.; Ooi, C.W.; Chandraju, S.; Quah, C.K.; Parlak, C. Synthesis, molecular structure, FT-IR and XRD investigations of 2-(4-chlorophenyl)-2-oxoethyl 2-chlorobenzoate: A comparative DFT study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 124C, 595–602. [Google Scholar]
- Kumar, C.S.C.; Panicker, C.Y.; Fun, H.K.; Sheena Mary, Y.; Harikumar, B.; Chandraju, S.; Quah, C.K.; Ooi, C.W. FT-IR, molecular structure, first order hyperpolarizability, HOMO and LUMO analysis, MEP and NBO analysis of 2-(4-chlorophenyl)-2-oxoethyl 3-nitrobenzoate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 126C, 208–219. [Google Scholar]
- Sivasothy, Y.; Sulaiman, S.F.; Ooi, K.L.; Ibrahim, H.; Awang, K. Antioxidant and antibacterial activities of flavonoids and curcuminoids from Zingiber spectabile Griff. Food Control 2013, 30, 714–720. [Google Scholar] [CrossRef]
- Ooi, K.L.; Muhammad, T.S.T.; Tan, M.L.; Sulaiman, S.F. Cytotoxic, poptotic and anti-α-glucosidase activities of 3,4-di-O-caffeoyl quinic acid, an antioxidant isolated from the polyphenolic-rich extract of Elephantopus mollis Kunth. J. Ethnopharmacol. 2011, 135, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 4(a–e) are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, C.S.C.; Then, L.Y.; Chia, T.S.; Chandraju, S.; Win, Y.-F.; Sulaiman, S.F.; Hashim, N.S.; Ooi, K.L.; Quah, C.K.; Fun, H.-K. Benzofuranyl Esters: Synthesis, Crystal Structure Determination, Antimicrobial and Antioxidant Activities. Molecules 2015, 20, 16566-16581. https://doi.org/10.3390/molecules200916566
Kumar CSC, Then LY, Chia TS, Chandraju S, Win Y-F, Sulaiman SF, Hashim NS, Ooi KL, Quah CK, Fun H-K. Benzofuranyl Esters: Synthesis, Crystal Structure Determination, Antimicrobial and Antioxidant Activities. Molecules. 2015; 20(9):16566-16581. https://doi.org/10.3390/molecules200916566
Chicago/Turabian StyleKumar, C. S. Chidan, Li Yee Then, Tze Shyang Chia, Siddegowda Chandraju, Yip-Foo Win, Shaida Fariza Sulaiman, Nurul Shafiqah Hashim, Kheng Leong Ooi, Ching Kheng Quah, and Hoong-Kun Fun. 2015. "Benzofuranyl Esters: Synthesis, Crystal Structure Determination, Antimicrobial and Antioxidant Activities" Molecules 20, no. 9: 16566-16581. https://doi.org/10.3390/molecules200916566